Feeding Patterns of Fish in Relation to the Trophic Status of Reservoirs: A Case Study of Rutilus rutilus (Linnaeus, 1758) in Five Fishing Waters in Serbia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Fish Sampling
2.2. Content Analysis of the Digestive Tract
2.3. Statistical Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wahltinez, S.J.; Kroll, K.J.; Behringer, D.C.; Arnold, J.E.; Whitaker, B.; Newton, A.L.; Edmiston, K.; Hewson, I.; Stacy, N.I. Common Sea Star (Asterias rubens) Coelomic Fluid Changes in Response to Short-Term Exposure to Environmental Stressors. Fishes 2023, 8, 51. [Google Scholar] [CrossRef]
- Bănăduc, D.; Simić, V.M.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simi, S.B. Freshwater as a Sustainable Resource and Generator of Secondary Resources in the 21st Century: Stressors, Threats, Risks, Management and Protection Strategies, and Conservation Approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef] [PubMed]
- Simian, C.; Georgiev, V.; Curtean-Bănăduc, A. Study on the biodiversity-biotope factors’ relations. In Proceedings of the WSEAS International Conference on Mathematics and Computers in Biology and Chemistry, Book Series Recent Advances in Biology and Biomedicine, Prague, Czech Republic, 23–25 March 2009; Volume 184. [Google Scholar]
- Bănăduc, D.; Barinova, S.; Cianfaglione, K.; Curtean-Bănăduc, A. Editorial: Multiple freshwater stressors-Key drivers for the future of freshwater environments. Front. Environ. Sci. 2023, 11, 92. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Olosutean, H.; Bănăduc, D. Influence of Environmental Variables on the Structure and Diversity of Ephemeropteran Communities: A Case Study of the Timiș River, Romania. Acta Zool. Bulg. 2016, 68, 215–224. [Google Scholar]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Villéger, S.; Brosse, S.; Mouchet, M.A.; Mouillot, D.; Vanni, M.J. Functional ecology of fish: Current approaches and future challenges. Aquat. Sci. 2017, 79, 783–801. [Google Scholar] [CrossRef]
- Simić, V.; Bănăduc, D.; Curtean-Bănăduc, A.; Petrović, A.; Veličković, T.; Stojković-Piperac, M.; Simić, S. Assessment of the ecological sustainability of river basins based on the modified the ESHIPPO fish model on the example of the Velika Morava basin (Serbia, Central Balkans). Front. Environ. Sci. 2022, 10, 952692. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Marić, S.; Gabor, G.; Didenko, A.; Rey Planellas, S.; Bănăduc, D. Hucho hucho (Linnaeus, 1758): Last natural viable population in the Eastern Carpathians—Conservation elements. Turk. J. Zool. 2019, 43, 215–223. [Google Scholar] [CrossRef]
- Bănăduc, D.; Sas, A.; Cianfaglione, K.; Barinova, S.; Curtean-Bănăduc, A. The role of aquatic refuge habitats for fish, and threats in the context of climate change and human impact, during seasonal hydrological drought in the Saxon Villages area (Transylvania, Romania). Atmosphere 2021, 12, 1209. [Google Scholar] [CrossRef]
- Zare-Shahraki, M.; Ebrahimi-Dorche, E.; Bruder, A.; Flotermersch, J.; Blocksom, K.; Bănăduc, D. Fish species composition, distribution and community structure in relation to environmental variation in a semi-arid mountainous river basin, Iran. Water 2022, 14, 2226. [Google Scholar] [CrossRef]
- Bănăduc, D.; Maric, S.; Cianfaglione, K.; Afanasyev, S.; Somogyi, D.; Nyeste, K.; Antal, L.; Kosco, J.; Caleta, M.; Wanzenbock, J.; et al. Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species drive a Fish to the Edge of Extinction. Sustainability 2022, 14, 13493. [Google Scholar] [CrossRef]
- Kryštufek, B.; Reed, J.M. Pattern and process in Balkan biodiversity—An overview. In Balkan Biodiversity Pattern and Process in the European Hotspot; Griffiths, H.I., Kryttufek, B., Reed, J.M., Eds.; Kluwer Publishers: Dordrecht, The Netherlands, 2004; pp. 203–217. [Google Scholar]
- Oikonomou, A.; Leprieur, F.; Leonardos, I.D. Biogeography of freshwater fishes of the Balkan Peninsula. Hydrobiologia 2014, 738, 205–220. [Google Scholar] [CrossRef]
- Web, I.T.; Bartlein, P.J. Global changes during the last 3 million years: Climatic controls and biotic responses. Annu. Rev. Ecol. Syst. 1992, 23, 141–172. [Google Scholar] [CrossRef]
- Gill, J.L.; Blois, J.L.; Benito, B.; Dombrowski, S.; Hunter, M.L., Jr.; McGuire, J.L. A 2.5-million-year perspective on coarse-filter strategies for conserving nature’s stage. Conserv. Biol. 2015, 29, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Shiklomanov, I.A. World Water Resources at the Beginning of the 21st Century; Monograph prepared and subimitted to UNESCO; Division of Water Sciences Hydrological Institute, International Hydrological Programme (IHP), UNESCO: Paris, France, 1998; p. 37. [Google Scholar]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Snickars, M.; Weigel, B.; Bonsdorff, E. Impact of eutrophication and climate change on fish and zoobenthos in coastal waters of the Baltic Sea. Mar. Biol. 2015, 162, 141–151. [Google Scholar] [CrossRef]
- Nazari-Sharabian, M.; Ahmad, S.; Karakouzian, M. Climate change and eutrophication: A short review. Eng. Technol. Appl. Sci. Res. 2018, 8, 3668–3672. [Google Scholar] [CrossRef]
- Ficke, A.; Myrick, C.; Hansen, L. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007, 17, 581–613. [Google Scholar] [CrossRef]
- Bobori, D.C.; Salvarina, I.; Michaloudi, E. Fish dietary patterns in the eutrophic lake Volvi (East mediterranean). J. Biol. Res. Thessalon. 2013, 19, 139–149. [Google Scholar]
- Buzhdygan, O.Y.; Stojković Piperac, M.; Stamenković, O.; Čerba, D.; Ostojić, A.; Tietjen, B.; Milošević, D. Human impact induces shifts in trophic composition and diversity of consumer communities in small freshwater ecosystems. In Small Water Bodies of the Western Balkans; Pešić, V., Milošević, D., Miliša, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 389–418. [Google Scholar]
- da Silveira, E.L.; Semmar, N.; Cartes, J.E.; Tuset, V.M.; Lombarte, A.; Ballester, E.L.C.; Vaz-dos-Santos, A.M. Methods for trophic ecology assessment in fishes: A critical review of stomach analyses. Rev. Fish. Sci. Aquac. 2020, 28, 71–106. [Google Scholar] [CrossRef]
- Dinh, M.Q.; Qin, J.G.; Dittmann, S.; Tran, D.D. Seasonal variation of food and feeding in burrowing goby Parapocryptes serperaster (Gobiidae) at different body sizes. Ichthyol. Res. 2017, 64, 179–198. [Google Scholar] [CrossRef]
- Alieva, A.K.; Nasibulina, B.M.; Bakhshalizadeh, S.; Kurochkina, T.F.; Popov, N.N.; Barbol, B.I.; Bănăduc, D.; Jussupbekova, N.M.; Kuanysheva, G.A.; Ali, A.M. The Low Ontogenetic Diet Diversity and Flexibility of the Pike-Perch, Sander lucioperca (Linnaeus, 1758) (Osteichthyes, Percidae): A Case Study. Fishes 2023, 8, 395. [Google Scholar] [CrossRef]
- Afanasyev, S.; Hupalo, O.; Tymoshenko, N.; Lietytska, O.; Roman, A.; Manturova, O.; Bănăduc, D. Morphological and trophic features of the invasive Babka gymnotrachelus (Gobiidae) in the plain and mountainous ecosystems of the Dniester Basin, spatiotemporal expansion and possible threats to native fishes. Fishes 2023, 8, 427. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Burcea, A.; Mihuţ, C.-M.; Bănăduc, D. The benthic trophic corner stone compartment in POPs transfer from abiotic environment to higher trophic levels—Trichoptera and Ephemeroptera pre-alert indicator role. Water 2021, 13, 1778. [Google Scholar] [CrossRef]
- Bănăduc, D.; Oprean, L.; Bogdan, A.; Curtean-Bănăduc, A. The analyse of the trophic resources utilisation by the congeneric species Barbus barbus (Linnaeus, 1758) and Barbus meridionalis Risso, 1827 in Târnava River Basin (Transylvania, Romania). Transylv. Rev. Syst. Ecol. Res. 2011, 12, 101–118. [Google Scholar]
- Curtean-Bănăduc, A.; Bănăduc, D. Trophic elements regarding the non-indigenous Pseudorasbora parva (Schlegel) 1842 fish species spreading success—Olt River Basin, a case study. Rom. J. Biol. 2008, 6, 185–196. [Google Scholar]
- Syvӓranta, J.; Jones, R.I. Changes in feeding niche widths of perch and roach following biomanipulation, revealed by stable isotope analysis. Freshw. Biol. 2008, 53, 425–434. [Google Scholar] [CrossRef]
- Persson, L.; De Roos, A.M. Mixed competition-predation: Potential vs. realized interactions. J. Anim. Ecol. 2012, 81, 483–493. [Google Scholar] [CrossRef]
- Kohonen, T. Self-organizing formation of topologically correct feature maps. Biol. Cybern. 1982, 43, 59–69. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Pavlović, M.; Simonović, P.; Stojković, M.; Simić, V. Analysis of diet of piscivorous fishes in Bovan, Gruža and Šumarice reservoir, Serbia. Iran. J. Fish. Sci. 2015, 14, 908–923. [Google Scholar]
- Radenković, M. Feeding and Importance of Predatory Fish Species in Maintenance of Ecosystem Stability in Reservoirs. Ph.D. Thesis, University of Kragujevac, Kragujevac, Serbia, 2019. (In Serbian). [Google Scholar]
- Laušević, R.; Cvijan, M. Seasonal and spatial dynamics of phytoplankton in Vlasinsko Jezero reservoir. In Vlasinsko Jezero—Hidrobiološka Studija; Blaženčić, J., Ed.; Biološki Fakultet Beograd: Beograd, Serbia, 1996; pp. 91–129. [Google Scholar]
- Ostojić, A.; Ćurčić, S.; Nedović, M. Trophic status of the Gruža reservoir. In The Reservoir Gruža—Monography; Čomić, L., Ostojić, A., Eds.; Faculty of Science: Kragujevac, Serbia, 2005; pp. 233–245. [Google Scholar]
- Urošević, V. Plankton Primary Production Changes in Gazivode Reservoir; Glasnik Instituta za Botaniku i Botaničke Bašte Univerziteta u Beogradu: Beogradu, Serbia, 1993; Volume XXIV–XXV, pp. 105–113. [Google Scholar]
- Ranković, B.; Simić, S.; Bogdanović, D. Phytoplankton as indicator of water quality of lakes Bubanj and Šumarice during autumn. Kragujev. J. Sci. 2006, 28, 107–114. [Google Scholar]
- Simić, S.; Đorđević, N.; Milošević, D. The relationship between the dominance of Cyanobacteria species and environmental variables in different seasons and after extreme percipitation. Fundam. Appl. Limnol. 2017, 190, 1–11. [Google Scholar] [CrossRef]
- Denić, L.; Đurković, A.; Čađo, S.; Dopuđa Glišić, T.; Novaković, B.; Stojanović, Z. Ocena Ekološkog Potencijala Akumulacije Vrutci na Osnovu Bioloških i Fizičko-Hemijskih Elemenata Kvaliteta; Srpsko Društvo za Zaštitu Voda i Institut za Vodoprivredu: Beograd, Srbija, 2014; pp. 41–47. [Google Scholar]
- Hickley, P.; North, R.; Muchiri, S.M.; Harper, D.M. The diet of largemouth bass, Micropterus salmoides, in Lake Naivasha, Kenya. J. Fish Biol. 1994, 44, 607–619. [Google Scholar] [CrossRef]
- Lorenzoni, M.; Corboli, N.; Dörr, A.J.M.; Giovinazzo, G.; Selvi, S.; Mearelli, M. Diets of Micropterus salmoides Lac. and Esox lucius L. in Lake Trasimeno (Umbria, Italy) and their diet overlap. Bull. Fr. De La Peche Et De La Piscic. 2002, 365–366, 537–547. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach content analysis: A review methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Costello, M.J. Predator feeding strategy and prey importance: A new graphical analysis. J. Fish Biol. 1990, 36, 261–263. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Gabler, H.M.; Staldvik, F.J. A new graphical approach to graphical analysis of feeding strategy from stomach contents data-modification of the Costello (1990) method. J. Fish Biol. 1996, 48, 607–614. [Google Scholar] [CrossRef]
- Cailliet, G.M.; Love, M.S.; Ebeling, A.W. Fishes: A Field and Laboratory Manual on Their Structure Identification and Natural History; Wadsworth Publishing: Belmont, CA, USA, 1986; p. 194. [Google Scholar]
- Lek, S.; Guégan, J.F. Artificial neural networks as a tool in ecological modelling, an introduction. Ecol. Model. 1999, 120, 65–73. [Google Scholar] [CrossRef]
- Park, Y.S.; Tison, J.; Lek, S.; Giraudel, J.L.; Coste, M.; Delmas, F. Application of a self-organizing map to select representative species in multivariate analysis: A case study determining diatom distribution patterns across France. Ecol. Inform. 2006, 1, 247–257. [Google Scholar] [CrossRef]
- Penczak, T.; Głowacki, Ł.; Kruk, A.; Galicka, W. Implementation of a self-organizing map for investigation of impoundment impact on fish assemblages in a large, lowland river: Long-term study. Ecol. Model. 2012, 227, 64–71. [Google Scholar] [CrossRef]
- Stojković, M.; Simić, V.; Milošević, D.; Mančev, D.; Penczak, T. Visualization of fish community distribution patterns using the self-organizing map: A case study of the Great Morava River system (Serbia). Ecol. Model. 2013, 248, 20–29. [Google Scholar] [CrossRef]
- Jain, A.K.; Dubes, R.C. Algorithms for Clustering Data; Prentice-Hall: Hoboken, NJ, USA, 1988; p. 334. [Google Scholar]
- Céréghino, R.; Park, Y.S. Review of the Self-Organizing Map (SOM) approach in water resources: Commentary. Environ. Model. Softw. 2009, 24, 945–947. [Google Scholar] [CrossRef]
- Vesanto, J.; Himberg, J.; Alhoniemi, E.; Parhankangas, J. Som Toolbox for Matlab 5; Techical Report A57; Neural Network Research Centre, Helsinki University of Technology: Helsinki, Finland, 2000; p. 60. [Google Scholar]
- Park, Y.S.; Céréghino, R.; Compin, A.; Lek, S. Applications of artificial neural networks for patterning and predicting aquatic insect species richness in running waters. Ecol. Model. 2003, 160, 265–280. [Google Scholar] [CrossRef]
- Lek, S.; Scardi, M.; Verdonschot, P.F.M.; Descy, J.P.; Park, Y.S. Modelling Community Structure in Freshwater Ecosystems; Springer: Berlin/Heidelberg, Germany, 2005; p. 518. [Google Scholar]
- McCune, B.; Mefford, M.S. PC-ORD: Multivariate Analysis of Ecological Data, version 6.06; MjM Software Design: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Dukowska, M.; Grzybkowska, M.; Kruk, A.; Szczerkowska-Majchrzak, E. Food niche partitioning between perch and ruffe: Combined use of a self-organizing map and IndVal index for analysing fish diet. Ecol. Model. 2013, 265, 221–229. [Google Scholar] [CrossRef]
- Dukowska, M.; Kruk, A.; Grzybkowska, M. Diet overlap between two cyprinids: Eurytopic roach and reophilic dace in tailwater submersed macrophyte patches. Ecol. Inform. 2014, 24, 112–123. [Google Scholar] [CrossRef]
- Manoel, P.S.; Azevedo-Santos, V.M. Fish gut content from biological collections as a tool for long-term environmental impacts studies. Environ. Biol. Fishes 2018, 101, 899–904. [Google Scholar] [CrossRef]
- da Silva, G.B.; Hazin, H.G.; Hazin, F.H.V.; Vaske, T., Jr. Diet composition of bigeye tuna (Thunnus obesus) and yellowfin tuna (Thunnus albacares) caught on aggregated schools in the western equatorial Atlantic Ocean. J. Appl. Ichthyol. 2019, 35, 1111–1118. [Google Scholar] [CrossRef]
- Persson, L. Food consumption and the significance of detritus and algae to intraspecific competition in roach Rutilus rutilus in a shallow eutrophic lake. Oikos 1983, 41, 118–125. [Google Scholar] [CrossRef]
- Brabrand, Å. Food of roach (Rutilus rutilus) and ide (Leuciscus idus): Significance of diet shift for interspecific competition in omnivorous fishes. Oecologia 1985, 437, 101–106. [Google Scholar]
- Zapletal, T.; Mareš, J.; Jurajda, P.; Všetičková, L. The food of roach, Rutilus rutilus (Actinopterygii: Cypriniformes: Cyprinidae), in biomanipulated water supply reservoir. Acta Ichthyol. Piscat. 2014, 44, 15–22. [Google Scholar] [CrossRef]
- Kornijów, R.; Vakkilainen, K.; Horppila, J.; Luokkanen, E.; Kairesalo, T. Impacts of a submerged plant (Elodea canadensis) on interactions between roach (Rutilus rutilus) and its invertebrate prey communities in lake littoral zone. Freshw. Biol. 2005, 50, 262–276. [Google Scholar] [CrossRef]
- Peterka, J.; Matěna, J. Differences in feeding selectivity and efficiency between young-of-the-year European perch (Perca fluviatilis) and roach (Rutilis rutilus)—Field observation and laboratory experiments on the importance of prey movement apparency vs. evasiveness. Biologia 2009, 64, 786–794. [Google Scholar] [CrossRef]
- Karus, K.; Paaver, T.; Agasild, H.; Zingel, P. The effect of predation by planktivorous juvenile fish on the microbial food web. Eur. J. Protistol. 2014, 50, 109–121. [Google Scholar] [CrossRef]
- Vašek, M.; Kubečka, J. In situ diel patterns of zooplankton consumption by subadult/adult roach Rutilus rutilus, bream Abramis brama, and bleak Alburnus alburnus. Folia Zool. 2004, 53, 203–214. [Google Scholar]
- Vašek, M.; Kubečka, J.; Matěna, J.; Sed’a, J. Distribution and diet of 0+ fish within a canyon-shaped European reservoir in late summer. Int. Rev. Hydrobiol. 2006, 91, 178–194. [Google Scholar] [CrossRef]
- Liu, Z.; Uiblein, F. Prey detectability mediates selectivity in a zooplanktivorous Cyprinid (Alburnus alburnus (L.)). Sitzungsber. Abt. I 1996, 20, 3–13. [Google Scholar]
- Tarvainen, M.; Sarvala, J.; Helminen, H. The role of phosphorus release by roach [Rutilus rutilus (L.)] in the water quality changes of a biomanipulated lake. Freshw. Biol. 2002, 47, 2325–2336. [Google Scholar] [CrossRef]
- Radenković, M.; Stojković Piperac, M.; Milošković, A.; Kojadinović, N.; Đuretanović, S.; Veličković, T.; Jakovljević, M.; Nikolić, M.; Simić, V. Diet seasonality and food overlap of Perca fluviatilis and Rutilus rutilus juveniles: A case study on Bovan Reservoir, Serbia. Acta Ichthyol. Et Piscat. 2022, 52, 77–90. [Google Scholar] [CrossRef]
- Bowen, S.H.; Lutz, E.V.; Ahlgren, M.O. Dietary protein and energy as determinants of food quality: Trophic strategies compared. Ecology 1995, 76, 899–907. [Google Scholar] [CrossRef]
- Bogacka-Kapusta, E.; Kapusta, A. The diet of roach, Rutilus rutilus (L.), and bleak, Alburnus alburnus (L.) larvae and fry in the shallow littoral zone of a heated lake. Arch. Pol. Fish 2007, 15, 401–413. [Google Scholar]
- Adamczuk, M.; Mieczan, T. Different levels of precision in studies on the alimentary tract content of omnivorous fish affect predictions of their food niche and competitive interactions. Comptes Rendus Biol. 2015, 338, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.K.; Bellwood, D.R.; Choat, J.H.; Furnas, M.J. Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr. Mar. Biol. 2003, 41, 279–309. [Google Scholar]
- Matěna, J. The role of ecotones as feeding grounds for fish fry in a Bohemian water suppy reservoir. Hydrobiologia 1995, 303, 31–38. [Google Scholar] [CrossRef]
- Matěna, J. Diet spectra and competition between juvenile fish in a pelagic zone of a deep stratified reservoir during the first year of life. Int. Rev. Hydrobiol. 1998, 83, 577–583. [Google Scholar]
- Lyagina, T.N. The seasonal dynamics of biological characteristics of the roach (Rutilus rutilus L.) under conditions of varying food availability. J. Ichthyol. 1972, 12, 210–226. [Google Scholar]
- Vøllestad, L.A. Resource partitioning of roach Rutilus rutilus and bleak Alburnus alburnus in two eutrophic lakes in SE Norway. Holarct. Ecol. 1985, 8, 88–92. [Google Scholar] [CrossRef]
- Brandl, Z. The seasonal dynamics of zooplankton biomass in two Czech reservoirs: A long-term study. Arch. Hydrobiol. Beih. Ergeb. Limnol. 1994, 40, 127–135. [Google Scholar]
- Ostojić, A.; Simić, V. Composition, structure and vertical distribution of zooplankton in Vlasinsko Jezero reservoir. In Vlasinsko Jezero—Hidrobiološka Studija; Blaženčić, J., Ed.; Biološki Fakultet Beograd: Beograd, Serbia, 1996; pp. 131–150. [Google Scholar]
- Vodopich, D.S.; Cowell, B.C. Interaction of factors governing the distribution of a predatory aquatic insect. Ecology 1984, 65, 39–52. [Google Scholar] [CrossRef]
- Schiemer, F.; Wieser, W. Epilogue: Food and feeding, ecomorphology, energy assimilation and conversion in cyprinids. Environ. Biol. Fishes 1992, 33, 223–227. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Hayden, B.; Harrod, C.; Kahilainen, K.K. Population niche breadth and individual trophic specialization of fish along a climate-productivity gradient. Rev. Fish Biol. Fish. 2021, 31, 1025–1043. [Google Scholar] [CrossRef]
- Specziár, A.; Rezsu, E.T. Feeding guilds and food resource partitioning in a lake fish assemblage: An ontogenetic approach. J. Fish Biol. 2009, 75, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Marszał, L.; Grzybkowska, M.; Penczak, T.; Galicka, W. Diet and feeding of dominant fish populations in the impounded Warta River, Poland. Pol. Arch. Hydrobiol. 1996, 43, 185–202. [Google Scholar]
- Marszał, L.; Grzybkowska, M.; Kostrzewa, J.; Kruk, A. Food resource partitioning between spined loach (Cobitis taenia L.) and golden loach (Sabanejewia aurata (Fil.)) in a lowland stream. Sci. Annu. Pol. Angling Assoc. 1998, 11, 51–64, (In Polish with English summary). [Google Scholar]
- Kruk, A.; Lek, S.; Park, Y.-S.; Penczak, T. Fish assemblages in the large lowland Narew River system (Poland): Application of the self-organizing map algorithm. Ecol. Model. 2007, 203, 45–61. [Google Scholar] [CrossRef]
- Chon, T.-S. Self-organizing maps applied to ecological sciences. Ecol. Inform. 2011, 6, 50–61. [Google Scholar] [CrossRef]
- Giraudel, J.L.; Lek, S. A comparison of self-organizing map algorithm and some conventional statistical methods for ecological community ordination. Ecol. Model. 2001, 146, 329–339. [Google Scholar] [CrossRef]
- Ha, J.-Y.; Saneyoshi, M.; Park, H.-D.; Toda, H.; Kitano, S.; Homma, T.; Shiina, T.; Moriyama, Y.; Chang, K.-H.; Hanazato, T. Lake restoration by biomanipulation using piscivore and Daphnia stocking; result of biomanipulation. Limnology 2013, 14, 19–30. [Google Scholar] [CrossRef]
- Persson, L.; Diehl, S.; Johansson, L.; Hamrin, S.F. Trophic interactions in lake ecosystems: A test of food chain theory. Am. Nat. 1992, 140, 59–84. [Google Scholar] [CrossRef]
- Lammens, E.H.R.R. Consequences of Biomanipulation for Fish and Fisheries; FAO Fisheries Circular: Rome, Italy, 2001; No. 952; p. 23. [Google Scholar]
- Shapiro, J. The importance of trophic-level interactions to the abundance and species composition of algae in lakes. In Development in Hypertrophic Ecosystems; Barica, J., Mur, L.R., Eds.; Dr W. Junk bv Publishers: The Hague, The Netherlands, 1980; pp. 105–115. [Google Scholar]
- Gulati, R.; Dionisio Pires, L.; Van Donk, E. Lake restoration studies: Failures bottleneck and prospects of new ecotechnological measures. Limnologica 2008, 38, 233–247. [Google Scholar] [CrossRef]
- Gao, J.; Zhong, P.; Ning, J.; Liu, Z.; Jeppesen, E. Herbivory of omnivorous fish shapes the food web structure of a Chinese tropical eutrophic lake: Evidence from stable isotope and fish gut content analysis. Water 2017, 9, 69. [Google Scholar] [CrossRef]

| Surface (km2) | Altitude (m) | Max Depth (m) | Mean Depth (m) | Trophic Status | |
|---|---|---|---|---|---|
| Vlasina Reservoir | 16 | 1211 | 35 | 10.3 | oligotrophic [37] |
| Gruža Reservoir | 9.34 | 269 | 35 | 6.5 | eutrophic [38] |
| Gazivode Reservoir | 11.9 | 694 | 107 | 36.6 | mesotrophic [39] |
| Šumarice Reservoir | 0.22 | 220 | 14 | 6.3 | eutrophic [40]; hypereutrophic [41] |
| Vrutci Reservoir | 2.7 | 700 | 64 | 20.9 | eutrophic [42] |
| Vlasina | Gruža | Gazivode | Šumarice | Vrutci | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %N | %FO | %PV | %N | %FO | %PV | %N | %FO | %PV | %N | %FO | %PV | %N | %FO | %PV | |
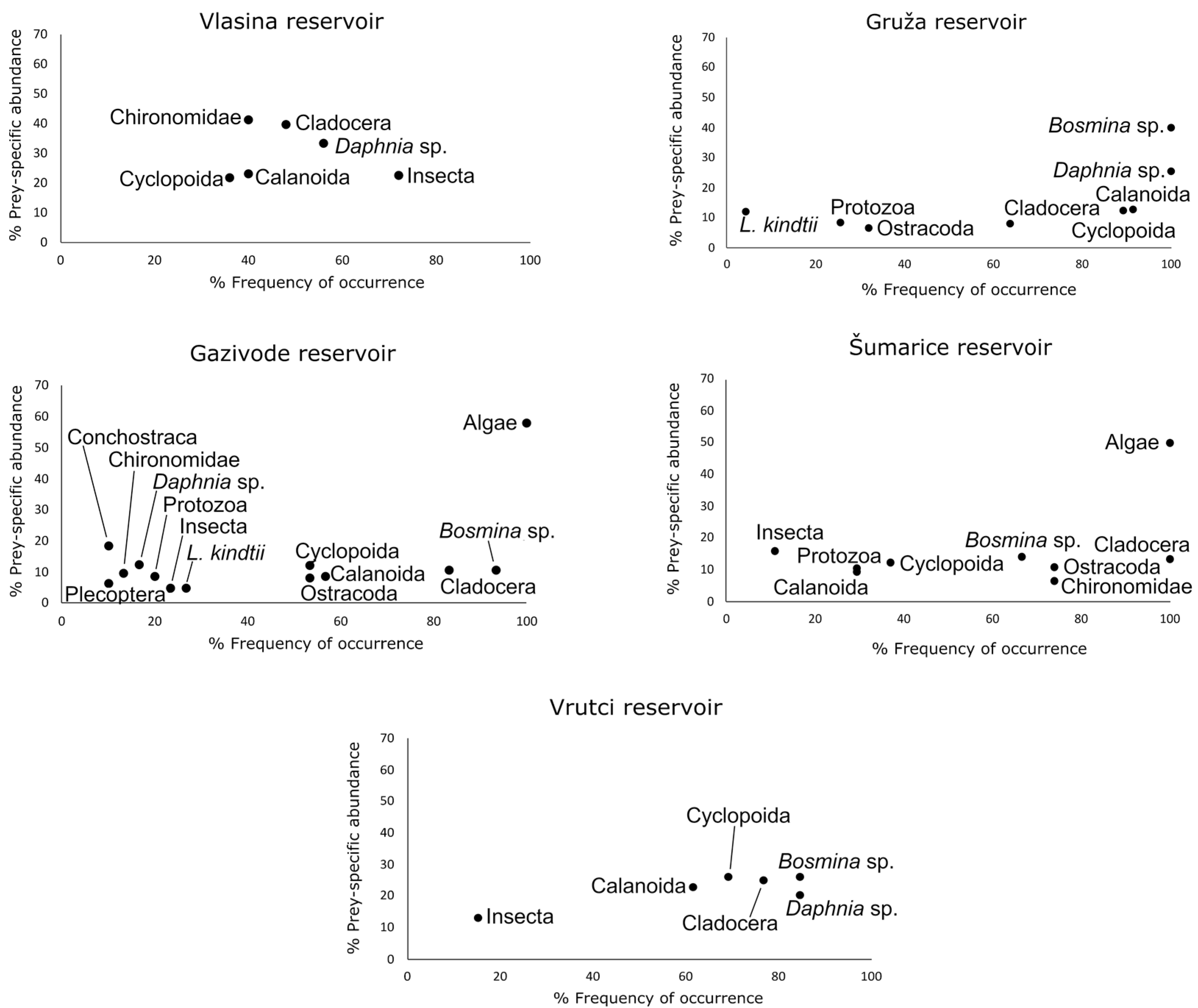
| Protozoa | - | - | - | 2.26 | 25.53 | 1.20 | 2.19 | 20.00 | 1.101 | 3.49 | 29.62 | 2.10 | - | - | - |
| Ostracoda | - | - | - | 2.42 | 31.91 | 1.43 | 4.49 | 53.33 | 3.69 | 8.31 | 74.07 | 7.93 | - | - | - |
| Conchostraca | - | - | - | - | - | - | 1.09 | 10.00 | 0.38 | - | - | - | - | - | - |
| Cladocera | 27.47 | 48.00 | 26.95 | 5.66 | 63.83 | 4.75 | 8.76 | 83.33 | 9.001 | 13.31 | 100.00 | 14.77 | 20.91 | 76.92 | 21.23 |
| Daphnia sp. | 25.82 | 56.00 | 27.36 | 25.48 | 100.00 | 26.78 | 2.19 | 16.66 | 1.005 | - | 18.49 | 84.61 | 19.69 | ||
| Bosmina sp. | - | - | - | 40.53 | 100.00 | 42.60 | 9.96 | 93.33 | 10.83 | 9.98 | 66.66 | 9.04 | 23.59 | 84.61 | 25.12 |
| Leptodora kindtii | - | - | - | 0.48 | 4.25 | 0.10 | 1.09 | 26.66 | 0.63 | - | - | - | - | - | - |
| Calanoida (Copepoda) | 12.08 | 40.00 | 10.82 | 11.01 | 91.48 | 11.06 | 5.47 | 56.66 | 6.63 | 3.32 | 29.62 | 2.04 | 16.08 | 61.53 | 14.61 |
| Cyclopoida (Copepoda) | 10.98 | 36.00 | 9.33 | 12.13 | 89.36 | 12.05 | 6.90 | 53.33 | 5.67 | 4.99 | 37.03 | 3.36 | 19.30 | 69.23 | 18.59 |
| Chironomidae | 9.34 | 40.00 | 0.08 | - | - | - | 1.20 | 13.33 | 0.49 | 4.99 | 74.07 | 4.76 | - | - | - |
| Plecoptera | - | - | - | - | - | - | 0.54 | 10.00 | 0.19 | - | - | - | - | - | - |
| Insecta | 14.28 | 72.00 | 17.16 | - | - | - | 1.31 | 23.33 | 0.71 | 1.66 | 11.11 | 0.61 | 1.61 | 15.38 | 0.73 |
| Algae | - | - | - | - | - | - | 54.76 | 100.00 | 61.64 | 49.91 | 100.00 | 55.38 | - | - | - |
| Detritus | 68.00 | - | - | - | 33.33 | 29.63 | 23.07 | ||||||||
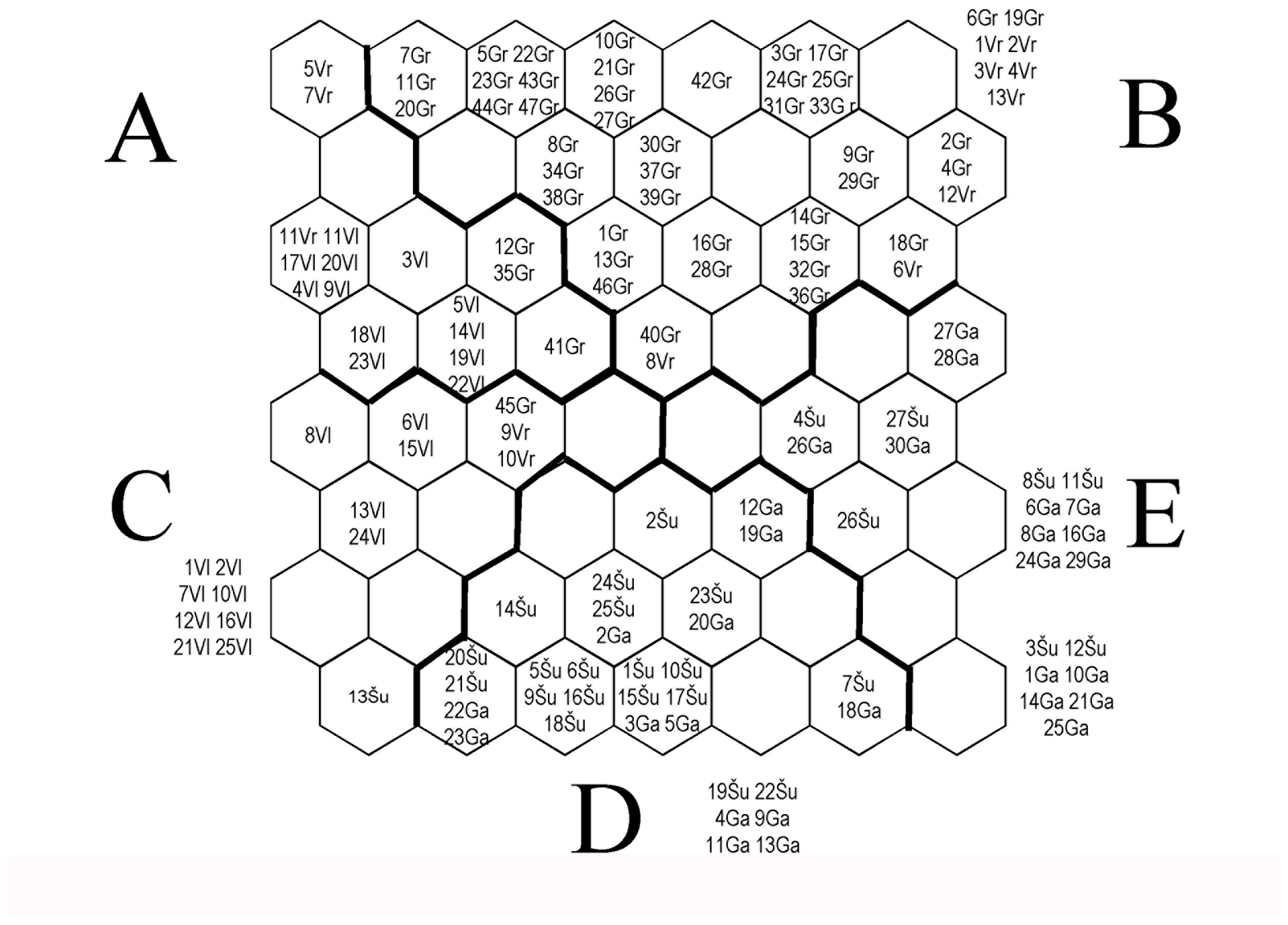
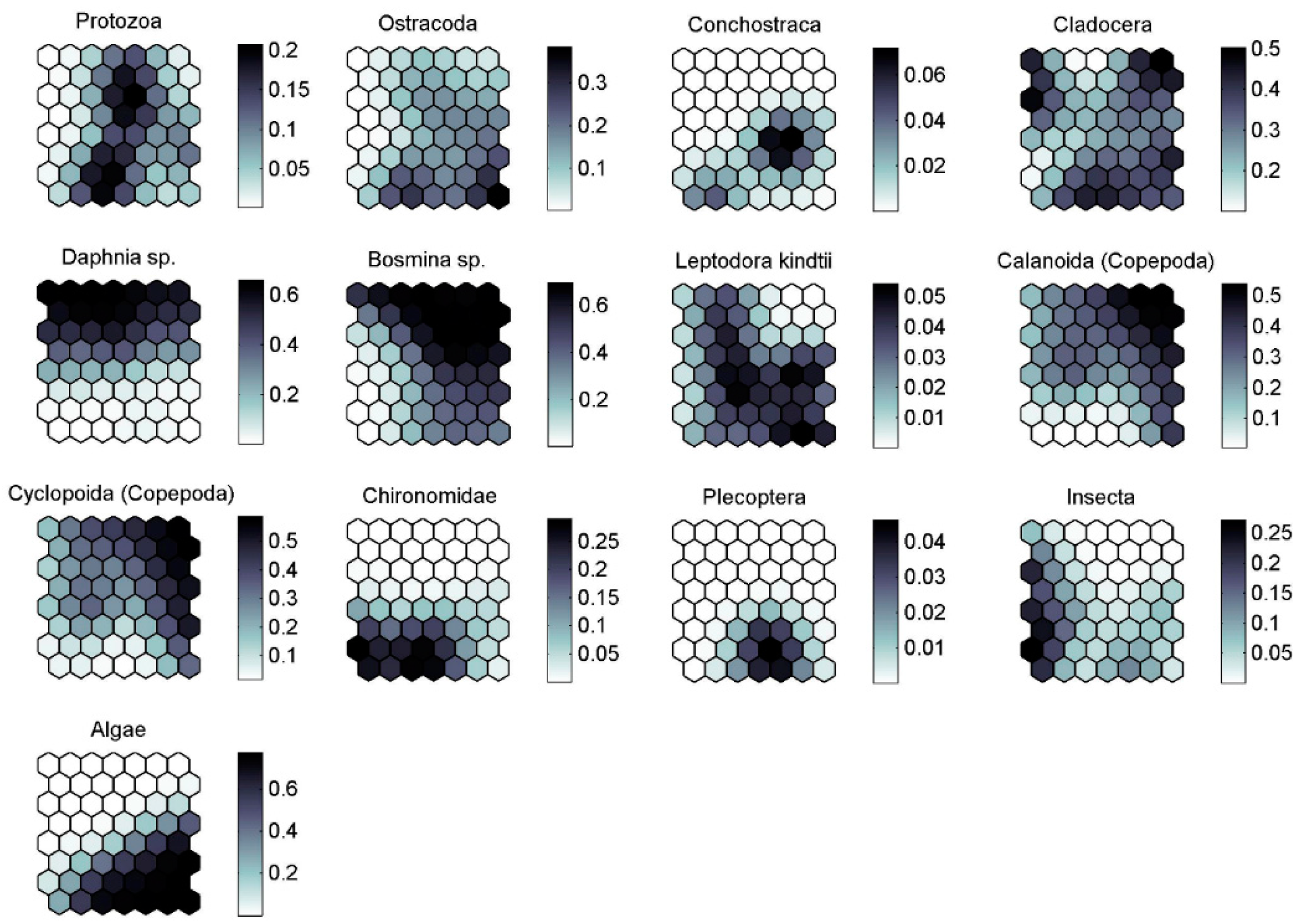
| Food Categories | A | B | C | D | E | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %F | %N | %I | %F | %N | %I | %F | %N | %I | %F | %N | %I | %F | %N | %I | |
| Protozoa | 0 | 0 | 0 | 24 | 28 | 7 | 0 | 0 | 0 | 29 | 42 | 12 | 18 | 30 | 5 |
| Ostracoda | 0 | 0 | 0 | 29 | 15 | 4 | 0 | 0 | 0 | 59 | 37 | 22 | 73 | 48 | 35 |
| Conchostraca | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 60 | 4 | 5 | 40 | 2 |
| Cladocera | 78 | 31 | 24 | 67 | 19 | 12 | 29 | 6 | 2 | 97 | 23 | 23 | 82 | 21 | 17 |
| Daphnia sp. | 94 | 35 | 33 | 100 | 56 | 56 | 18 | 1 | 0 | 9 | 1 | 0 | 9 | 6 | 1 |
| Bosmina sp. | 33 | 7 | 2 | 100 | 61 | 61 | 6 | 0 | 0 | 76 | 10 | 8 | 91 | 21 | 19 |
| Leptodora kindtii | 6 | 30 | 2 | 2 | 5 | 0 | 6 | 16 | 1 | 15 | 24 | 4 | 14 | 25 | 3 |
| Calanoida (Copepoda) | 56 | 14 | 8 | 90 | 38 | 34 | 35 | 14 | 5 | 9 | 1 | 0 | 100 | 33 | 33 |
| Cyclopoida (Copepoda) | 50 | 9 | 5 | 90 | 38 | 34 | 41 | 15 | 6 | 12 | 3 | 0 | 95 | 36 | 34 |
| Chironomidae | 6 | 2 | 0 | 0 | 0 | 0 | 59 | 44 | 26 | 59 | 48 | 28 | 14 | 6 | 1 |
| Plecoptera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 100 | 9 | 0 | 0 | 0 |
| Insecta | 39 | 34 | 13 | 0 | 0 | 0 | 82 | 42 | 35 | 18 | 16 | 3 | 14 | 9 | 1 |
| Algae | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 100 | 44 | 44 | 100 | 56 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radenković, M.; Milošković, A.; Stojković Piperac, M.; Veličković, T.; Curtean-Bănăduc, A.; Bănăduc, D.; Simić, V. Feeding Patterns of Fish in Relation to the Trophic Status of Reservoirs: A Case Study of Rutilus rutilus (Linnaeus, 1758) in Five Fishing Waters in Serbia. Fishes 2024, 9, 21. https://doi.org/10.3390/fishes9010021
Radenković M, Milošković A, Stojković Piperac M, Veličković T, Curtean-Bănăduc A, Bănăduc D, Simić V. Feeding Patterns of Fish in Relation to the Trophic Status of Reservoirs: A Case Study of Rutilus rutilus (Linnaeus, 1758) in Five Fishing Waters in Serbia. Fishes. 2024; 9(1):21. https://doi.org/10.3390/fishes9010021
Chicago/Turabian StyleRadenković, Milena, Aleksandra Milošković, Milica Stojković Piperac, Tijana Veličković, Angela Curtean-Bănăduc, Doru Bănăduc, and Vladica Simić. 2024. "Feeding Patterns of Fish in Relation to the Trophic Status of Reservoirs: A Case Study of Rutilus rutilus (Linnaeus, 1758) in Five Fishing Waters in Serbia" Fishes 9, no. 1: 21. https://doi.org/10.3390/fishes9010021






