Assessing the Speciation of Lutjanus campechanus and Lutjanus purpureus through Otolith Shape and Genetic Analyses
Abstract
:1. Introduction
2. Materials and Methods
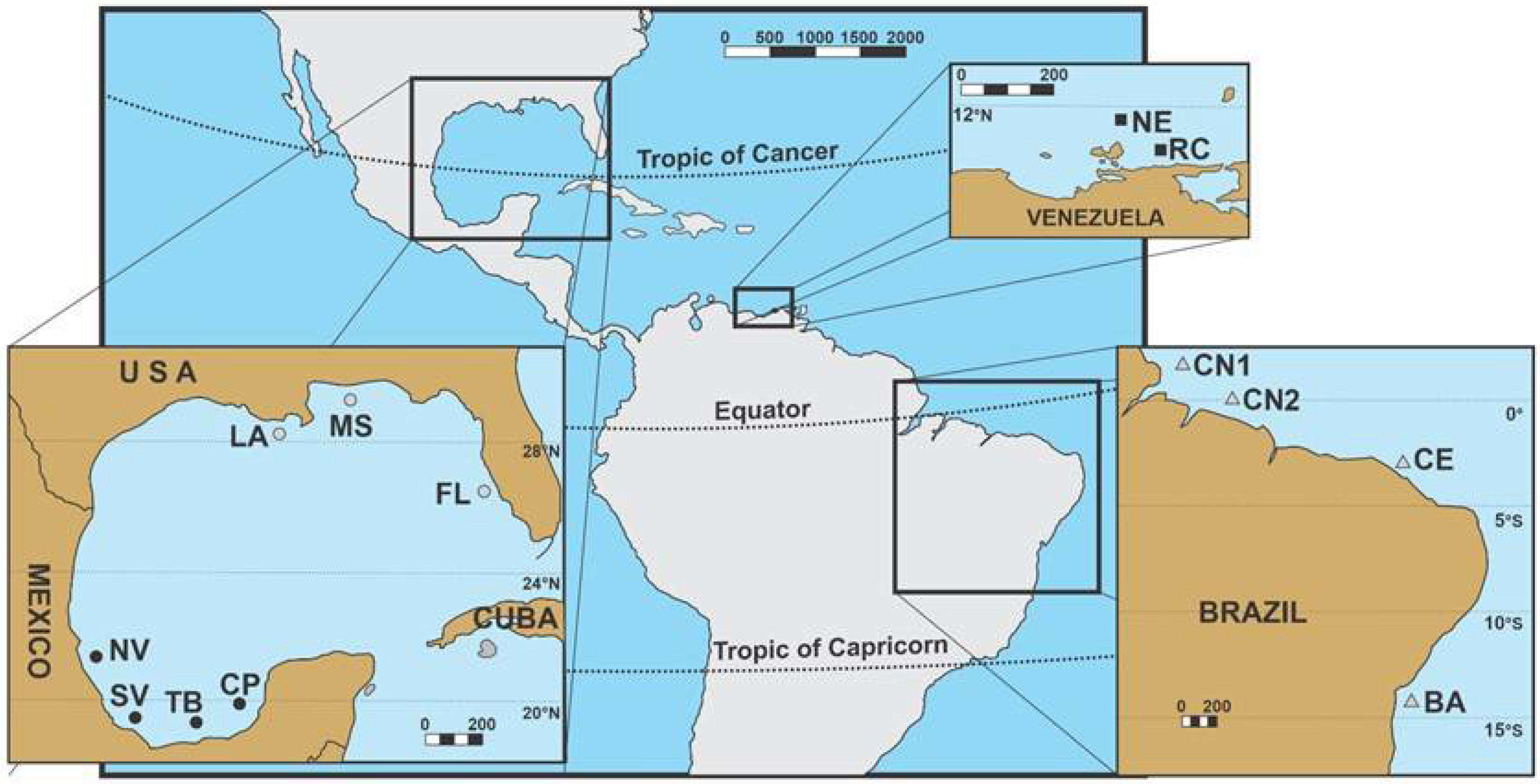
2.1. Sample Collection
2.2. Otolith Analysis
2.2.1. Otolith Morphological Description
2.2.2. Otolith Shape Analyses
2.2.3. Otolith Statistical Analysis
2.3. Molecular Analysis
2.3.1. Sample Processing, Fragment Amplification and Sequencing
2.3.2. Population Analysis
2.3.3. Phylogenetic Analysis
3. Results
3.1. Otolith Analysis
3.1.1. Otolith Morphological Description
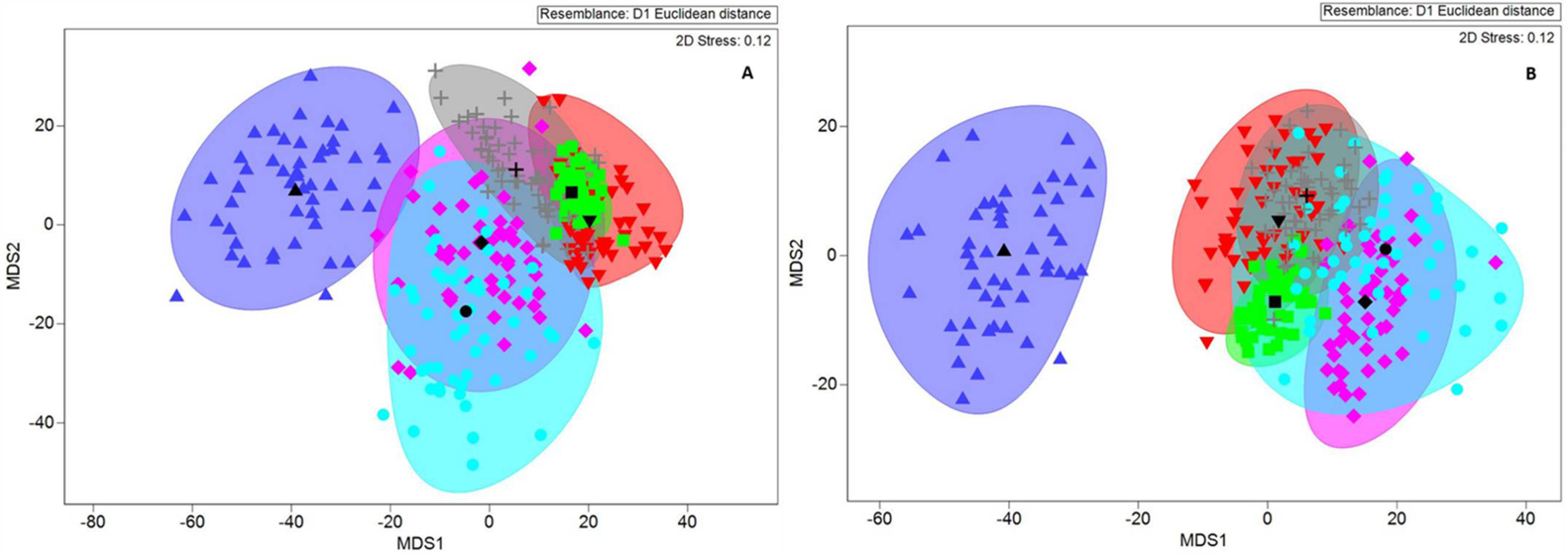
3.1.2. Inter-region Factor Variation
3.1.3. Intrapopulation Factor Variation
3.2. Molecular Analysis
3.2.1. Intrapopulation Factor Variation
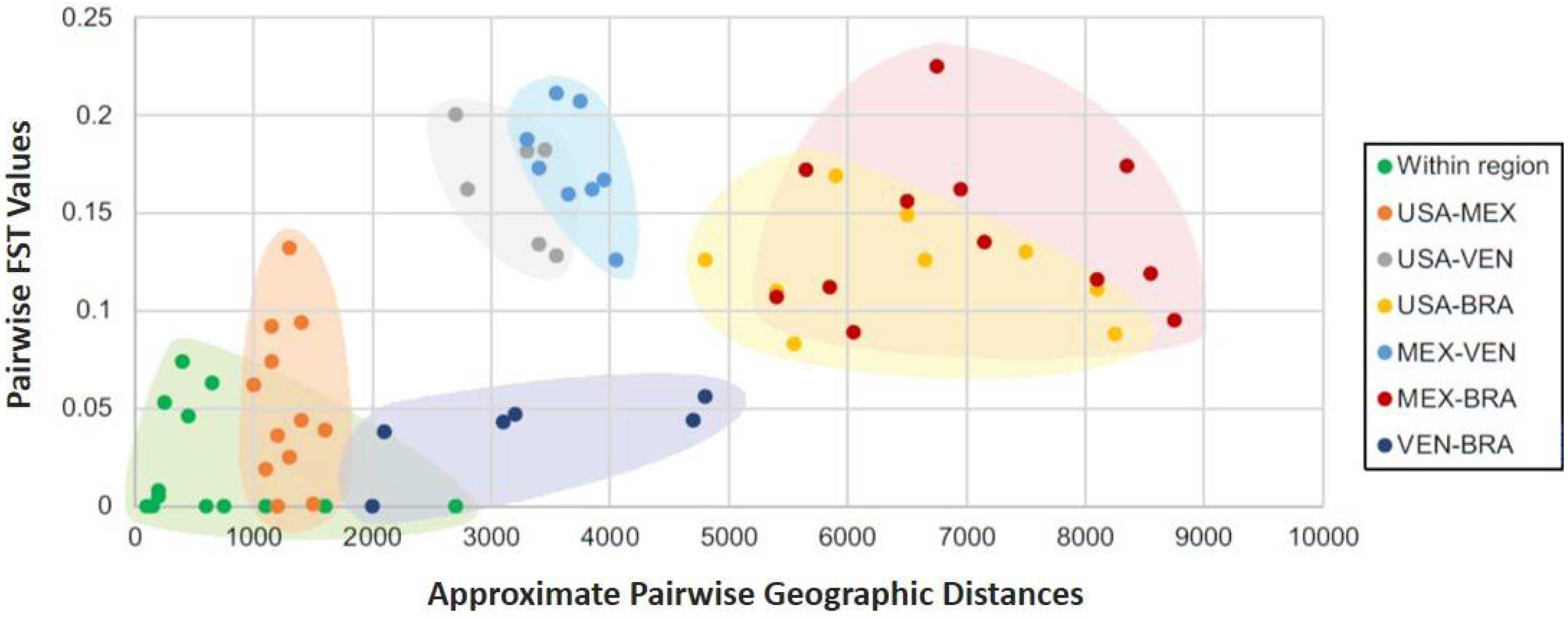
3.2.2. Interpopulation Factor Differentiation
3.2.3. Haplotype Network and Phylogenetic Analysis
4. Discussion
4.1. Otolith Analysis
4.2. Genetic Differentiation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryant, D.; Bouckaert, R.; Felsenstein, J.; Rosenberg, N.A.; Roychoudhury, A. Inferring species trees directly from biallelic genetic markers: Bypassing gene trees in a full coalescent analysis. Mol. Biol. Evol. 2012, 29, 1917–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaché, A.; Fujita, M.; Minin, V.; Bouckaert, R. Species delimitation using genome-wide SNP data. Syst. Biol. 2014, 63, 534–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowen, R.K.; Paris, C.B.; Srinivasan, A. Scaling of connectivity in marine populations. Science 2006, 311, 522–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturrock, A.M.; Trueman, C.N.; Darnaude, A.M.; Hunter, E. Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? J. Fish Biol. 2012, 81, 766–795. [Google Scholar] [CrossRef]
- Corrigan, S.; Maisano, P.; Eddy, C.; Duffy, C.; Yang, L.; Li, C.; Bazinet, A.L.; Mona, S.; Naylor, G.J. Historical introgression drives pervasive mitochondrial admixture between two species of pelagic sharks. Mol. Phylogenet. Evol. 2017, 110, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Vignon, M.; Morat, F. Environmental and genetic determinant of otolith shape revealed by a non-indigenous tropical fish. Mar. Ecol. Prog. Ser. 2010, 411, 231–241. [Google Scholar] [CrossRef]
- Waples, R.S.; Do, C. LDNE: A program for estimating effective population size from data on linkage disequilibrium. Mol. Ecol. Resour. 2017, 8, 753–756. [Google Scholar] [CrossRef] [Green Version]
- Tuset, V.M.; Lozano, I.J.; González, J.A.; Pertusa, J.F.; García-Díaz, M.M. Shape indices to identify regional differences in otolith morphology of comber Serranus cabrilla (L., 1758). J. Appl. Ichthyol. 2003, 19, 88–93. [Google Scholar] [CrossRef]
- Tuset, V.; Rosin, P.; Lombarte, A. Sagittal otolith shape used in the identification of fishes of the genus Serranus. Fish. Res. 2006, 81, 316–325. [Google Scholar] [CrossRef]
- Tuset, V.M.; Imondi, R.; Aguado, G.; Otero-Ferrer, J.L.; Santschi, L.; Lombarte, A.; Love, M. Otolith Patterns of Rockfishes from the Northeastern Pacific. J. Morphol. 2015, 276, 458–469. [Google Scholar] [CrossRef]
- DeVries, D.A.; Grimes, C.B.; Prager, M.H. Using otolith shape analysis to distinguish eastern Gulf of Mexico and Atlantic Ocean stocks of king mackerel. Fish. Res. 2002, 57, 51–62. [Google Scholar] [CrossRef]
- Volpedo, A.V.; Echevarría, D.D. Ecomorphological patterns of the sagitta in fish on the continental shelf off Argentine. Fish. Res. 2003, 60, 551–560. [Google Scholar] [CrossRef]
- Galley, E.A.; Wright, P.J.; Gibb, F.M. Combined methods of otolith shape analysis improve identification of spawning areas of Atlantic cod. ICES J. Mar. Sci. 2006, 63, 1710–1717. [Google Scholar] [CrossRef]
- Avigliano, E.; Martínez-Riaños, F.; Volpedo, A.V. Combined use of otolith microchemistry and morphometry as indicators of the habitat of the silverside (Odontesthes bonariensis) in a freshwater-estuarine environment. Fish. Res. 2014, 149, 55–60. [Google Scholar] [CrossRef]
- Callicó Fortunato, R.; Benedito Durà, V.; González-Castro, M.; Volpedo, A. Morphological and morphometric changes of sagittae otoliths related to fish growth in three Mugilidae species. J. Appl. Ichthyol 2017, 33, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Santos, R.; Costa de Azevedo, M.C.; Queiroz de Albuquerque, C.; Gerson Araújo, F. Different sagitta otolith morphotypes for the whitemouth croaker Micropogonias furnieri in the Southwestern Atlantic coast. Fish. Res. 2017, 195, 222–229. [Google Scholar] [CrossRef]
- Vignon, M.; Morat, F.; Galzin, R.; Sasal, P. Evidence for spatial limitation of the bluestripe snapper Lutjanus kasmira in French Polynesia from parasite and otolith shape analysis. J. Fish Biol. 2008, 73, 2305–2320. [Google Scholar] [CrossRef]
- Beyer, S.G.; Szedlmayer, S.T. The use of otolith shape analysis for ageing juvenile red snapper, Lutjanus campechanus. Environ. Biol. Fishes 2010, 89, 333–340. [Google Scholar] [CrossRef]
- Sadighzadeh, Z.; Tuset, V.M.; Valinassab, T.; Dadpour, M.R.; Lombarte, A. Comparison of different otolith shape descriptors and morphometrics for the identification of closely related species of Lutjanus spp. from the Persian Gulf. Mar. Biol. Res. 2012, 8, 802–814. [Google Scholar] [CrossRef]
- Sadighzadeh, Z.; Valinassab, T.; Vosugi, G.; Motallebi, A.A.; Fatemi, M.R.; Lombarte, A.; Tuset, V.M. Use of otolith shape for stock identification of John’s Snapper, Lutjanus johnii (Pisces: Lutjanidae), from the Persian Gulf and the Oman Sea. Fish. Res. 2014, 155, 59–63. [Google Scholar] [CrossRef]
- Puentes-Granada, V.; Rojas, P.; Pavolini, G.; Gutiérrez, C.F.; Villa, A.A. Morphology and morphometric relationships for sagitta otoliths in Lutjanus argentiventris (Pisces: Lutjanidae) and Hyporthodus acanthistius (Pisces: Serranidae) from the Colombian Pacific Ocean. Univ. Sci. 2019, 24, 337–361. [Google Scholar] [CrossRef]
- Stevens, M.M. Commercially Important Gulf of Mexico/South Atlantic Snappers; Monterey Bay Aquarium: Monterey, CA, USA, 2009; pp. 2–4. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 366–367. [Google Scholar]
- FAO: Yearbook of Fishery Statistics. Available online: http://www.fao.org/fi/statist/statist.asp (accessed on 23 June 2021).
- Anderson, W.D. Lutjanidae. In The living Marine Resources of the Western Central Atlantic, FAO Species Identification Guide for Fishery Purposes; Carpenter, K.E., Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; pp. 1479–1504. [Google Scholar]
- Moura, R.L.; Lindeman, K.C. A new species of snapper (Perciformes: Lutjanidae) from Brazil, with comments on the distribution of Lutjanus griseus and L. apodus. Zootaxa 2007, 1422, 31–43. [Google Scholar] [CrossRef]
- Monroy-García, C.; Garduño-Andrade, M.; Espinosa, J.C. Análisis de la pesquería de huachinango (Lutjanus campechanus) en el Banco de Campeche. Proc. Gulf Caribb. Fish. Inst. 2002, 53, 507–515. [Google Scholar]
- Drass, D.M.; Bootes, K.L.; Lyczkowski-Shultz, J.; Comyns, B.H.; Holt, G.H.; Riley, C.M.; Phelps, R.P. Larval development of red snapper, Lutjanus campechanus, and comparisons with co-occurring snapper species. Fish. Bull. 2000, 98, 507–527. [Google Scholar]
- Gomes, G.; Schneider, H.; Vallinoto, M.; Santos, S.; Ortil, G.; Sampaio, I. Can Lutjanus purpureus (South red snapper) be “legally” considered a red snapper (Lutjanus campechanus)? Genet. Mol. Biol. 2008, 31, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Cervigón, F.; Cipriani, R.; Fischer, W.; Garibaldi, L.; Hendrickx, M.; Lemus, A.; Márquez, R.; Poutiers, J.; Robaina, G.; Rodríguez, B. Fichas FAO de identificación de especies para los fines de la pesca. In Guía de Campo de las Especies Comerciales Marinas y de Aguas Salobres de la Costa Septentrional de Sur América; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; pp. 353–355. [Google Scholar]
- Gomes, G.; Sampaio, I.; Schneider, H. Population Structure of Lutjanus purpureus (Lutjanidae-Perciformes) on the Brazilian coast: Further existence evidence of a single species of red snapper in the western Atlantic. An. Acad. Bras. Ciênc. 2012, 84, 979–999. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.; Pedraza-Marrón, C.R.; Sampaio, I.; Betancur-R, R.; Gomes, G.; Schneider, H. New insights about species delimitation in red snappers (Lutjanus purpureus and L. campechanus) using multilocus data. Mol. Phylogenet. Evol. 2020, 147, 106780. [Google Scholar] [CrossRef]
- Assis, C.A. Guia Para a Identificação de Algumas Famílias de Peixes Ósseos de Portugal Continental, Através da Morfologia dos Seus Otólitos Sagitta; Câmara Municipal de Cascais: Cascais, Portugal, 2004. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Pavlov, D.A. Differentiation of three species of Genus Upeneus (Mullidae) based on otolith shape analysis. J. Ichthyol. 2016, 56, 37–51. [Google Scholar] [CrossRef]
- Parisi-Baradad, V.; Lombarte, A.; García-Ladona, E.; Cabestany, J.; Piera, J.; Chic, O. Otolith shape contour analysis using affine transformation invariant wavelet transforms and curvature scale space representation. Mar. Freshw. Res. 2005, 56, 795–804. [Google Scholar] [CrossRef]
- Parisi-Baradad, V.; Manjabacas, A.; Lombarte, A.; Olivella, R.; Chic, Ò.; Piera, J.; García-Ladona, E. Automatic taxon identification of teleost fishes in an otolith online database. Fish. Res. 2010, 105, 13–20. [Google Scholar] [CrossRef]
- Renán, X.; Montero-Muñoz, J.; Garza-Pérez, J.R.; Brulé, T. Age and stock analysis using otolith shape in gags from the southern Gulf of Mexico. Trans. Am. Fish. Soc. 2016, 145, 1252–1265. [Google Scholar] [CrossRef]
- Lleonart, J.; Salat, J.; Torres, G.J. Removing allometric effects of body size in morphological analysis. J. Theor. Biol. 2000, 205, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Doering-Arjes, P.; Kastowsky, M.; Mosegaard, H. Effects of sex, stock, and environment on the shape of known-age Atlantic Cod (Gadus morhua) otoliths. Can. J. Fish. Aquat. Sci. 2004, 61, 158–167. [Google Scholar] [CrossRef]
- Watkinson, D.A.; Gills, D.M. Stock discrimination of Lake Winnipeg walleye based on Fourier and wavelet description of scale outline signals. Fish. Res. 2005, 72, 193–203. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists, 2nd ed.; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, MA, USA, 2008. [Google Scholar]
- Lee, W.; Coroy, J.; Howell, W.H.; Koocher, T.D. Structure and evolution of teleost mitochondrial control regions. J. Mol. Evol. 1995, 41, 54–66. [Google Scholar] [CrossRef]
- da Silva, R.; Sampaio, I.; Schneider, H.; Gomes, G. Lack of spatial subdivision for the snapper Lutjanus purpureus (Lutjanidae-Perciformes) from southwest Atlantic based on multi-locus analyses. PLoS ONE 2016, 11, e0161617. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Rohl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Smouse, P.E.; Banks, S.C.; Peakall, R. Converting quadratic entropy to diversity: Both animals and alleles are diverse, but some are more diverse than others. PLoS ONE 2017, 12, e0185499. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mier-Uco, L.A. Catálogo de otolitos de Peces Capturados en el Parque Nacional Sistema Arrecifal Veracruzano; Instituto Tecnológico de Boca del Río: Veracruz, México, 2011; 119p. [Google Scholar]
- Capoccioni, F.; Costa, C.; Aguzzi, J.; Menesatti, P.; Lombarte, A.; Ciccotti, E. Ontogenetic and environmental effects on otolith shape variability in three Mediterranean European eel (Anguilla anguilla) local stocks. J. Exp. Mar. Biol. Ecol. 2011, 397, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mille, T.; Mahé, K.; Cahcera, M.; Villanueva, M.C.; de Pontual, H.; Ernande, B. Diet is correlated with otolith shape in marine fish. Mar. Ecol. Prog. Ser. 2016, 555, 167–184. [Google Scholar] [CrossRef] [Green Version]
- Begg, G.A.; Brown, R.W. Stock identification of haddock Melanogrammus aeglefinus on Georges Bank based on otolith shape analysis. Trans. Am. Fish. Soc. 2001, 129, 935–945. [Google Scholar] [CrossRef]
- Hüssy, K. Otolith shape in juvenile cod (Gadus morhua): Ontogenetic and environmental effects. J. Exp. Mar. Biol. Ecol. 2008, 364, 35–41. [Google Scholar] [CrossRef]
- Vignon, M. Ontogenetic trajectories of otolith shape during shift in habitat use: Interaction between otolith growth and environment. J. Exp. Mar. Biol. Ecol. 2012, 420–421, 26–32. [Google Scholar] [CrossRef]
- Grant, W.S.; Bowen, B.W. Shallow population histories in deep evolutionary lineages of marine fishes: Insights from sardines and anchovies and lessons for conservation. Genetics 1998, 89, 415–426. [Google Scholar] [CrossRef]
- Floeter, S.R.; Rocha, L.A.; Robertson, D.R.; Joyeux, J.C.; Smith-Vaniz, W.F.; Wirtz, P.; Edwards, A.J.; Barreiros, J.P.; Ferreira, C.E.; Gasparini, J.L.; et al. Atlantic reef fish biogeography and evolution. J. Biogeogr. 2008, 35, 22–47. [Google Scholar] [CrossRef] [Green Version]
- Rosado-Nic, O.J.; Hogan, J.D.; Lara-Arenas, J.H.; Rosas-Luis, R.; Carrillo, L.; Villegas-Sánchez, C.A. Gene flow between subpopulations of gray snapper (Lutjanus griseus) from the Caribbean and Gulf of Mexico. PeerJ 2020, 8, e8485. [Google Scholar] [CrossRef] [Green Version]
- Jue, N.K.; Brulé, T.; Coleman, F.C.; Koenig, C.C. From Shelf to Shelf: Assessing Historical and Contemporary Genetic Differentiation and Connectivity across the Gulf of Mexico in Gag, Mycteroperca microlepis. PLoS ONE 2015, 10, e0120676. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Marrón, C.D.R.; Silva, R.; Deeds, J.; Van Belleghem, S.M.; Mastretta-Yanes, A.; Domínguez-Domínguez, O.; Rivero-Vega, R.A.; Lutackas, L.; Murie, D.; Parkyn, D.; et al. Genomics overrules mitochondrial DNA, siding with morphology on a controversial case of species delimitation. Proc. Biol. Sci. 2019, 286, 20182924. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.A.; Rocha, C.R.; Robertson, D.R.; Bowen, B.W. Comparative phylogeography of Atlantic reef fishes indicates both origin and accumulation of diversity in the Caribbean. Evol. Biol. 2008, 8, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, S.; Carrillo, L.; Marinone, S.G. Potential connectivity between marine protected areas in the Mesoamerican Reef for two species of virtual fish larvae: Lutjanus analis and Epinephelus striatus. Ecol. Ind. 2019, 102, 10–20. [Google Scholar] [CrossRef]
- Silva, D.; Martins, K.; Oliveira, J.; da Silva, R.; Sampaio, I.; Schneider, H.; Gomes, G. Genetic differentiation in populations of lane snapper (Lutjanus synagris-Lutjanidae) from Western Atlantic as revealed by multilocus analysis. Fish. Res. 2018, 198, 138–149. [Google Scholar] [CrossRef]

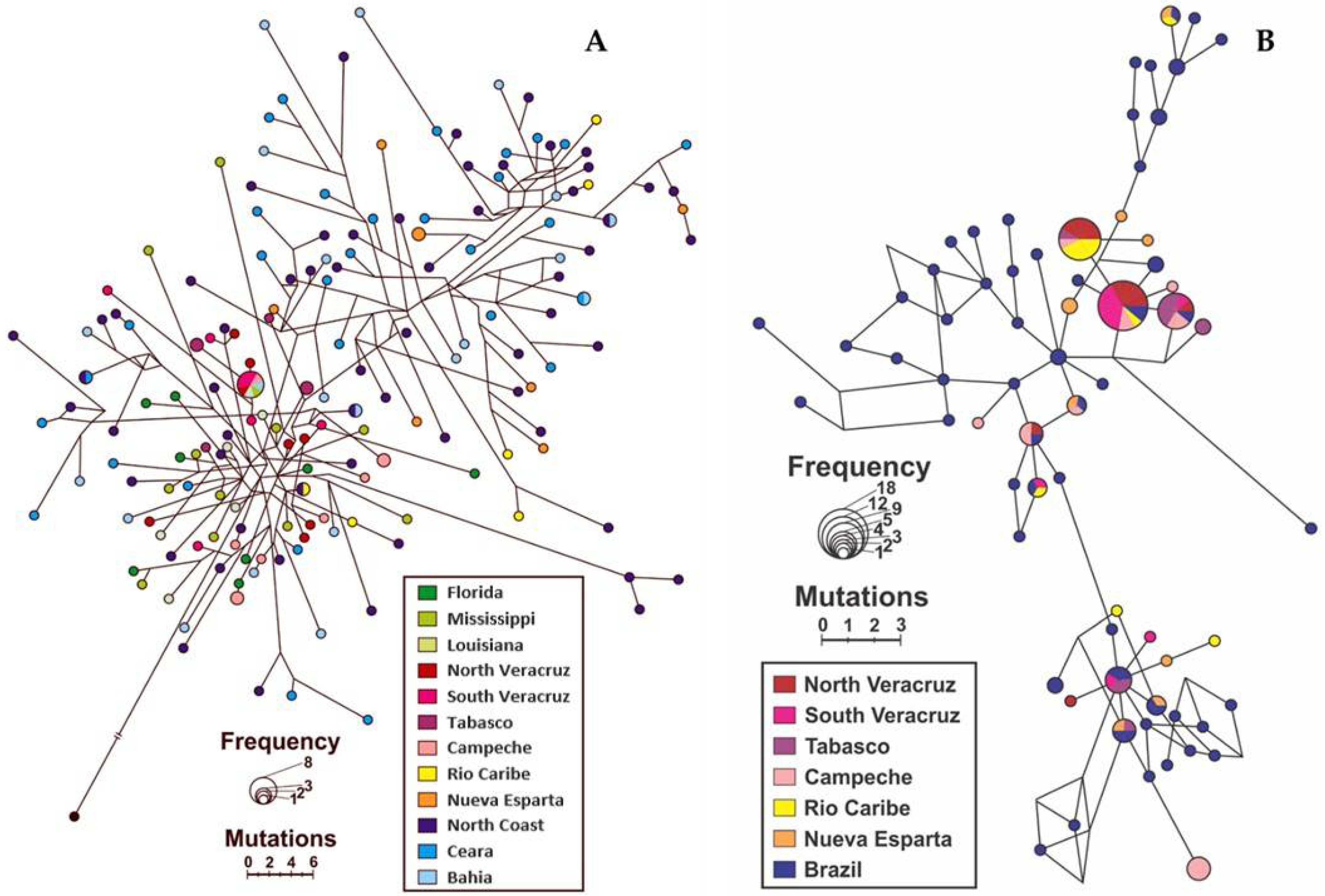
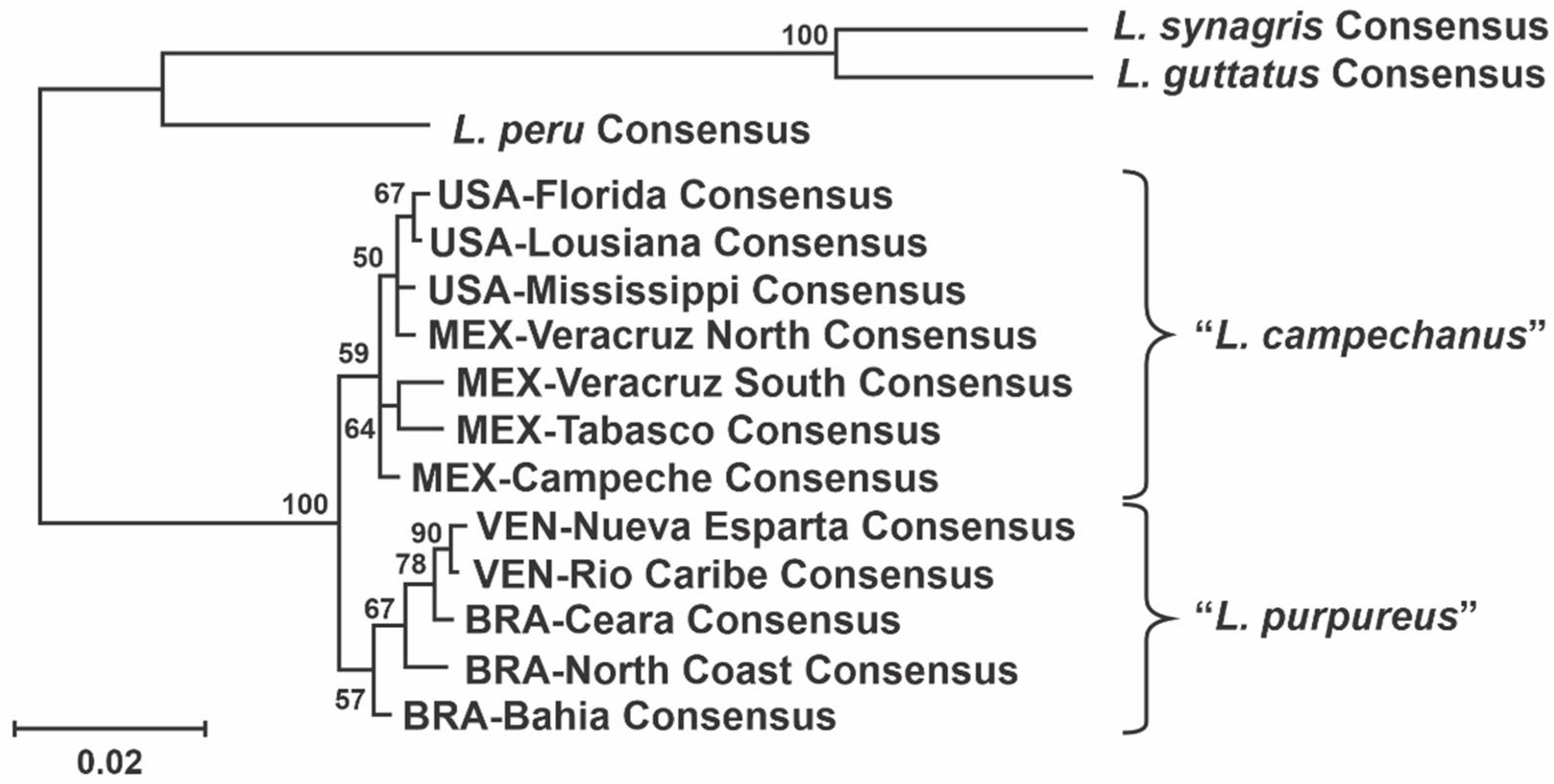
| Gene | Source of Variation | DF | SS | VC | % Variation |
|---|---|---|---|---|---|
| mtDNA D-loop | Inter-regional | 4 | 1231.22 | 8.59 | 47.86 |
| Interpopulational within regions | 8 | 57.24 | −0.17 | −0.94 | |
| Intrapopulational | 199 | 1896.17 | 9.53 | 53.08 | |
| Total | 211 | 3184.62 | 17.95 | ||
| FADS2, intron 8 | Inter-regional | 2 | 25.60 | 0.49 | 18.21 |
| Interpopulational within regions | 4 | 3.09 | −0.23 | −8.44 | |
| Intrapopulational | 99 | 241.61 | 2.44 | 90.23 | |
| Total | 105 | 270.30 | 2.70 |
| Regions | USA | Mexico | Venezuela | Brazil | L. peru |
|---|---|---|---|---|---|
| USA | _ | 0.050 | 0.255 | 0.144 | 0.751 |
| Mexico | 0.121 | _ | 0.273 | 0.155 | 0.765 |
| Venezuela | <0.001 * | <0.001 * | _ | 0.003 | 0.716 |
| Brazil | <0.001 * | <0.001 * | 0.387 | _ | 0.659 |
| L. peru | <0.001 * | <0.001 * | <0.001 * | <0.001 * | _ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marval-Rodríguez, A.; Renán, X.; Galindo-Cortes, G.; Acuña-Ramírez, S.; Jiménez-Badillo, M.d.L.; Rodulfo, H.; Montero-Muñoz, J.L.; Brulé, T.; De Donato, M. Assessing the Speciation of Lutjanus campechanus and Lutjanus purpureus through Otolith Shape and Genetic Analyses. Fishes 2022, 7, 85. https://doi.org/10.3390/fishes7020085
Marval-Rodríguez A, Renán X, Galindo-Cortes G, Acuña-Ramírez S, Jiménez-Badillo MdL, Rodulfo H, Montero-Muñoz JL, Brulé T, De Donato M. Assessing the Speciation of Lutjanus campechanus and Lutjanus purpureus through Otolith Shape and Genetic Analyses. Fishes. 2022; 7(2):85. https://doi.org/10.3390/fishes7020085
Chicago/Turabian StyleMarval-Rodríguez, Angel, Ximena Renán, Gabriela Galindo-Cortes, Saraí Acuña-Ramírez, María de Lourdes Jiménez-Badillo, Hectorina Rodulfo, Jorge L. Montero-Muñoz, Thierry Brulé, and Marcos De Donato. 2022. "Assessing the Speciation of Lutjanus campechanus and Lutjanus purpureus through Otolith Shape and Genetic Analyses" Fishes 7, no. 2: 85. https://doi.org/10.3390/fishes7020085