Seabream Larval Physiology under Ocean Warming and Acidification
Abstract
:1. Introduction
2. Results
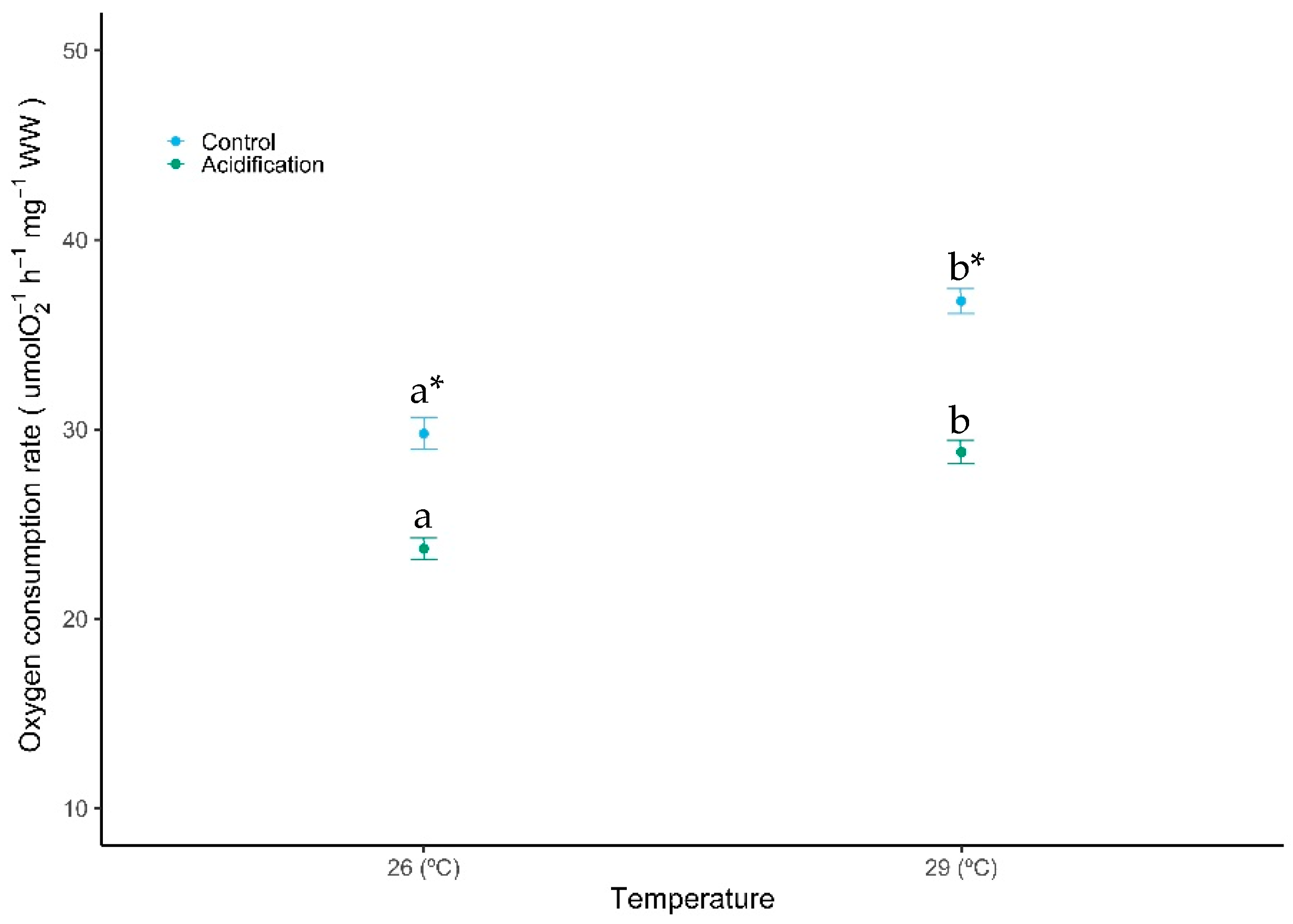
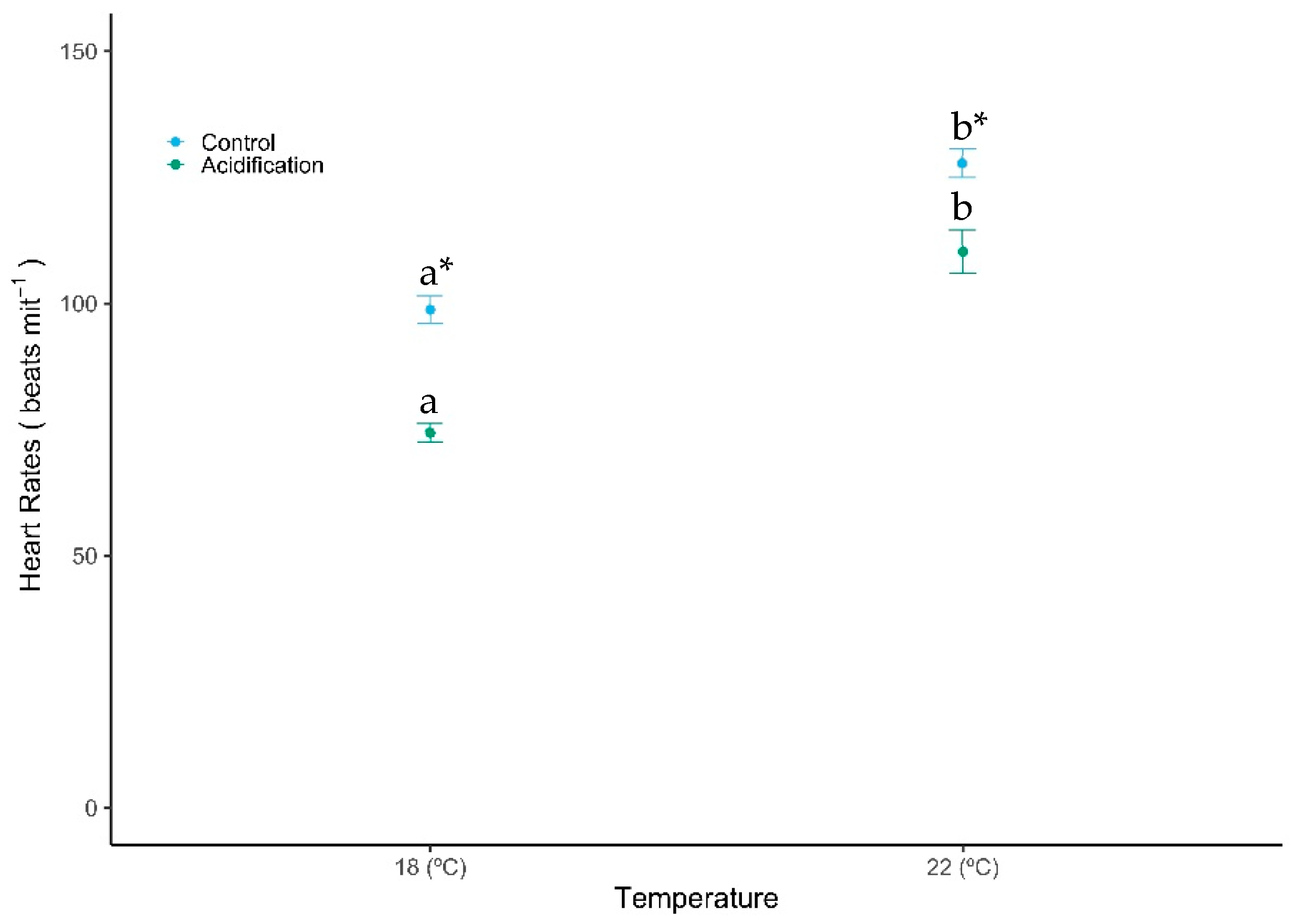
2.1. Oxygen Consumption and Routine Heart Rates
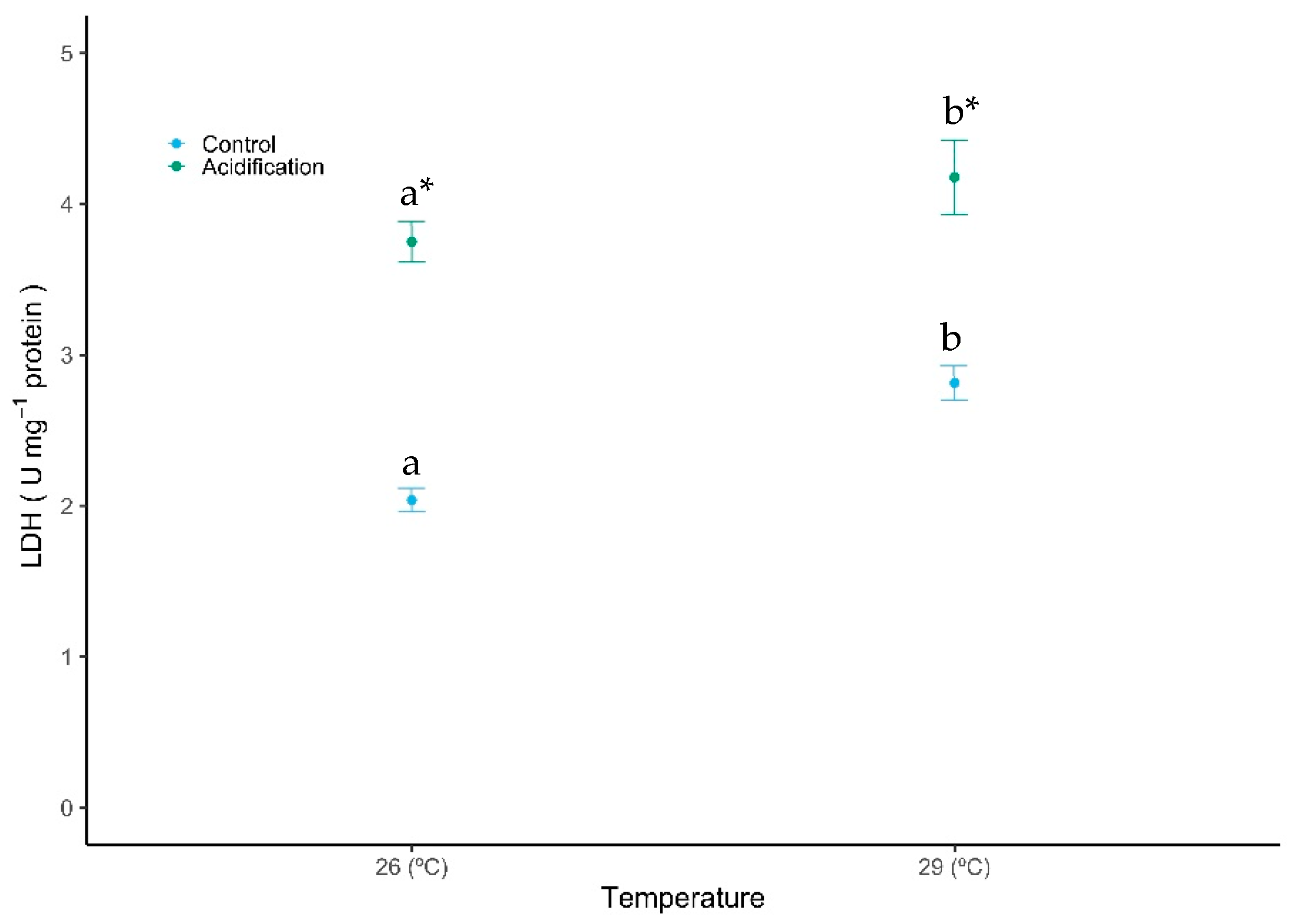
2.2. Enzyme Activity
3. Discussion
4. Materials and Methods
4.1. Larval Rearing
4.2. Oxygen Consumption and Routine Heart Rates
4.3. Enzyme Activity
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caldeira, K.; Wickett, M.E. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. 2005, 110. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H.; Karl, D.; Boyd, P.; Cheung, W.; Lluch-Cota, S.; Nojiri, Y.; Schmidt, D.; Zavialov, P. Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Ocean Systems; Field, C., Barros, V., Dokken, D., Mach, K., Mastrandrea, M., Bilir, T., Chatterjee, M., Ebi, K., Estrada, Y., Genova, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 411–484. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.; Johns, T.; Krinner, G.; et al. Long-Term Climate Change: Projections, Commitments and Irreversibility. In Climate Change 2013: the Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Mar. Res. 2008, 65, 414–432. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Todgham, A.E. Living in the now: Physiological mechanisms to tolerarte a rapidly changing environment. Ann. Rev. Physiol. 2010, 27, 127–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, G.E.; Barry, J.P.; Edmunds, P.J.; Gates, R.D.; Hutchuns, D.A.; Klinger, K.; Sewell, M.A. The effects of ocean acidification in polar, tropical and temperate marine calcifying organisms: An organism to ecosystem perspective. Ann. Rev. Ecol. Evol. Syst. 2010, 41, 127–147. [Google Scholar] [CrossRef]
- Melzner, F.; Gutowska, M.A.; Langenbuch, M.; Dupont, S.; Lucassen, M.; Thorndyke, M.C.; Bleich, M.; Portner, H.O. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny? Biogeosciences 2009, 6, 2313–2331. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H.; Farrell, A. Physiology and Climate Change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef]
- Pörtner, H.O. Ecosystem effects of ocean acidification in times of ocean warming: A physiologist’s view. Mar. Ecol. Prog. 2008, 373, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, J.G.; Cushing, D.H. A mechanism for density-dependent survival of larval fish as the basis of a stock-recruitment relationship. ICES J. Mar. Res. 1980, 39, 160–167. [Google Scholar] [CrossRef]
- Houde, E.D. Fish early life dynamics and recruitment variability. Am. Fish. Soc. Symp. 1987, 2, 17–29. [Google Scholar]
- Frommel, A.; Maneja, F.; Lowe, D.; Pascoe, C.; Geffen, A.; Folkvord, A.; Piatkowski, U.; Clemmensen, C. Organ damage in Atlantic herring larvae as a result of ocean acidification. Ecol. Appl. 2014, 24, 1131–1143. [Google Scholar] [CrossRef] [Green Version]
- Frommel, A.Y.; Maneja, R.; Lowe, D.; Malzahn, A.M.; Geffen, A.J.; Folkvord, A.; Piatkowski, U.; Reusch, T.B.U.; Clemmesen, C. Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat. Clim. Chang. 2012, 2, 42–46. [Google Scholar] [CrossRef]
- Munday, P.L.; McCormick, M.I.; Nilsson, G.E. Impact of global warming and rising CO2 levels on coral reef fishes: What hope for the future? J. Exp. Biol. 2012, 215, 3865–3873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, M.S.; Faleiro, F.; Dionísio, G.; Repolho, T.; Pousão-Ferreira, P.; Machado, J.; Rosa, R. Defective skeletogenesis and oversized otoliths in fish early stages in a changing ocean. J. Exp. Biol. 2014, 217, 2062–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, M.S.; Faleiro, F.; Marques, T.; Bispo, R.; Dionísio, G.; Faria, A.M.; Machado, J.; Peck, M.A.; Pörtner, H.O.; Pousão-Ferreira, P.; et al. Foraging behaviour, swimming performance and malformations of early stages of commercially important fishes under ocean acidification and warming. Clim. Chang. 2016, 137, 495–509. [Google Scholar] [CrossRef] [Green Version]
- Tseng, Y.C.; Hu, M.U.; Stumpp, M.; Lin, L.Y.; Melzner, F.; Hwang, P.P. CO2-driven seawater acidification differentially affects development and molecular plasticity along life history of fish (Oryzias latipes). Comp. Biochem. Physiol. Part A 2013, 165, 119–130. [Google Scholar] [CrossRef]
- Pimentel, M.S.; Faleiro, F.; Diniz, M.; Machado, M.; Pousão-Ferreira, P.; Peck, M.; Pörtner, H.O.; Rosa, R. Oxidative stress and digestive enzyme activity of flatfish larvae in a changing ocean. PLoS ONE 2015, 10, e0134082. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.; Taylor, E.W.; Brown, D.J.A.; Brown, J.A. Acid Toxicity and Aquatic Animals; Society for Experimental Biology Seminar Series; Cambridge University Pres: Cambridge, UK, 1989. [Google Scholar]
- Sayer, M.D.J.; Reader, J.P.; Dalziel, T.R.K. Fresh-water acidification—Effects on the early-life stages of fish. Rev. Fish Biol. Fish. 1993, 3, 298. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Langenbuch, M.; Reipschlager, A. Biological impact of elevated ocean CO2 concentrations: Lessons from animal physiology and earth history. J. Ocean. 2004, 60, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Munday, P.L.; Donelson, J.M.; Dixson, D.L.; Endo, G.K. Effects of ocean acidification on the early life history of a tropical marine fish. Proc. R. Soc. B Biol. Sci. 2009, 276, 3275–3283. [Google Scholar] [CrossRef] [Green Version]
- Rummer, J.L.; Stecyk, J.A.; Couturier, C.S.; Watson, S.A.; Nilsson, G.E.; Munday, P.L. Elevated CO2 enhances aerobic scope of a coral reef fish. Conserv. Physiol. 2013, 1, cot023. [Google Scholar] [CrossRef] [Green Version]
- Couturier, C.S.; Stecyk, J.A.W.; Rummer, J.L.; Munday, P.L.; Nilsson, G.E. Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp. Biochem. Phys. A 2013, 166, 482–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, D.F.; Jesus, T.F.; Kochhann, D.; Heinrichs-Caldas, W.; Coelho, M.M.; Almeida-Val, V.M.F. Metabolic rate and thermal tolerance in two congeneric Amazon fishes: Paracheirodon axelrodi Schultz, 1956 and Paracheirodon simulans Géry, 1963 (Characidae). Hydrobiologia 2017, 789, 133–142. [Google Scholar] [CrossRef]
- Jesus, T.F.; Rosa, I.C.; Repolho, T.; Lopes, A.R.; Pimentel, M.S.; Almeida-Val, V.M.F.; Coelho, M.M.; Rosa, R. Different ecophysiological responses of freshwater fish to warming and acidification. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 216, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pörtner, H. Integrating climate-related stressor effects on marine organisms: Unifying principles linking molecule to ecosystem-level changes. Mar. Ecol. Prog. Ser. 2012, 470, 273–290. [Google Scholar] [CrossRef] [Green Version]
- Rosa, R.; Ricardo Paula, J.; Sampaio, E.; Pimentel, M.; Lopes, A.R.; Baptista, M.; Guerreiro, M.; Santos, C.; Campos, D.; Almeida-Val, V.M.F.; et al. Neuro-oxidative damage and aerobic potential loss of sharks under elevated CO2 and warming. Mar. Biol. 2016, 163, 119. [Google Scholar] [CrossRef]
- Strobel, A.E.; Leo, E.; Pörtner, H.O.; Mark, F.C. Elevated temperature and PCO2 shift metabolic pathways in differentially oxidative tissues of Notothenia rossii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 166, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Feidantsis, K.; Portner, H.O.; Antonopoulou, E.; Michaelidis, B. Synergistic effects of acute warming and low pH on cellular stress responses of the gilthead seabrea Sparus aurata. J. Comp. Physiol. B 2015, 185, 185–205. [Google Scholar] [CrossRef]
- Michaelidis, B.; Spring, A.; Pörtner, H.O. Effects of long-term acclimation to environmental hypercapnia on extracellular acid–base status and metabolic capacity in Mediterranean fish Sparus aurata. Mar. Biol. 2007, 150, 1417–1429. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanisms and Process in Physiological Evolution; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Bennet, A.F. Activity metabolism of the lower vertebrates. Ann. Rev. Physiol. 1979, 400, 447–469. [Google Scholar] [CrossRef]
- McClelland, G.B.; Craig, P.M.; Dhekney, K.; Dipardo, S. Temperature- and exerciseinduced gene expression and metabolic enzyme changes in skeletal muscle of adult zebrafish (Danio rerio). J. Physiol. 2006, 577, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Somero, G.; Childress, J. A violation of the metabolism-size scaling paradigm—Activities of glycolytic-enzymes in muscle increase in larger-size fish. Physiol. Zool. 1980, 53, 322–337. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Demirel, H.A.; Coombes, J.S.; Fletcher, L.; Calliaud, C.; Vrabas, I.; Prezant, D. Myosin phenotype and bioenergetic characteristics of rat respiratory muscles. Med. Sci. Sports Exerc. 1977, 29, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.; Leech, A. Biochemistry for the Medical Sciences; Wiley and Sons: New York, NY, USA, 1988. [Google Scholar]
- Hochachka, P.W.; Stanley, C.; Merkt, J.; Sumar-Kalinowski, J. Metabolic meaning of elevated levels of oxidative enzymes in high altitude adapted animals: An interpretive hypothesis. Respir. Physiol. 1983, 52, 303–313. [Google Scholar] [CrossRef]
- Bauchot, M.L.; Hureau, J.C. Sparidae. In Checklist of the Fishes of the Eastern Tropical Atlantic (CLOFETA); Quero, J.C., Hureau, J.C., Karrer, C., Post, A., Saldanha, L., Eds.; JNICT: Lisbon, Portugal; SEI: Paris, France; UNESCO: Paris, France, 1990; Volume 2, pp. 790–812. [Google Scholar]
- Pörtner, H.O.; Langenbuch, M.; Michaelidis, B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: From Earth history to global change. J. Geophys. Res. Oceans 2005, 110. [Google Scholar] [CrossRef]
- Perrichon, P.; Pasparakis, C.; Mager, E.M.; Stieglitz, J.D.; Benetti, D.D.; Grosell, M.; Burggren, W.W. Morphology and cardiac physiology are differentially affected by temperature in developing larvae of the marine fish mahi-mahi (Coryphaena hippurus). Biol. Open 2017, 6, 800–809. [Google Scholar] [CrossRef] [Green Version]
- Storey, K.B.; Storey, J.M. Oxygen limitation and metabolic ratedepression. In Functional Metabolism. Regulation and Adaptation; Storey, K.B., Ed.; Wiley: Hobocken, NJ, USA, 2004; pp. 415–442. [Google Scholar]
- Perry, S.F.; Reid, S.G. Cardiorespiratory adjustments during hypercarbia in rainbow trout Oncorhynchus mykiss are initiated by external CO2 receptors on the first gill arch. J. Exp. Biol. 2002, 205 Pt 21, 3357–3365. [Google Scholar]
- Ishimatsu, A.; Kikkawa, T.; Hayashi, M.; Lee, K.S.; Kita, J. Effects of CO2 on marine fish: Larvae and adults. J. Oceanogr. 2004, 60, 731–741. [Google Scholar] [CrossRef]
- Lee, K.S.; Kita, J.; Ishimatsu, A. Effects of lethal levels of environmental hypercapnia on cardiovascular and blood-gas status in yellowtail Seriola quinqueradiata. Zool. Sci. 2003, 20, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Crocker, E.C.; Cech, J.J.J. Effects of hypercapnia on bloodgas and acid–base status in the white sturgeon, Acipenser transmontanus. J. Comp. Physiol. 1998, 168B, 50–60. [Google Scholar] [CrossRef]
- Reid, S.G.; Sundin, L.; Kalinin, A.L.; Rantin, F.T.; Milsom, W.K. Cardiovascular and respiratory reflexes in the tropical fish, traira (Hoplias malabaricus): CO2/pH chemoresponses. Respir. Physiol. 2000, 120, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Farrell, A.P.; Eliason, E.J.; Sandblom, E.; Clark, T.D. Fish cardiorespiratory physiology in an era of climate change. Can. J. Zool. 2009, 87, 835–851. [Google Scholar] [CrossRef] [Green Version]
- Gilmour, K.M.; Perry, S.F. Branchial chemoreceptor regulation of cardiorespiratory function. In Fish Physiology; Hara, T., Zielinksi, B., Eds.; Elsevier: San Diego, CA, USA, 2007; pp. 97–151. [Google Scholar]
- Perry, S.F.; Abdallah, S. Mechanisms and consequences of carbon dioxide sensing in fish. Respir. Physiol. Neurobiol. 2012, 184, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Smart, G.R.; Knox, D.; Harrison, J.G.; Ralph, J.A.; Richards, R.H.; Cowey, C.B. Nephrocalcinosis in rainbow trout Salmo gairdneri Richardson; the effect of exposure to elevated CO2 concentration. J. Fish Dis. 1979, 2, 279–289. [Google Scholar] [CrossRef]
- Hayashi, M.; Kita, J.; Ishimatsu, A. Acid-base responses to lethal aquatic hypercapnia in three marine fishes. Mar. Biol. 2004, 144, 153–160. [Google Scholar] [CrossRef]
- Perry, S.F.; Fritsche, R.; Hoagland, T.M.; Duff, D.W.; Olson, K.R. The control of blood pressure during external hypercapnia in the rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 1999, 202, 2177–2190. [Google Scholar]
- McKendry, J.E.; Milsom, W.K.; Perry, S.F.; Steffensen, J.F. Branchial CO2 receptors and cardiorespiratory adjustments during hypercarbia in Pacific spiny dogfish (Squalus acanthias). J. Exp. Biol. 2001, 204, 1519–1527. [Google Scholar]
- Sundin, L.; Reid, S.G.; Rantin, F.T.; Milsom, W.K. Branchial receptors and cardiorespiratory reflexes in the neotropical fish, Tambaqui (Colossoma macropomum). J. Exp. Biol. 2000, 203, 1225–1239. [Google Scholar]
- Fivelstad, S.; Olsen, A.B.; Kluften, H.; Ski, H.; Stefansson, S. Effects of carbon dioxide on Atlantic salmon (Salmo salar L.) smolts, at constant pH in bicarbonate rich freshwater. Aquaculture 1999, 178, 171–187. [Google Scholar] [CrossRef]
- Blancheton, J.P. Developments in recirculation systems for Mediterranean fish species. Aquac. Eng. 2000, 22, 17–31. [Google Scholar] [CrossRef]
- Davis, B.E.; Miller, N.A.; Flynn, E.E.; Todgham, A.E. Juvenile Antarctic rockcod (Trematomus bernacchii) are physiologically robust to CO2-acidified seawater. J. Exp. Biol. 2016, 219, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, B.E.; Flynn, E.E.; Miller, N.A.; Nelson, F.A.; Fangue, N.A.; Todgham, A. Antarctic emerald rockcod have the capacity to compensate for warming when uncoupled from CO2-acidification. Glob. Chang. Biol 2018, 24, e655–e670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobel, A.; Bennecke, S.; Leom, E.; Mintenbeck, K.; Pörtner, H.O.; Mark, F.C. Metabolic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and pCO2. Front. Zool. 2012, 9, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, B.E.; Komoroske, L.M.; Hansen, M.; Poletto, J.B.; Perry, E.N.; Miller, N.A.; Ehlman, S.M.; Wheeler, S.G.; Sih, A.; Todgham, A.E.; et al. Juvenile rockfish show resilience to CO2-acidification and hypoxia across multiple biological scales. Conserv. Physiol. 2018, 6, coy038. [Google Scholar] [CrossRef]
- Strobel, A.; Graeve, M.; Pörtner, H.O.; Mark, F.C. Mitochondrial acclimation capacities to ocean warming and acidification are limited in the Antarctic nototheniid fish, Notothenia rossii and Lepidonotothen squamifrons. PLoS ONE 2013, 8, e68865. [Google Scholar] [CrossRef] [Green Version]
- Flynn, E.E.; Bjelde, B.E.; Miller, N.A.; Todgham, A.E. Ocean acidification exerts negative effects during warming conditions in a developing Antarctic fish. Conserv. Physiol. 2015, 3, cov033. [Google Scholar] [CrossRef] [Green Version]
- Enzor, L.A.; Hunter, E.M.; Place, S.P. The effects of elevated temperature and ocean acidification on the metabolic pathways of notothenioid fish. Conserv. Physiol. 2017, 5, cox019. [Google Scholar] [CrossRef] [Green Version]
- Brauner, C.J.; Baker, D.W. Patterns of Acid-Base Regulation during Exposure to Hypercarbia in Fishes. In Cardio-Respiratory Control in Vertebrates: Comparative and Evolutionary Aspects; Glass, M.L., Wood, S.C., Eds.; Springer: Berlin, Germany, 2009; pp. 43–63. [Google Scholar]
- Arias, A. Crecimiento, regimen alimentario y reproducción de la dorada (Sparus aurata L.) y del robalo (Dicentrarchus labrax L.) en los esteros de Cadiz. Investig. Pesq. 1980, 44, 59–83. [Google Scholar]
- Kissil, G.; Lupatsch, I.; Elizur, A.; Zohar, Y. Long photoperiod delayed spawning and increased somatic growth in gilthead seabream (Sparus aurata). Aquaculture 2001, 200, 363–379. [Google Scholar] [CrossRef]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Firedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, E.A. Climate Change 2007: The Physical Science Basis; Contribution of working group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Sarazin, G.; Michard, G.; Prevot, F. A rapid and accurate spectroscopic method for alkalinity measurements in sea water samples. Water Res. 1999, 33, 290–294. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D.W.R. CO2SYS-Program Developed for the CO2 System Calculations; Report ORNL/CDIAC-105; Carbon Dioxide Inf Anal Center, Oak Ridge Natl. Lab.: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Fernández, I.; Hontoria, F.; Ortiz-Delgado, J.B.; Kotzamanis, Y.; Estevez, A.; Zambonino-Infante, J.L.; Gisbert, E. Larval performance and skeletal deformities in farmed gilthead sea bream (Sparus aurata) fed with graded levels of vitamin A enriched rotifers (Brachionus plicatilis). Aquaculture 2008, 283, 102–115. [Google Scholar] [CrossRef] [Green Version]
- Driedzic, W.R.; deAlmeidaVal, V.M.F. Enzymes of cardiac energy metabolism in Amazonian teleosts and the fresh-water stingray (Potamotrygon hystrix). J. Exp. Zool. 1996, 274, 327–333. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2019. Available online: www.R-project.org (accessed on 10 February 2019).
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]


| Model | Terms | Estimate | Std. Error | t value | Pr ( > |t|) | ||
|---|---|---|---|---|---|---|---|
| Oxygen Consumption rate | GLM, family = Gamma AIC = 159.097 | Intercept | 0.0338 | 0.00069 | 49.401 | <2 × 10−16 | *** |
| Temperature | −0.0068 | 0.00079 | −8.678 | 4.98 × 10−10 | *** | ||
| pH | 0.0079 | 0.00079 | 10.065 | 1.38 × 10−11 | *** | ||
| Heart rates | GLM, family = Gaussian AIC = 368.672 | Intercept | 98.75 | 3.080 | 32.060 | <2 × 10−16 | *** |
| Temperature | 29.00 | 4.356 | 6.657 | 3.64 × 10−8 | *** | ||
| pH | −24.42 | 4.356 | −5.605 | 1.28 × 10−6 | *** | ||
| CS activity | GLM, family = Gaussian AIC = 4.623 | Intercept | 1.9027 | 0.1121 | 15.678 | 7.68 × 10−8 | *** |
| Temperature | −0.4852 | 0.1401 | −3.462 | 0.30063 | |||
| pH | 0.1539 | 0.1401 | 1.098 | 0.00714 | ** | ||
| LDH activity | GLM, family = Gaussian AIC = 7.542 | Intercept | 2.1260 | 0.1371 | 15.511 | 8.43 × 10−8 | *** |
| Temperature | 0.6013 | 0.1583 | 3.799 | 0.00422 | ** | ||
| pH | 1.5380 | 0.1583 | 9.718 | 4.54 × 10−6 | *** | ||
| HOAD activity | GLM, family = Gaussian AIC = 38.672 | Intercept | 8.9219 | 0.5015 | 17.792 | 2.54 × 10−8 | *** |
| Temperature | 1.7513 | 0.5709 | 3.024 | 0.1437 | |||
| pH | −2.2089 | 0.5709 | −3.815 | 0.00412 | ** | ||
| LDH/CS ratio | GLM, family = Gaussian AIC = 16.023 | Intercept | 1.1267 | 0.2020 | 5.577 | 0.00344 | *** |
| Temperature | 0.2237 | 0.2333 | 0.959 | 0.362656 | |||
| pH | 1.4555 | 0.2333 | 6.239 | 0.000152 | *** |
| Treatments | LDH/CS Ratio |
|---|---|
| 18 °C, pH 8.0 | 1.12 ± 0.22 a |
| 18 °C, pH 7.5 | 2.59 ± 0.23 b |
| 22 °C, pH 8.0 | 1.36 ± 0.19 a |
| 22 °C, pH 7.5 | 2.78 ± 0.77 b |
| Temperature (°C) | pH (Total Scale) | AT (µmol kg−1SW) | CT (µmol/kg−1 SW) | pCO2 (µatm) | HCO3− (µmol kg−1) | Ωarag |
|---|---|---|---|---|---|---|
| 18.4 ± 0.3 | 8.09 ± 0.07 | 2331.7 ± 67.0 | 2055.2 ± 60.5 | 342.3 ± 73.7 | 1836.2 ± 70.2 | 3.19 ± 0.56 |
| 18.2 ± 0.3 | 7.53 ± 0.04 | 2318.6 ± 50.7 | 2284.0 ± 57.0 | 1484.7 ± 91.0 | 2169.1 ± 54.2 | 0.97 ± 0.08 |
| 22.3 ± 0.3 | 8.09 ± 0.07 | 2308.3 ± 78.8 | 1970.5 ± 80.2 | 337.0 ± 73.4 | 1736.9 ± 98.7 | 3.49 ± 0.49 |
| 22.1 ± 0.5 | 7.53 ± 0.04 | 2321.0 ± 93.3 | 2292.2 ± 83.2 | 1473.0 ± 92.0 | 2137.0 ± 99.5 | 1.16 ± 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel, M.S.; Faleiro, F.; Machado, J.; Pousão-Ferreira, P.; Rosa, R. Seabream Larval Physiology under Ocean Warming and Acidification. Fishes 2020, 5, 1. https://doi.org/10.3390/fishes5010001
Pimentel MS, Faleiro F, Machado J, Pousão-Ferreira P, Rosa R. Seabream Larval Physiology under Ocean Warming and Acidification. Fishes. 2020; 5(1):1. https://doi.org/10.3390/fishes5010001
Chicago/Turabian StylePimentel, Marta S., Filipa Faleiro, Jorge Machado, Pedro Pousão-Ferreira, and Rui Rosa. 2020. "Seabream Larval Physiology under Ocean Warming and Acidification" Fishes 5, no. 1: 1. https://doi.org/10.3390/fishes5010001






