Invasive Rainbow Trout (Oncorhynchus mykiss) Are Not Affected by Different Land Uses in a Multi-Use, Mediterranean Climate Landscape
Abstract
:1. Introduction
1.1. Negative Impacts of Forestry on Trout
1.2. Measures of Trout Quality
1.3. Central Chilean Headwaters
2. Results
2.1. Oncorhynchus mykiss
2.2. Nematogenys inermis
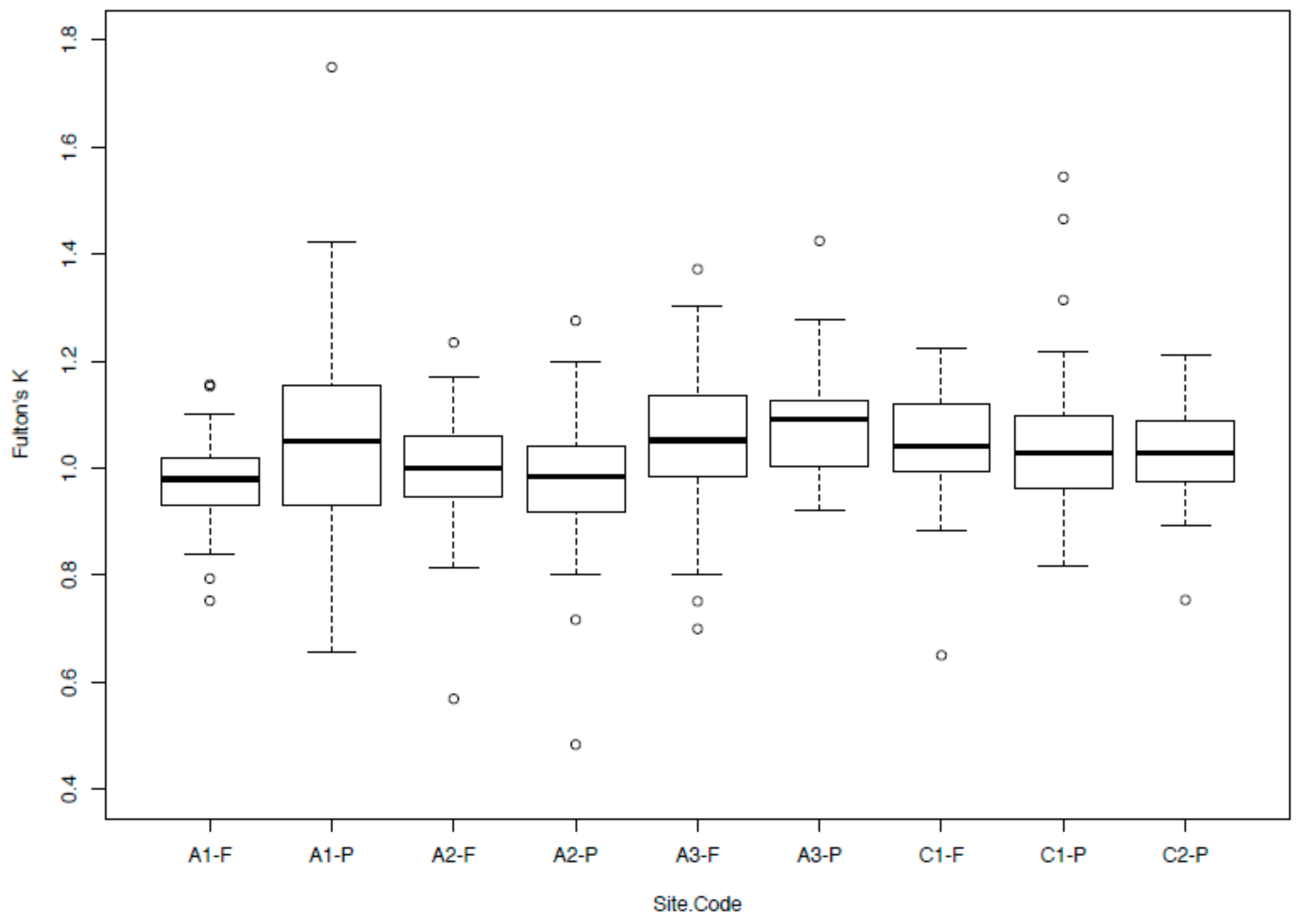
2.3. Body Condition Index
3. Discussion
3.1. Oncorhynchus mykiss
3.2. Nematogenys inermis
3.3. Body Condition Index
3.4. Implications for Invasive Species and Conservation Management
4. Materials and Methods
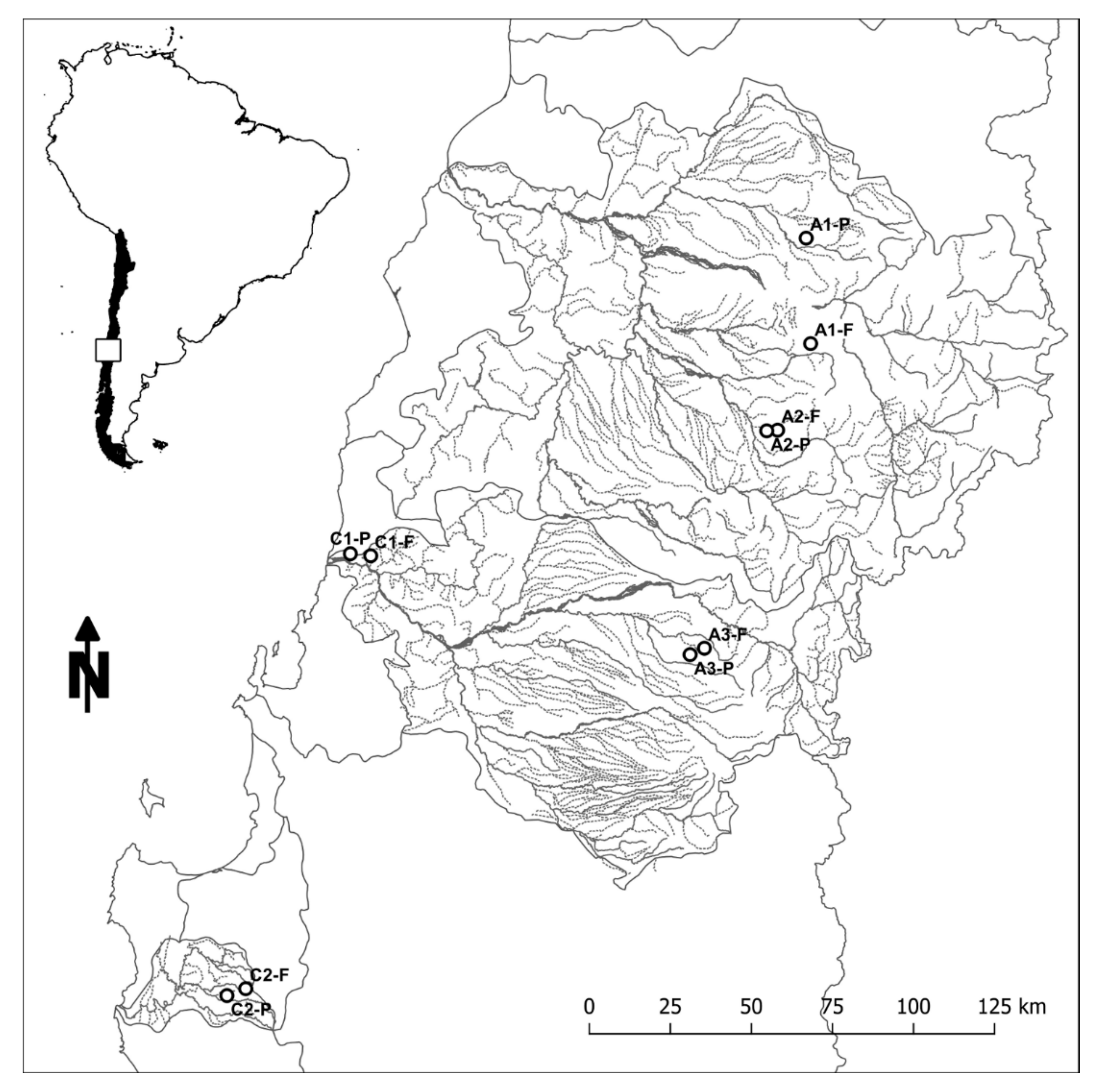
4.1. Study Area
4.2. Field Sampling
4.3. Comparative Fish Condition Assessment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reiners, W.A.; Reiners, D.S.; Lockwood, J.A. Traits of a good ecologist: What do ecologists think? Ecosphere 2013, 4, 1–22. [Google Scholar] [CrossRef]
- Justice, C.; White, S.M.; McCullough, D.A.; Graves, D.S.; Blanchard, M.R. Can stream and riparian restoration offset climate change impacts to salmon populations? J. Environ. Manag. 2017, 188, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.K.; Taylor, W.W.; Schlee, K.M.; Zorn, T.G.; Infante, D.M. Projected impacts of climate change on stream salmonids with implications for resilience-based management. Ecol. Freshw. Fish 2017, 26, 190–204. [Google Scholar] [CrossRef]
- Santiago, J.M.; García de Jalón, D.; Alonso, C.; Solana, J.; Ribalaygua, J.; Pórtoles, J.; Monjo, R. Brown trout thermal niche and climate change: Expected changes in the distribution of cold-water fish in central Spain. Ecohydrology 2016, 9, 514–528. [Google Scholar] [CrossRef]
- Crawford, S.S.; Muir, A.M. Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Rev. Fish Biol. Fish. 2008, 18, 313–344. [Google Scholar] [CrossRef]
- Arismendi, I.; Penaluna, B.E.; Dunham, J.B.; García de Leaniz, C.; Soto, D.; Fleming, I.A.; Gomez-Uchida, D.; Gajardo, G.; Vargas, P.V.; León-Muñoz, J.; et al. Differential invasion success of salmonids in southern Chile: Patterns and hypotheses. Rev. Fish Biol. Fish. 2014, 24, 919–941. [Google Scholar] [CrossRef]
- Vila, I.; Fuentes, L.; Contreras, M. Peces Límnicos de Chile. Bol. Mus. Nac. Hist. Nat. Chile 1999, 48, 61–75. (In Spanish) [Google Scholar]
- Shelton, J.M.; Weyl, O.L.F.; Impson, N.D.; Dallas, H.F.; Esler, K.J.; Paxton, B.R. Temperature mediates the impact of non-native rainbow trout on native freshwater fishes in South Africa’s Cape Fold Ecoregion. Biol. Invasions 2018, 1–18. [Google Scholar] [CrossRef]
- Penaluna, B.E.; Arismendi, I.; Soto, D. Evidence of Interactive Segregation between Introduced Trout and Native Fishes in Northern Patagonian Rivers, Chile. Trans. Am. Fish. Soc. 2009, 138, 839–845. [Google Scholar] [CrossRef]
- Bøhn, T.; Amundsen, P.A.; Sparrow, A. Competitive exclusion after invasion? Biol. Invasions 2008, 10, 359–368. [Google Scholar] [CrossRef]
- Soto, D.; Arismendi, I.; González, J.; Sanzana, J.; Jara, F.; Jara, C.; Guzman, E.; Lara, A. Southern Chile, trout and salmon country: Invasion patterns and threats for native species. Rev. Chil. Hist. Nat. 2006, 79, 97–117. [Google Scholar] [CrossRef]
- OECD. OECD Environmental Performance Reviews: Chile 2005; OECD: Paris, France, 2005. [Google Scholar]
- Schlaepfer, M.A.; Sax, D.F.; Olden, J.D. The Potential Conservation Value of Non-Native Species. Conserv. Biol. 2011, 25, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Leach, J.H.; Carlton, J.T.; Secor, C.L. Exotic Species in the Great Lakes: A History of Biotic Crises and Anthropogenic Introductions. J. Great Lakes Res. 1993, 19, 1–54. [Google Scholar] [CrossRef]
- Prichard, C.G.; Bence, J.R. Estimating wounding of lake trout by sea lamprey in the upper Great Lakes: Allowing for changing size-specific patterns. J. Great Lakes Res. 2013, 39, 110–119. [Google Scholar] [CrossRef]
- Freeman, R.; Bowerman, W. Opening Rivers to Trojan Fish The Ecological Dilemma of Dam Removal in the Great Lakes. Conserv. Pract. 2002, 3, 35–39. [Google Scholar] [CrossRef]
- Dodd, H.R.; Hayes, D.B.; Baylis, J.R.; Carl, L.M.; Goldstein, J.D.; McLaughlin, R.L.; Noakes, D.L.G.; Porto, L.M.; Jones, M.L. Low-head Sea Lamprey Barrier Effects on Stream Habitat and Fish Communities in the Great Lakes Basin. J. Great Lakes Res. 2003, 29, 386–402. [Google Scholar] [CrossRef]
- Hauer, C.; Pulg, U.; Gabrielsen, S.E.; Barlaup, B.T. Application of step-backwater modelling for salmonid spawning habitat restoration in Western Norway. Ecohydrology 2015, 8, 1239–1261. [Google Scholar] [CrossRef]
- Person, E.; Bieri, M.; Peter, A.; Schleiss, A.J. Mitigation measures for fish habitat improvement in Alpine rivers affected by hydropower operations. Ecohydrology 2014, 7, 580–599. [Google Scholar] [CrossRef]
- Imholt, C.; Soulsby, C.; Malcolm, I.A.; Gibbins, C.N. Influence of contrasting riparian forest cover on stream temperature dynamics in salmonid spawning and nursery streams. Ecohydrology 2013, 6, 380–392. [Google Scholar] [CrossRef]
- Habit, E.M.; Dyer, B.S.; Vila, I. Estado de conocimiento de los peces dulceacuícolas de Chile. Gayana (Concepción) 2006, 70, 100–113. (In Spanish) [Google Scholar] [CrossRef]
- Orrego, R.; Marshall Adams, S.; Barra, R.; Chiang, G.; Gavilan, J.F. Patterns of fish community composition along a river affected by agricultural and urban disturbance in south-central Chile. Hydrobiologia 2009, 620, 35–46. [Google Scholar] [CrossRef]
- Stehr, A.; Aguayo, M.; Link, O.; Parra, O.; Romero, F.; Alcayaga, H. Modelling the hydrologic response of a mesoscale Andean watershed to changes in land use patterns for environmental planning. Hydrol. Earth Syst. Sci. 2010, 14, 1963–1977. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Jorde, K.; Habit, E.; Caamaño, D.; Parra, O. Downstream environmental effects of dam operations: Changes in habitat quality for native fish species. River Res. Appl. 2011, 27, 312–327. [Google Scholar] [CrossRef]
- Wang, L.; Lyons, J.; Kanehl, P.; Bannerman, R. Impacts of urbanization on stream habitat and fish across multiple spatial scales. Environ. Manag. 2001, 28, 255–266. [Google Scholar] [CrossRef]
- Richter, A.; Kolmes, S.A. Maximum temperature limits for chinook, coho, and chum salmon, and steelhead trout in the Pacific Northwest. Rev. Fish. Sci. 2005, 13, 23–49. [Google Scholar] [CrossRef]
- Ellender, B.R.; Rivers-Moore, N.A.; Coppinger, C.R.; Bellingan, T.A.; Weyl, O.L.F. Towards using thermal stress thresholds to predict salmonid invasion potential. Biol. Invasions 2016, 18, 3513–3525. [Google Scholar] [CrossRef]
- Syrjänen, J. Ecology, Fisheries and Management of Wild Brown Trout Populations in Boreal Inland Waters; University of Jyväskylä: Jyväskylä, Finland, 2010. [Google Scholar]
- Baxter, C.V.; Frissell, C. a.; Hauer, F.R. Geomorphology, logging roads, and the distribution of bull trout spawning in a forested river basin: Implications for management and conservation. Trans. Am. Fish. Soc. 1999, 128, 854–867. [Google Scholar] [CrossRef]
- Steedman, R.J. Effects of experimental clearcut logging on water quality in three small boreal forest lake trout (Salvelinus namaycush) lakes. Can. J. Fish. Aquat. Sci. 2000, 57, 92–96. [Google Scholar] [CrossRef]
- Rosenberger, A.E.; Dunham, J.B.; Neuswanger, J.R.; Railsback, S.F. Legacy effects of wildfire on stream thermal regimes and rainbow trout ecology: an integrated analysis of observation and individual-based models. Freshw. Sci. 2015, 34, 1571–1584. [Google Scholar] [CrossRef]
- Lara, A.; Little, C.; Urrutia, R.; McPhee, J.; Álvarez-Garretón, C.; Oyarzún, C.; Soto, D.; Donoso, P.; Nahuelhual, L.; Pino, M.; et al. Assessment of ecosystem services as an opportunity for the conservation and management of native forests in Chile. For. Ecol. Manag. 2009, 258, 415–424. [Google Scholar] [CrossRef]
- Meador, M.R.; Goldstein, R.M. Assessing water quality at large geographic scales: Relations among land use, water physicochemistry, riparian condition, and fish community structure. Environ. Manag. 2003, 31, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.R.; Taylor, W.D.; Biette, R.M. Dimensions of riparian buffer strips required to maintain habitat in southern Ontario streams. North Am. J. Fish. Manag. 1985, 5, 364–378. [Google Scholar] [CrossRef]
- Burt, T.P.; Matchett, L.S.; Goulding, K.W.T.; Webster, C.P.; Haycock, N.E. Denitrification in riparian buffer zones: The role of floodplain hydrology. Hydrol. Process. 1999, 13, 1451–1463. [Google Scholar] [CrossRef]
- Hefting, M.M.; Clement, J.C.; Bienkowski, P.; Dowrick, D.; Guenat, C.; Butturini, A.; Topa, S.; Pinay, G.; Verhoeven, J.T.A. The role of vegetation and litter in the nitrogen dynamics of riparian buffer zones in Europe. Ecol. Eng. 2005, 24, 465–482. [Google Scholar] [CrossRef]
- Dosskey, M.G.; Vidon, P.; Gurwick, N.P.; Allan, C.J.; Duval, T.P.; Lowrance, R. The role of riparian vegetation in protecting and improving chemical water quality in streams. J. Am. Water Resour. Assoc. 2010, 46, 261–277. [Google Scholar] [CrossRef]
- Davies, P.; Nelson, M. Relationships between riparian buffer widths and the effects of logging on stream habitat, invertebrate community composition and fish abundance. Mar. Freshw. Res. 1994, 45, 1289–1305. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.; Smyth, C.; Boutin, S. Quantitative review of riparian buffer width guidelines from Canada and the United States. J. Environ. Manag. 2004, 70, 165–180. [Google Scholar] [CrossRef]
- Romero, F.I.; Cozano, M.A.; Gangas, R.A.; Naulin, P.I. Zonas ribereñas: Protección, restauración y contexto legal en Chile. Bosque (Valdivia) 2014, 35, 1–2. (In Spanish) [Google Scholar] [CrossRef]
- Habit, E.; Piedra, P.; Ruzzante, D.E.; Walde, S.J.; Belk, M.C.; Cussac, V.E.; Gonzalez, J.; Colin, N. Changes in the distribution of native fishes in response to introduced species and other anthropogenic effects. Glob. Ecol. Biogeogr. 2010, 19, 697–710. [Google Scholar] [CrossRef]
- Lacy, S.N.; Meza, F.J.; Marquet, P.A. Can environmental impact assessments alone conserve freshwater fish biota? Review of the Chilean experience. Environ. Impact Assess. Rev. 2017, 63, 87–94. [Google Scholar] [CrossRef]
- Arismendi, I.; Penaluna, B.; Soto, D. Body condition indices as a rapid assessment of the abundance of introduced salmonids in oligotrophic lakes of southern Chile. Lake Reserv. Manag. 2011, 27, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Milner, N.J.; Elliott, J.M.; Armstrong, J.D.; Gardiner, R.; Welton, J.S.; Ladle, M. The natural control of salmon and trout populations in streams. Fish. Res. 2003, 62, 111–125. [Google Scholar] [CrossRef]
- Valdes-Pineda, R.; Pizarro, R.; Garcia-Chevesich, P.; Valdes, J.B.; Olivares, C.; Vera, M.; Balocchi, F.; Perez, F.; Vallejos, C.; Fuentes, R.; et al. Water governance in Chile: Availability, management and climate change. J. Hydrol. 2014, 519, 2538–2567. [Google Scholar] [CrossRef]
- O’Briain, R.; Shephard, S.; Coghlan, B. River reaches with impaired riparian tree cover and channel morphology have reduced thermal resilience. Ecohydrology 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Arismendi, I.; Gonzáles, J.; Soto, D.; Penaluna, B.E. Piscivory and diet overlap between two non-native fishes in southern Chilean streams. Austral Ecol. 2012, 37, 346–354. [Google Scholar] [CrossRef]
- Montgomery, D.R.; Buffington, J.M. Channel-reach morphology in mountain drainage basins. Bull. Geol. Soc. Am. 1997, 109, 596–611. [Google Scholar] [CrossRef]
- Hitt, N.P.; Angermeier, P.L. Evidence for fish dispersal from spatial analysis of stream network topology. J. N. Am. Benthol. Soc. 2008, 27, 304–320. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Ugalde, F.; Iroume, A.; Lacy, S.N. The Effects of Replacing Native Forest on the Quantity and Impacts of In-Channel Pieces of Large Wood in Chilean Streams. River Res. Appl. 2017, 33, 73–88. (In Spanish) [Google Scholar] [CrossRef]
- Arratia F., G. Preferencias de habitat de peces siluriformes de aguas continentales de Chile (Fam. Diplomystidae y Trichomycteridae). Stud. Neotrop. Fauna Environ. 1983, 18, 217–237. [Google Scholar] [CrossRef]
- Roni, P.; Beechie, T.; Pess, G.; Hanson, K. Wood placement in river restoration: fact, fiction, and future direction. Can. J. Fish. Aquat. Sci. 2015, 72, 466–478. [Google Scholar] [CrossRef] [Green Version]
- Pardo, R.; Vila, I.; Capella, J.J. Competitive interaction between introduced rainbow trout and native silverside in a Chilean stream. Environ. Biol. Fishes 2009. [Google Scholar] [CrossRef]
- Jowet, I.G.; Richardson, J.; Boubée, J.A.T. Effects of riparian manipulation on stream communities in small streams: Two case studies. N. Z. J. Mar. Freshw. Res. 2009, 43, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Tschaplinski, P.J.; Pike, R.G. Carnation Creek watershed experiment—long-term responses of coho salmon populations to historic forest practices. Ecohydrology 2017, 10. [Google Scholar] [CrossRef]
- QGIS Development Team QGIS Geographic Information System; Open Source Geospatial Foundation Project; American Scientific Publishers: Stevenson Ranch, CA, USA, 2015.
- Lamothe, K.A.; Allen, R.M.; Winningham, K.; Dennis, C.; Johnson, R.L. Stocking for a trophy bass fishery: searching for size differences among largemouth bass and hybrids in southern Arkansas reservoirs. Lake Reserv. Manag. 2016, 32, 194–207. [Google Scholar] [CrossRef]
- Seber, G.A.F.; Cren, E.D. Le Estimating Population Parameters from Catches Large Relative to the Population. J. Anim. Ecol. 1967, 36, 631. [Google Scholar] [CrossRef]
- R Core Team R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3-900051-07-0. [Google Scholar]

| Species | Grouping | Location | N | F-value | R2 | m | b | CPUE | k |
|---|---|---|---|---|---|---|---|---|---|
| O. mykiss | Site | A1-F | 47 | 2170.67 | 0.98 | 3.056 | −2.08 | 90.7 | 0.97 |
| A1-P | 10 | 747.13 | 0.99 | 2.501 | −1.366 | 26.8 | 1.08 | ||
| A2-F | 34 | 969.78 | 0.97 | 2.736 | −1.722 | 88.3 | 0.99 | ||
| A2-P | 41 | 532.35 | 0.93 | 2.746 | −1.737 | 204.2 | 0.97 | ||
| A3-F | 48 | 420.18 | 0.9 | 2.927 | −1.912 | 33.8 | 1.06 | ||
| A3-P | 24 | 677.33 | 0.97 | 3.039 | −2.009 | 13.6 | 1.10 | ||
| C1-F | 41 | 2668.69 | 0.99 | 2.915 | −1.885 | 43.7 | 1.04 | ||
| C1-P | 42 | 1055.01 | 0.96 | 2.969 | −1.957 | 93.0 | 1.06 | ||
| C2-P | 28 | 718.54 | 0.97 | 2.865 | −1.84 | 26.6 | 1.02 | ||
| Land Use | Forest | 170 | 6264.85 | 0.97 | 2.904 | −1.891 | 64.1 | 1.02 | |
| Plantation | 146 | 3591.47 | 0.96 | 2.837 | −1.81 | 73.9 | 1.02 | ||
| Range | Andes | 205 | 5330.64 | 0.96 | 2.834 | −1.813 | 76.2 | 1.02 | |
| Coast | 111 | 4666.36 | 0.98 | 2.941 | −1.923 | 54.4 | 1.00 | ||
| N. inermis | Sampling Site | A1-P | 3 | - | - | - | - | 12.6 | 0.83 |
| C1-P | 17 | 1275.98 | 0.99 | 3.459 | −2.682 | 21.8 | 0.76 | ||
| Combined | 20 | 1307.6 | 0.99 | 3.378 | −2.581 | 17.2 | 0.77 |
| Catchment Code | A1-F | A1-P | A2-F | A2-P | A3-F | A3-P | C1-F | C1-P | C2-F | C2-P |
|---|---|---|---|---|---|---|---|---|---|---|
| Location | Andes | Andes | Andes | Andes | Andes | Andes | Coast | Coast | Coast | Coast |
| Catchment area (km2) | 11.86 | 8.68 | 2.35 | 3.53 | 10.21 | 11.22 | 4.92 | 4.83 | 1.43 | 2.54 |
| Max elevation (m) | 2087 | 913 | 1153 | 1078 | 1551 | 1168 | 571 | 558 | 982 | 730 |
| Min elevation (m) | 660 | 452 | 520 | 530 | 565 | 576 | 78 | 75 | 560 | 224 |
| Average slope | 0.086 | 0.016 | 0.047 | 0.046 | 0.043 | 0.015 | 0.08 | 0.029 | 0.168 | 0.073 |
| Native forest (%) | 100 | 32 | 100 | 34 | 98 | 29 | 62 | 25 | 99 | 40 |
| Pine plantation (%) | 0 | 68 | 0 | 66 | 2 | 71 | 38 | 75 | 1 | 60 |
| Water temperature (°C) | 16.5 | 15.6 | 15.4 | 17.1 | 14.6 | 12.8 | 14.1 | 14.1 | 13.2 | 14.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacy, S.; Ugalde, F.; Mao, L. Invasive Rainbow Trout (Oncorhynchus mykiss) Are Not Affected by Different Land Uses in a Multi-Use, Mediterranean Climate Landscape. Fishes 2018, 3, 37. https://doi.org/10.3390/fishes3040037
Lacy S, Ugalde F, Mao L. Invasive Rainbow Trout (Oncorhynchus mykiss) Are Not Affected by Different Land Uses in a Multi-Use, Mediterranean Climate Landscape. Fishes. 2018; 3(4):37. https://doi.org/10.3390/fishes3040037
Chicago/Turabian StyleLacy, Shaw, Fernando Ugalde, and Luca Mao. 2018. "Invasive Rainbow Trout (Oncorhynchus mykiss) Are Not Affected by Different Land Uses in a Multi-Use, Mediterranean Climate Landscape" Fishes 3, no. 4: 37. https://doi.org/10.3390/fishes3040037





