Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review
Abstract
:1. Introduction
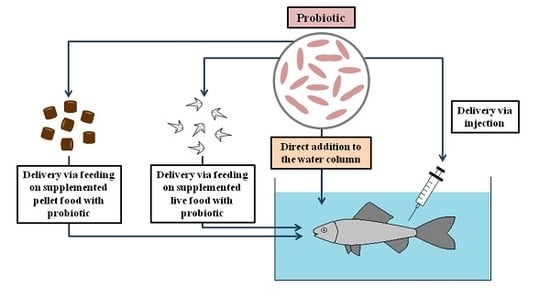
2. The Range of Probiotic Administration through Water
3. Benefits of Probiotic Administration through Water
3.1. Improvement of Water Quality
3.2. Inhibitory Activity against Fish Pathogens
3.2.1. Improvement of Non-Specific Defense in Marine Fish Larvae
3.2.2. Treatment of Fungal Infections
3.2.3. Improving the Substrate Spawners Aquaculture
4. The Major Factors Regulating the Benefits of this Administration Method
4.1. Temperature
4.2. Treatment Dose
4.3. Inoculation Times
4.4. Age of Treated Fishes
4.5. Salinity
5. Research Gaps and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martínez Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of Probiotics in Aquaculture. ISRN Microbiol. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S. Recent advances in the role of probiotics and prebiotics in carp aquaculture: A review. Aquaculture 2016, 454, 243–251. [Google Scholar] [CrossRef]
- Guerreiro, I.; Couto, A.; Machado, M.; Castro, C.; Pousão-Ferreira, P.; Oliva-Teles, A.; Enes, P. Prebiotics effect on immune and hepatic oxidative status and gut morphology of white sea bream (Diplodus sargus). Fish Shellfish Immunol. 2016, 50, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Song, S.K.; Beck, B.R.; Kim, D.; Park, J.; Kim, J.; Kim, H.D.; Ringø, E. Prebiotics as immunostimulants in aquaculture: A review. Fish Shellfish Immunol. 2014, 40, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and managing microbes in aquaculture—Towards a sustainable industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Magnadottir, B. Immunological control of fish diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.Á.; Cordero, H.; Martínez-Tomé, M.; Jiménez-Monreal, A.M.; Bakhrouf, A.; Mahdhi, A. Effect of dietary supplementation of probiotics and palm fruits extracts on the antioxidant enzyme gene expression in the mucosae of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2014, 39, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, T.; Ruiz-Zarzuela, J.; de Blas, I.; Balcázar, J.L. Probiotics in aquaculture: A current assessment. Rev. Aquac. 2013, 6, 1–14. [Google Scholar] [CrossRef]
- Muñoz-Atienza, E.; Araújo, C.; Magadán, S.; Hernández, P.E.; Herranz, C.; Santos, Y.; Cintas, L.M. In vitro and in vivo evaluation of lactic acid bacteria of aquatic origin as probiotics for turbot (Scophthalmus maximus L.) farming. Fish Shellfish Immunol. 2014, 41, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization and World Health Organization. Antimicrobial Resistance Fact Sheet 194. Available online: http://www.who.int/inf-fs/en/fact194html (accessed on 7 July 2018).
- Mohapatra, S.; Chakraborty, T.; Kumar, V.; De Boeck, G.; Mohanta, K.N. Aquaculture and stress management: A review of probiotic intervention. J. Anim. Physiol. Anim. Nutr. 2013, 97, 405–430. [Google Scholar] [CrossRef] [PubMed]
- C. De, B.; Meena, D.K.; Behera, B.K.; Das, P.; Das Mohapatra, P.K.; Sharma, A.P. Probiotics in fish and shellfish culture: Immunomodulatory and ecophysiological responses. Fish Physiol. Biochem. 2014, 40, 921–971. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.G.; Shiu, Y.L.; Nguyen, T.P.; Truong, Q.P.; Chen, J.C.; Liu, C.H. Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: A review. Fish Shellfish Immunol. 2017, 64, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Kiron, V. Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 2012, 173, 111–133. [Google Scholar] [CrossRef]
- Lauzon, H.L.; Pérez-Sánchez, T.; Merrifield, D.L.; Ringø, E.; Balcázar, J.L. Probiotic application in cold water fish species. In Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; Merrifield, D.L., Ringø, E., Eds.; Wiley-Blackwell Publishing: Oxford, UK, 2014; pp. 223–252. [Google Scholar]
- Carnevali, O.; Sun, Y.Z.; Merrifield, D.L.; Zhou, Z.; Picchietti, S. Probiotic application in temperate and warm water fish species. In Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; Merrifield, D.L., Ringø, E., Eds.; Wiley-Blackwell Publishing: Oxford, UK, 2014; pp. 253–289. [Google Scholar]
- Hoseinifar, S.H.; Ringø, E.; ShenavarMasouleh, A.; Esteban, M.Á. Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: A review. Rev. Aquac. 2014, 6, 1–14. [Google Scholar] [CrossRef]
- Ringø, E.; Gatesoupe, F.J. Lactic acid bacteria in fish: A review. Aquaculture 1998, 160, 177–203. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Dossou, S.; Moss, A.S. Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shellfish Immunol. 2016, 49, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.K.; Jena, P.K.; Nagar, N.; Patel, A.K.; Seshadri, S. In vitro evaluation of probiotic properties of lactic acid bacteria from the gut of Labeo tohita and Catla catla. Probiotics Antimicrob. Proteins 2015, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Strøm, E.; Ringø, E. Changes in the bacterial composition of early developing cod, Gadus morhua (L.) larvae following inoculation of Lactobacillus plantarum into the water. In Physiology and Biochemical Aspects of Fish Development; Walther, B.T., Fyhn, H.J., Eds.; University of Bergen: Bergen, Norway, 1993; pp. 226–228. [Google Scholar]
- Gobi, N.; Malaikozhundan, B.; Sekar, V.; Shanthi, S.; Vaseeharan, B.; Jayakumar, R.; Nazar, A.K. GFP tagged Vibrio parahaemolyticus Dahv2 infection and the protective effects of probiotic Bacillus licheniformis Dahb1 on the growth, immune and antioxidant responses in Pangasius hypophthalmus. Fish Shellfish Immunol. 2016, 52, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Irianto, A.; Austin, B. Probiotics in aquaculture. J. Fish Dis. 2002, 25, 633–642. [Google Scholar] [CrossRef]
- Das, S.; Ward, L.R.; Burke, C. Prospects of using marine actinobacteria as probiotics in aquaculture. Appl. Microbiol. Biotechnol. 2008, 81, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Cordero, H.; Guardiola, F.A.; Tapia-Paniagua, S.T.; Cuesta, A.; Meseguer, J.; Balebona, M.C. Modulation of immunity and gut microbiota after dietary administration of alginate encapsulated Shewanella putrefaciens Pdp11 to gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2015, 45, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, F.A.; Bahi, A.; Bakhrouf, A.; Esteban, M.Á. Effects of dietary supplementation with fenugreek seeds, alone or in combination with probiotics, on gilthead seabream (Sparus aurata L.) skin mucosal immunity. Fish Shellfish Immunol. 2017, 65, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E.; Birkbeck, T.H.; Munro, P.D.; Vadstein, O.; Hjelmeland, K. The effect of early exposure to Vibrio Pelagius on the aerobic bacterial flora of turbot, Scophthalmus maximus (L.) larvae. J. Appl. Bacteriol. 1996, 81, 207–211. [Google Scholar] [CrossRef]
- Ringø, E.; Vadstein, O. Colonization of Vibrio Pelagius and Aeromonas caviae in early developing turbot (Scophthalmus maximus L.) larvae. J. Appl. Microbiol. 1998, 84, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E. Does Carnobacterium divergens isolated from Atlantic salmon, Salmo salar L., colonize the gut of early developing turbot, Scophthalmus maximus L., larvae? Aquac. Res. 1999, 30, 229–232. [Google Scholar] [CrossRef]
- Park, Y.H.; Hwang, S.Y.; Hong, M.K.; Kwon, K.H. Use of antimicrobial agents in aquaculture. Rev. Sci. Tech. 2012, 31, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, D.J.W. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture 1998, 164, 351–358. [Google Scholar] [CrossRef]
- Skjermo, J.; Vadstein, O. Techniques for microbial control in the intensive rearing of marine larvae. Aquaculture 1999, 177, 333–343. [Google Scholar] [CrossRef]
- LaPatra, S.E.; Fehringer, T.R.; Cain, K.D. A probiotic Enterobacter sp. provides significant protection against Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss) after injection by two different routes. Aquaculture 2014, 433, 361–366. [Google Scholar] [CrossRef]
- Sveinsdottir, H.; Steinarsson, A.; Gudmundsdottir, A. Differential protein expression in early Atlantic cod larvae (Gadus morhua) in response to treatment with probiotic bacteria. Comp. Biochem. Physiol. 2009, 4, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Olafsen, J.A. Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture 2001, 200, 223–247. [Google Scholar] [CrossRef]
- Olafsen, J.A.; Hansen, G.H. Intact antigen uptake in intestinal epithelial cells of marine fish larvae. J. Fish Biol. 1992, 40, 141–156. [Google Scholar] [CrossRef]
- Skjermo, J.; Vadstein, O. Characterization of the bacterial flora of mass cultivated Brachionu splicatilis. Hydrobiologia 1993, 255, 185–191. [Google Scholar] [CrossRef]
- Villamil, L.; Figueras, A.; Planas, M.; Novoa, B. Pediococcus acidilactici in the culture of turbot (Psettamaxima) larvae: Administration pathways. Aquaculture 2010, 307, 83–88. [Google Scholar] [CrossRef]
- Wang, Y.B.; Tian, Z.Q.; Yao, J.T.; Li, W.F. Effects of probiotics, Enterococcus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture 2008, 277, 203–207. [Google Scholar] [CrossRef]
- Ruangsri, J.; Lokesh, J.; Fernandes, J.M.O.; Kiron, V. Transcriptional regulation of antimicrobial peptides in mucosal tissues of Atlantic cod Gadus morhua L. in response to different stimuli. Aquac. Res. 2014, 45, 1893–1905. [Google Scholar] [CrossRef]
- Shaheen, A.A.; Eissa, N.; Abou-ElGheit, E.N.; Yao, H.; Wang, H.P. Effect of probiotic on growth performance and growth-regulated genes in yellow perch (Perca flavescens). GJFAR 2014, 1, 1–15. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, P.; Dhawan, A. Paenibacillus polymyxa as a water additive improved immune response of Cyprinus carpio and disease resistance against Aeromonas hydrophila. Aquac. Rep. 2016, 4, 86–92. [Google Scholar] [CrossRef]
- Schaeck, M.; Reyes-López, F.E.; Vallejos-Vidal, E.; Van Cleemput, J.; Duchateau, L.; Van den Broeck, W.; Tort, L.; Decostere, A. Cellular and transcriptomic response to treatment with the probiotic candidate Vibrio lentus in gnotobiotic sea bass (Dicentrarchus labrax) larvae. Fish Shellfish Immunol. 2017, 63, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Han, W. Reuse strategy of wastewater in prawn nursery by microbial remediation. Aquaculture 2004, 230, 281–296. [Google Scholar] [CrossRef]
- Gross, A.; Abutbul, S.; Zilberg, D. Acute and chronic effects of nitrite on white shrimp, Litopenaeus vannamei, cultured in low-salinity brackish water. J. World Aquac. Soc. 2004, 35, 315–321. [Google Scholar] [CrossRef]
- Su, Z.; Li, Y.; Pan, L.; Xue, F. An investigation on the immunoassays of an ammonia nitrogen-degrading bacterial strain in aquatic water. Aquaculture 2016, 450, 17–22. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, J.C. Effect of ammonia on the immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus. Fish Shellfish Immunol. 2004, 16, 321–334. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, T.; Espinosa, G.; Hernández-López, J.; Gollas-Galván, T.; Marrero, J.; Borrell, Y.; Alonso, M.E.; Becquer, U. Effects of Echerichia coli lipopolysaccharides and dissolved ammonia on immune response in southern white shrimp Litopenaeus schmitti. Aquaculture 2008, 274, 118–125. [Google Scholar] [CrossRef]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 2007, 270, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.P.; d’Orbcastel, E.R.; Verreth, J.A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Chun, S.J.; Cui, Y.; Ahn, C.Y.; Oh, H.M. Improving water quality using settleable microalgae Ettlia sp. and the bacterial community in freshwater recirculating aquaculture system Danio rerio. Water Res. 2018, 135, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Burford, M.A.; Thompson, P.J.; McIntosh, P.R.; Bauman, R.H.; Pearson, D.C. The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero exchange system. Aquaculture 2004, 232, 525–537. [Google Scholar] [CrossRef]
- López-Elías, J.A.; Moreno-Arias, A.; Miranda-Baeza, A.; Martínez-Córdova, L.R.; Rivas-Vega, M.E.; Márquez-Ríos, E. Proximate composition of bioflocs in culture systems containing hybrid red tilapia fed diets with varying levels of vegetable meal inclusion. N. Am. J. Aquac. 2015, 77, 102–109. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Cody, J.J.; Conquest, L.D.; Divakaran, S.; Forster, I.P.; Decamp, O.E. Effect of culture system on the nutrition and growth performance of Pacific white shrimp Litopenaeus vannamei (Boone) fed different diets. Aquac. Nutr. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Martínez-Córdova, L.R.; Martínez-Porchas, M.; Emerenciano, M.G.C.; Miranda-Baeza, A.; Gollas-Galván, T. From microbes to fish the next revolution in food production. Crit. Rev. Biotechnol. 2017, 37, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arias, A.; López-Elías, J.A.; Martínez-Córdova, L.R.; Ramírez-Suárez, J.C.; Carvallo-Ruiz, M.G.; García-Sánchez, M.E.; Miranda-Baeza, A. Effect of fishmeal replacement with a vegetable protein mixture on the amino acid and fatty acid profiles of diets, biofloc and shrimp cultured in BFT system. Aquaculture 2018, 483, 53–62. [Google Scholar] [CrossRef]
- Aguilera-Rivera, D.; Prieto-Davó, A.; Escalante, K.; Chávez, C.; Cuzon, G.; Gaxiola, G. Probiotic effect of FLOC on Vibrios in the pacific white shrimp Litopenaeus vannamei. Aquaculture 2014, 424, 215–219. [Google Scholar] [CrossRef]
- Arias-Moscoso, J.L.; Espinoza-Barrón, L.G.; Miranda-Baeza, A.; Rivas-Vega, M.E.; Nieves-Soto, M. Effect of commercial probiotics addition in a biofloc shrimp farm during the nursery phase in zero water exchange. Aquac. Rep. 2018, 11, 47–52. [Google Scholar] [CrossRef]
- Banerjee, G.; Ray, A.K. The advancement of probiotics research and its application in fish farming industries. Res. Vet. Sci. 2017, 115, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, D.D.; Peng, K.S.; Cui, Z.W.; Zhang, X.J.; Li, S.; Zhang, Y.A. Identification and characterization of Bacillus subtilis from grass carp (Ctenopharyngodon idellus) for use as probiotic additives in aquatic feed. Fish Shellfish Immunol. 2016, 52, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Rahimnejad, S.; Yang, S.Y.; Kim, K.W.; Lee, K.J. Evaluations of Bacillus spp. as dietary additives on growth performance, innate immunity and disease resistance of olive flounder (Paralichthys olivaceus) against Streptococcus iniae and as water additives. Aquaculture 2013, 402–403, 50–57. [Google Scholar] [CrossRef]
- Balcazar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Cunningham, D.; Vendrell, D.; Muzquiz, J.L. The role of probiotics in aquaculture. Vet. Microbiol. 2006, 114, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Zorriehzahra, M.J.; Torabi Delshad, S.; Adel, M.; Tiwari, R.; Karthik, K.; Dhama, K.; Lazado, C.C. Probiotics as beneficial microbes in aquaculture: An update on their multiple modes of action: A review. Vet. Quart. 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Feng, Y.H.; Wang, J.; Guo, J.; Zhang, Y.H.; Gao, J.Z.; Song, Z.F. Study on the characteristics of the ammonia-nitrogen and residual feeds degradation in aquatic water by Bacillus licheniformis. Acta Hydrobiol. Sin. 2011, 35, 498–503. [Google Scholar]
- Jóźwiakowski, K.; Czernaś, K.; Szczurowska, A. Preliminary results of studies on the purification of water in a pond using the SCD Probiotics technology. Ecohydrol. Hydrobiol. 2009, 9, 307–312. [Google Scholar] [CrossRef]
- Banerjee, S.; Khatoon, H.; Shariff, M.; Yusoff, F.M. Enhancement of Penaeus monodon shrimp post larvae growth and survival without water exchange using marine Bacillus pumilus and periphytic microalgae. Fish. Sci. 2010, 76, 481–487. [Google Scholar] [CrossRef]
- Aguirre-Guzman, G.; Lara-Flores, M.; Sanchez-Martinez, J.G.; Campa-Cordova, A.I.; Luna-Gonzalez, A. The use of probiotics in aquatic organisms: A review. Afr. J. Microbiol. Res. 2012, 2, 4854–4857. [Google Scholar] [CrossRef]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Ibrahem, M.D. Evolution of probiotics in aquatic world: Potential effects, the current status in Egypt and recent prospective. J. Adv. Res. 2015, 6, 765–791. [Google Scholar] [CrossRef] [PubMed]
- Irianto, A.; Austin, B. Use of dead probiotic cells to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2003, 26, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Austin, B. Cytokine expression in leucocytes and gut cells of rainbow trout, Oncorhynchus mykiss Walbaum, induced by probiotics. Vet. Immunol. Immunopathol. 2006, 114, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Austin, B. Innate immune responses in rainbow trout (Oncorhynchus mykiss, Walbaum) induced by probiotics. Fish Shellfish Immunol. 2006, 21, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mukherjee, S.C.; Ranjan, R.; Nayak, S.K. Enhanced innate immune parameters in Labeo rohita (Ham.) following oral administration of Bacillus subtilis. Fish Shellfish Immunol. 2008, 24, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K.; Swain, P.; Mukherjee, S.C. Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (Ham.). Fish Shellfish Immunol. 2007, 23, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Widanarni; Tanbiyaskur. Application of probiotic, prebiotic and symbiotic for the control of Streptococcosis in Tilapia Oreochromis niloticus. Pak. J. Biol. Sci. 2015, 18, 59–66. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Carnevali, O.; Picchietti, S.; Avella, M.; Daniels, C.; Güroy, D.; Davies, S.J. Microbial manipulations to improve fish health and production—A Mediterranean perspective. Fish Shellfish Immunol. 2011, 30, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazado, C.C.; Caipang, C.M.A. Atlantic cod in the dynamic probiotics research in aquaculture. Aquaculture 2014, 424–425, 53–62. [Google Scholar] [CrossRef]
- Picchietti, S.; Fausto, A.M.; Randelli, E.; Carnevali, O.; Taddei, A.R.; Buonocore, F.; Scapigliati, G.; Abelli, L. Early treatment with Lactobacillus delbrueckii strain induces an increase in intestinal T-cells and granulocytes and modulates immune-related genes of larval Dicentrarchuslabrax (L.). Fish Shellfish Immunol. 2009, 26, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picchietti, S.; Mazzini, M.; Taddei, A.R.; Renna, R.; Fausto, A.M.; Mulero, V.; Carnevali, O.; Cresci, A.; Abelli, L. Effects of administration of probiotic strains on GALT of larval gilthead sea bream: Immunohistochemical and ultrastructural studies. Fish Shellfish Immunol. 2007, 22, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Lazado, C.C.; Caipang, C.M.A. Mucosal immunity and probiotics in fish. Fish Shellfish Immunol. 2014, 39, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.E. Ontogeny of the immune system in teleost fish. In Fish Vaccination; Ellis, A.E., Ed.; Academic Press: London, UK, 1998; pp. 20–31. [Google Scholar]
- Chantanachookhin, C.; Seikai, T.; Tanaka, M. Comparative study of the ontogeny of the lymphoid organs in three species of marine fish. Aquaculture 1991, 99, 143–155. [Google Scholar] [CrossRef]
- Ottasen, O.H.; Olafsen, J.A. Effects on survival and mucous cell proliferation of Atlantic halibut, Hippoglossus hippoglossus L., larvae following microflora manipulation. Aquaculture 2000, 187, 225–238. [Google Scholar] [CrossRef]
- Hatai, K.; Hoshiai, G. Mass mortality in cultured coho salmon (Oncorhynchus kisutch) due to Saprolagnia parasitica coker. J. Wildl. Dis. 1992, 28, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Song, P.; Ou, R.; Fang, W.; Lin, M.; Ruan, J.; Yang, X.; Hu, K. Sequence analysis and typing of Saprolegnia strains isolated from freshwater fish from Southern Chinese regions. Aquac. Fish. 2017, 2, 227–233. [Google Scholar] [CrossRef]
- Lategan, M.J.; Gibson, L.F. Antagonistic activity of Aeromonas media strain A199 against Saprolegnia sp., an opportunistic pathogen of the eel, Anguilla australis Richardson. J. Fish Dis. 2003, 26, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lategan, M.J.; Torpy, F.R.; Gibson, L.F. Biocontrol of saprolegniosis in silver perch Bidyanus bidyanus (Mitchell) by Aeromonas media strain A100. Aquaculture 2004, 235, 77–88. [Google Scholar] [CrossRef]
- Lategan, M.J.; Torpy, F.R.; Gibson, L.F. Control of saprolegniosis in the eel Anguilla australis Richardson, by Aeromonas media strain 199. Aquaculture 2004, 2004, 19–27. [Google Scholar] [CrossRef]
- Wesseling, W.; Wittka, S.; Kroll, S.; Soltmann, C.; Kegler, P.; Kunzmann, A.; Riss, H.W.; Lohmeyer, M. Functionalised ceramic spawning tiles with probiotic Pseudoalteromonas biofilms designed for clownfish aquaculture. Aquaculture 2015, 446, 57–66. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and β-glucan on growth performance, digestibility and immune response of juvenile red sea bream, Pagrus major. Fish Shellfish Immunol. 2015, 45, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hai, N.V. Research findings from the use of probiotics in tilapia aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Mandiki, S.N.M.; Milla, S.; Wang, N.; Blanchard, G.; Djonkack, T.; Tanascaux, S.; Kestemont, P. Effects of probiotic on growth parameters and immune defense in Eurasian perch Perca fluviatilis L. larvae under intensive culture conditions. Aquac. Res. 2011, 42, 693–703. [Google Scholar] [CrossRef]
- Suzer, C.; Çoban, D.; OkanKamaci, H.; Saka, Ş.; Firat, K.; Otgucuoğlu, Ö.; Küçüksari, H. Lactobacillus spp. bacteria as probiotics in gilthead sea bream (Sparus aurata L.) larvae: Effects on growth performance and digestive enzyme activities. Aquaculture 2008, 280, 140–145. [Google Scholar] [CrossRef]
- Lauzon, H.L.; Gudmundsdottir, S.; Steinarsson, A.; Oddgeirsson, M.; Petursdottir, S.K.; Reynisson, E.; Bjornsdottir, R.; Gudmundsdottir, B.K. Effects of bacterial treatment at early stages of Atlantic cod (Gadus morhua L.) on larval survival and development. J. Appl. Microbiol. 2010, 108, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Gatesoupe, F.J. Updating the importance of lactic acid bacteria in fish farming: Natural occurrence and probiotic treatments. J. Mol. Microbiol. Biotechnol. 2008, 14, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chinabut, S.; Puttinaowarat, S. The choice of disease control strategies to secure international market access for aquaculture products. Dev. Biol. 2005, 121, 255–261. [Google Scholar]
- Skjermo, J.; Bakke, I.; Dahle, S.W.; Vadstein, O. Probiotics strains introduced through live feed and rearing water have low colonizing success in developing Atlantic cod larvae. Aquaculture 2015, 438, 17–23. [Google Scholar] [CrossRef]
- Widanarni, W.; Yuniasari, D.; Sukenda, S.; Ekasari, J. Nursery culture performance of Litopenaeus vannamei with probiotics addition and different C/N ratio under laboratory condition. Hayati J. Biosci. 2010, 17, 115–119. [Google Scholar] [CrossRef]
- Hostins, B.; Lra, G.; Decamp, O.; Cesar, D.E.; Wasielesky, W. Efficacy and variations in bacterial density in the gut of Litopenaeus vannamei reared in a BFT system and in clear water supplemented with a commercial probiotic mixture. Aquaculture 2017, 480, 58–64. [Google Scholar] [CrossRef]
- Reyes-López, F.E.; Romeo, J.S.; Vallejos-Vidal, E.; Reyes-Cerpa, S.; Sandino, A.M.; Tort, L.; Mackenzie, S.; Imarai, M. Differential immune gene expression profiles in susceptible and resistant full-sibling families of Atlantic salmon (Salmo salar) challenged with infectious pancreatic necrosis virus (IPNV). Dev. Comp. Immunol. 2015, 53, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.M.; Lall, S.P.; Rajaselvam, R.; Boutilier, L.A.; Blanchard, B.; Flight, R.M.; Colombo, S.; Mohindra, V.; Douglas, S.E. A nutrigenomic analysis of intestinal response to partial soybean meal replacement in diets for juvenile Atlantic halibut, Hippoglossus hipoglossus, L. Aquaculure 2010, 298, 282–293. [Google Scholar] [CrossRef]

| Probiotics | Biological Model | Concentration | Fish Density, Age, Average Weight | Inoculation Times | Contact Duration | Major Outcomes | References |
|---|---|---|---|---|---|---|---|
| Lactobacillus plantarum (later classified as Carnobacterium divergens) | Atlantic cod (Gadus morhua L.) | 105 CFU mL−1 | 5 dph | - | 9 days | Strain colonization Decreased opportunistic bacteria | [24] |
| Vibrio pelagius | Turbot (Scophthalmus maximus L.) | 4 × 105 bacteria mL−1 | 0 dph | - | 14 days | Influenced gut microbiota | [31] |
| V. pelagius and Aeromonas caviae | Turbot | 105 bacteria mL−1 | 2–8 dph | - | 16 days | Colonization of probiotic bacteria in the group received the bacteria at earlier days. Increased survival in group received V. pelagius | [32] |
| C. divergens and V. pelagius | Turbot | 105 bacteria mL−1 | 0 dph | 15 days | Higher colonization of V. pelagius. No significant difference in survival | [33] | |
| Enterococcus faecium ZJ4 (isolated from intestinal tract of piglet) | Tilapia (Oreochromis niloticus) | 107 CFU mL−1 | 30 fishes (with average weight 6.83 ± 0.18 g) per aquarium | Every 4 days | 40 days | Significantly higher final weight, DWG, complement component 3 content, MPO activity and RBA of blood phagocytes in group treated with probiotic (p < 0.05). No significant difference in total serum protein, albumin and globulin concentration and lysozyme activity between treated group and control | [43] |
| Pseudomonas sp. (isolate GP21) isolated from the intestine of Atlantic cod | Atlantic cod | 3.8 × 108 CFU mL−1 | 8 fishes (with average weight 150 g) per tank | - | 1 h | Downregulated defb expression in gills and upregulated defb expression in skin after treatment with probiotic | [44] |
| Commercial probiotic Fishery Prime™ (Keeton Industries, Wellington, CO, USA) | Perca flavescens | 5 g to each 100 L tank | 30 fishes (with average weight 6.17 ± 2.27 g) per tank | Every day | 6 weeks | Significantly higher weight gain (p < 0.05) and higher levels of GH and IGF-I transcription in group treated with probiotic | [45] |
| Paenibacillus polymyxa | Cyprinus carpio | 103, 104 and 105 CFU mL−1 | 20 fishes (with average weight 23.17 g) per tank | 3 times a week | 8 weeks | Significantly improved survival, lysozyme activity, MPO content, RBA, catalase and superoxidase dismutase activity and resistance against Aeromonas hydrophila in groups treated with probiotic (p < 0.05) | [46] |
| Bacillus licheniformis Dahb1 | Asian catfish (Pangasius hypophthalmus) | 105 and 107 CFU mL−1 | 12 fishes (with average weight 15 ± 2.5 g) per group | - | 24 days | Significantly higher weight gain, survival (with no mortality during contact time), RBA, GST activity, total glutathione activity (p < 0.05) and higher MPO and lysozyme activity in group treated with probiotic. No significant difference in ACH50 and GR in group treated with probiotic | [25] |
| Vibrio lentus | Sea bass (Dicentrarchus labrax) | 106 CFU mL−1 | 12 larvae (4, 6 and 8 dph) inoculated via immersion in well plate | - | - | Significantly modified gene expression (i.e., immune response, cell proliferation and death, cell adhesion, ROS metabolism and iron transport (p < 0.05). No significant differences in apoptotic and cell proliferative indexes | [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahangiri, L.; Esteban, M.Á. Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review. Fishes 2018, 3, 33. https://doi.org/10.3390/fishes3030033
Jahangiri L, Esteban MÁ. Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review. Fishes. 2018; 3(3):33. https://doi.org/10.3390/fishes3030033
Chicago/Turabian StyleJahangiri, Ladan, and María Ángeles Esteban. 2018. "Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review" Fishes 3, no. 3: 33. https://doi.org/10.3390/fishes3030033