Transmission Strategies Used by Gyrodactylus gasterostei (Monogenea) on Its Host, the Three-Spined Stickleback Gasterosteus aculeatus
Abstract
:1. Introduction
2. Results
2.1. Experiment 1. Parasite Migration from Dead Hosts
2.2. Experiment 2. Parasite Choice of Live or Dead Hosts
2.3. Experiment 3. One-to-One Fish Cohabitation
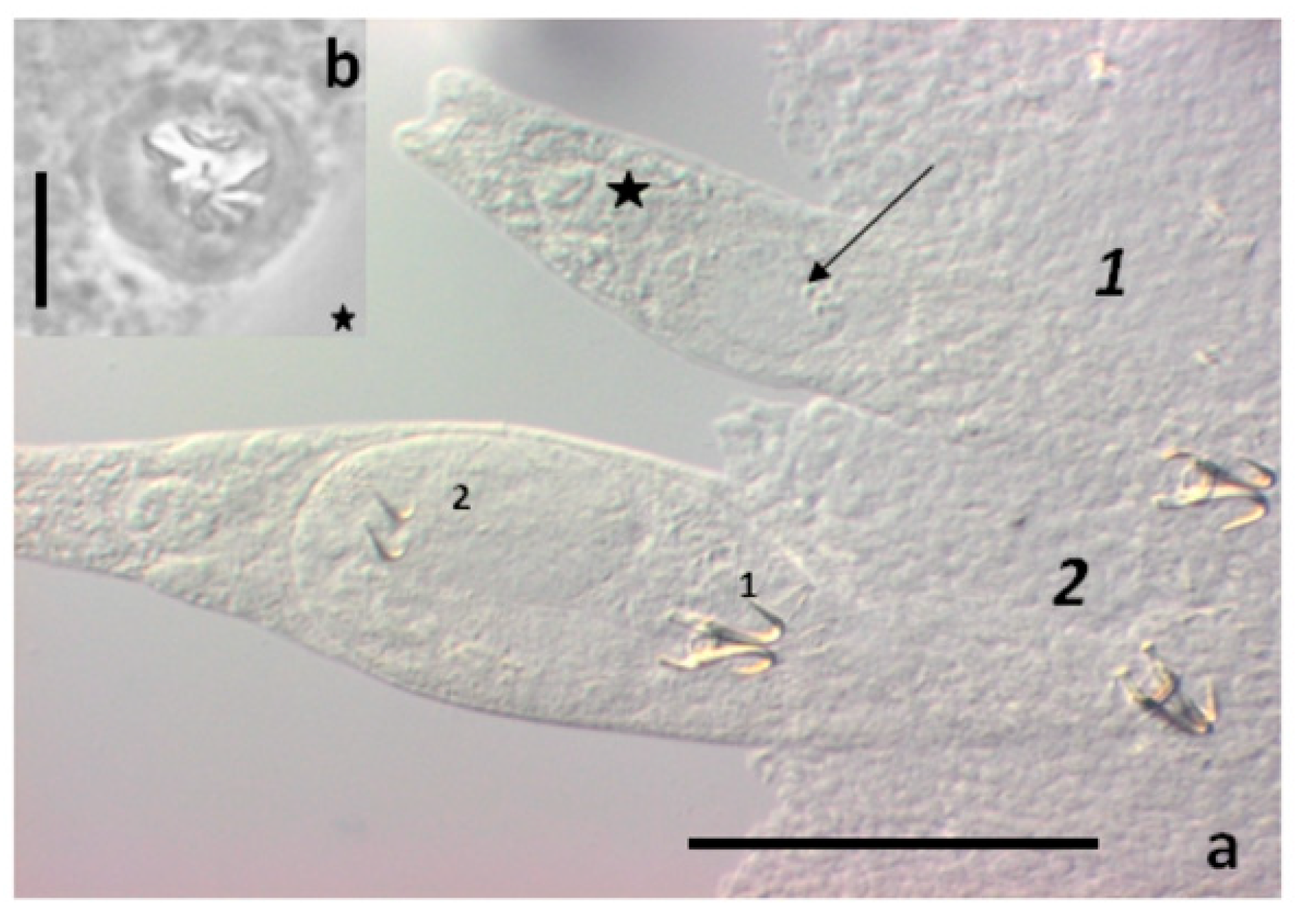
2.4. Experiment 4. Video Monitoring of Gyrodactylid Transmission In Vivo
3. Discussion
4. Materials and Methods
4.1. Source of Hosts and Parasites
4.2. Parasite Free-Hosts
4.3. Experiment 1. Parasite Migration from Dead Hosts
4.4. Experiment 2. Parasite Choice of Live or Dead Hosts
4.5. Experiment 3. One-to-One Fish Cohabitation
4.6. Experiment 4. Video Monitoring of Gyrodactylid Transmission In Vivo
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Harris, P.D. Interactions between reproduction and population biology in gyrodactylid monogeneans—A review. Bull. Fr. Peche Piscic. 1993, 1, 47–65. [Google Scholar] [CrossRef]
- Bakke, T.A.; Harris, P.D.; Cable, J. Host specificity dynamics: Observations on gyrodactylid monogeneans. Int. J. Parasitol. 2002, 32, 281–308. [Google Scholar] [CrossRef]
- Cable, J.; Harris, P.D. Gyrodactylids developmental biology historical review, current status and future trends. Int. J. Parasitol. 2002, 32, 255–280. [Google Scholar] [CrossRef]
- Harris, P.D. Changes in the site specificity of Gyrodactylus turnbulli Harris, 1986 (Monogenea) during infections of individual guppies (Poecilia reticulata Peters, 1859). Can. J. Zool. 1988, 66, 2854–2857. [Google Scholar] [CrossRef]
- Cable, J.; Scott, E.C.G.; Tinsley, R.C.; Harris, P.D. Behaviour favoring transmission in the viviparous monogenean Gyrodactylus turnbulli. J. Parasitol. 2002, 88, 183–184. [Google Scholar] [CrossRef]
- El-Naggar, M.M.; El-Naggar, A.M.; Kearn, G.C. Swimming in Gyrodactylus rysavyi (Monogenea: Gyrodactylidae) from the Nile catfish Clarias gariepinus. Acta Parasitol. 2004, 49, 102–107. [Google Scholar]
- Dmitrieva, E.V. Transmission triggers and pathways in Gyrodactylus sphinx (Monogenea, Gyrodactylidae). Vestnik Zool. 2003, 37, 67–72. [Google Scholar]
- Bakke, T.A.; Cable, J.; Harris, P.D. The biology of gyrodactylid monogeneans: The “Russian Doll-killers”. Adv. Parasitol. 2007, 64, 161–376. [Google Scholar] [PubMed]
- Grano-Maldonado, M.I. The Biological and Behavioural Basis of Host Selection in Transmission of Gyrodactylus (Monogenea). Ph.D. Thesis, Stirling Univeristy, Stirling, UK, 2011; p. 228. [Google Scholar]
- Grano-Maldonado, M.I.; Palaiokostas, C. Does the anaesthetic influence behavioural transmission of the monogenean Gyrodactylus gasterostei Gläser, 1974 off the host? Helminthologia 2015, 52, 144–147. [Google Scholar] [CrossRef]
- Olstad, K.; Cable, J.; Robertsen, G.; Bakke, T.A. Unpredicted transmission strategy of Gyrodactylus salaris (Monogenea: Gyrodactylidae): Survival and infectivity of parasites on dead hosts. Parasitology 2006, 133, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.D. Observations on the development of the male reproductive system in Gyrodactylus gasterostei Gläser, 1974 (Monogenea, Gyrodactylidae). Parasitology 1985, 91, 519–529. [Google Scholar] [CrossRef]
- Raeymaekers, J.; Wegner, K.; Huyse, T.; Volckaert, F. Infection dynamics of the monogenean parasite Gyrodactylus gasterostei on sympatric and allopatric populations of the three-spined stickleback Gasterosteus aculeatus. Folia Parasitol. 2011, 58, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Grano-Maldonado, M.I.; Bron, J.; Longshaw, M.; Shinn, A. The accidental transfer of Gyrodactylus (Monogenea) during short duration fish transportation. Fish Pathol. 2011, 46, 71–79. [Google Scholar] [CrossRef]
- Grano-Maldonado, M.I. Ultrastructure of the external sensory apparatus of Gyrodactylus gasterostei Gläser, 1974. Microsc. Res. Tech. 2014, 77, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Grano-Maldonado, M.I. Gyrodactylus gasterostei a difficult meal to swallow for the three-spined sticklebacks, Gasterosteus aculeatus L. Scanning 2014, 36, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Brooker, A.J.; Grano-Maldonado, M.I.; Irving, S.; Bron, J.; Longshaw, M.; Shinn, A.P. The effect of octopaminergic compounds on the behaviour and transmission of Gyrodactylus. Parasite Vectors 2011, 4, 207. [Google Scholar] [CrossRef] [PubMed]
- Grano-Maldonado, M.I. Ultrastructure study of the stored lipid reserves in Gyrodactylus gasterostei Gläser, 1974 using confocal microscopy. J. Microsc. Ultrastruct. 2017. [Google Scholar] [CrossRef]
- Lester, R.J.G.; Adams, J.R. Gyrodactylus alexanderi: Reproduction, mortality and effect on its host Gasterosteus aculeatus. Can. J. Zool. 1974, 52, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Scott, M. Reproductive potential of Gyrodactylus bullatarudis (Monogenea) on guppies (Poecilia reticulata). Parasitology 1982, 85, 217–236. [Google Scholar] [CrossRef]
- Schelkle, B.; Paladini, G.; Shinn, A.; King, S.; Johnson, M.; van Oosterhout, C.; Mohammed, R.; Cable, J. Ieredactylus rivuli gen. et sp. nov. (Monogenea, Gyrodactylidae) from Ivulus hartii (Cyprinodontiformes, Rivulidae) in Trinida. Acta Parasitol. 2011, 56, 360–370. [Google Scholar] [CrossRef]
- Lavin, P.; McPhail, J. The evolution of freshwater diversity in the three-spined stickleback (Gasterosteus aculeatus): Site-specific differentation of trophic morphology. Can. J. Zool. 1985, 63, 2632–2638. [Google Scholar] [CrossRef]
- Bell, M.; Foster, S. The Evolutionary Biology of the Three spined Stickleback; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Grano-Maldonado, M.I.; Bruno de Sousa, C.; Rodriguez-Santiago, A. First insights into the ultrastructure of myosin and actin bands using Transmission Electron Microscopy in Gyrodactylus (Monogenea). J. Microsc. Ultrastruct. 2017. [Google Scholar] [CrossRef]
- Malmberg, G. The excretory system and marginal hooks as a basis for the systematics of Gyrodactylus (Trematoda, Monogenea). Arch. Zool. 1970, 23, 1–237. [Google Scholar]
- De Roij, J.; Harris, P.; MacColl, A. Divergent resistance to a monogenean flatworm among three-spined stickleback populations. Funct. Ecol. 2011, 25, 211–217. [Google Scholar] [CrossRef]
- Harris, P.D.; Shinn, A.P.; Cable, J.; Bakke, T.A. Nominal species of the genus Gyrodactylus von Nordmann 1832 (Monogenea: Gyrodactylidae), with a list of principal host species. Syst. Parasitol. 2004, 59, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Shinn, A.P.; Hansen, H.; Olstad, K.; Bachmann, L.; Bakke, T.A. The use of morphometric characters to discriminate species of laboratory-reared and wild populations of Gyrodactylus salaris and G. thymalli (Monogenea). Folia Parasitol. 2004, 51, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.D.; Shinn, A.P.; Cable, J.; Bakke, T.A.; Bron, J.E. GyroDb: Gyrodactylid monogeneans on the web. Trends Parasitol. 2008, 24, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Shinn, A.P.; Harris, P.D.; Cable, J.; Bakke, T.A.; Paladini, G.; Bron, J.E. GyroDb—A Home for Gyrodactylids on the Web. Editors 2010. GyroDb. World Wide Web Electronic publicatIon, Version (06/2010). Available online: www.gyrodb.net (accessed on 1 August 2016).
- Konijnendijk, N.; Raeymaekers, J.A.; Vandeuren, S.; Jacquemin, L.; Volckaert, F.A. Testing for local adaptation in the Gasterosteus–Gyrodactylus host–parasite system. Evol. Ecol Res. 2013, 15, 489–502. [Google Scholar]
- Neiman, M.; Sharbel, T.F.; Schwander, T. Genetic causes of transitions from sexual reproduction to asexuality in plants and animals. J. Evol. Biol. 2014, 27, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Lumme, J.; Ziętara, M.S. Horizontal transmission of the ectoparasite Gyrodactylus arcuatus (Monogenea: Gyrodactylidae) to the next generation of the three-spined stickleback Gasterosteus aculeatus. Folia Parasitol. 2018, 65, 006. [Google Scholar] [CrossRef] [PubMed]
- Tytell, E.D. Median fin function in bluegill sunfish, Lepomis macrochirus: Streamwise vortex structure during steady swimming. J. Exp. Biol. 2006, 209, 1516–1534. [Google Scholar] [CrossRef] [PubMed]
- Bozkurttas, M.; Dong, H.; Mittal, R.; Madden, P.; Lauder, G.V. Hydrodynamic performance of deformable fish fins and flapping foils. In Proceedings of the 44th AIAA Aerospace Sciences Meeting and Exhibit, Reno, Nevada, 9–12 January 2006. 2006 AIAA Paper 2006-1392. [Google Scholar]
- Drucker, E.G.; Lauder, G.V. Function of pectoral fins in rainbow trout: Behavioral repertoire and hydrodynamic forces. J. Exp. Biol. 2003, 206, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Pahor-Filho, E.; Miranda Filho, K.C.; Klosterhoff, M.; Romano, L.A.; Pereira Júnior, J. Histopathological and behaviour effects of formaldehyde treatment in juvenile mullet, Mugil liza (Valenciennes). Aquac. Res. 2015, 46, 3040–3045. [Google Scholar] [CrossRef]
- Harris, P.D. Ecological and genetic evidence for clonal reproduction in Gyrodactylus gasterostei Gläser, 1974. Int. J. Parasitol. 1998, 28, 1595–1607. [Google Scholar] [CrossRef]
| PRS | [A] n = 240 | [B] n = 213 | p-Value (A vs. B) |
|---|---|---|---|
| N MCO/ND | 36 (15.0%) | 9 (4.2%) | <0.001 *** |
| N MCO/D | 136 (56.7%) | 53 (24.9%) | <0.001 *** |
| MCO/ND | 25 (10.4%) | 67 (31.5%) | <0.001 *** |
| MCO/D | 43 (17.92%) | 84 (39.4%) | <0.001 *** |
| Total N MCO | 172 (71.7%) | 62 (29.1%) | <0.001 *** |
| Total MCO | 68 (28.3%) | 151 (70.9%) | <0.001 *** |
| Location | Maturity Status of the Gyrodactylid | ||||
|---|---|---|---|---|---|
| N MCO/ND | N MCO/D | MCO/ND | MCO/D | Total | |
| Dead-un | 4 (4.2%) | 1 (0.2%) | 8 (2.1%) | 1 (0.3%) | 14 (1.1%) *** |
| Live-un | 1 (1.0%) | 6 (1.1%) | 11 (2.9%) | 5 (1.7%) | 23 (1.7%) *** |
| Live-inf | 79 (82.3%) | 510 (93.4%) | 346 (90.6%) | 274 (93.8%) | 1209 (91.9%) |
| Dislodged | 12 (12.5%) | 29 (5.3%) | 17 (4.5%) | 12 (4.1%) | 70 (5.3%) |
| Total | 96 | 546 | 382 | 292 | 1316 |
| Location | MCO/D | % | NMCO/D | % | MCO/ND | % | NMCO/ND | % | Total DS | Total | % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Live-un | 46 | 33.3% | 21 | 15.21% | 49 | 35.50% | 22 | 15.94% | 138 (10.49%) | 1315 | 10.49 |
| Live-inf | 291 | 27.6% | 447 | 42.36% | 184 | 17.44% | 133 | 12.60% | 1055 (80.22%) | 80.22 | |
| Dislodged | 18 | 14.8% | 13 | 10.65% | 66 | 54.09% | 25 | 20.49% | 122 (9.2%) | 9.27 | |
| Dead-un | 104 | 35.86% | 56 | 19.3% | 85 | 29.31% | 45 | 15.51% | 290 (21.96%) | 1320 | 21.96 |
| Live-inf | 283 | 30.49% | 399 | 42.99% | 162 | 17.45% | 84 | 9.05% | 928 (70.30%) | 70.30 | |
| Dislodged | 25 | 24.50% | 9 | 8.82% | 53 | 51.96% | 15 | 14.70% | 102 (7.72%) | 7.72 | |
| Control | 314 | 27.86% | 430 | 38.15% | 243 | 21.56% | 140 | 12.42% | 1127 (90.74%) | 1242 | 90.74 |
| Dislodged | 28 | 24.34% | 25 | 21.73% | 47 | 40.86% | 15 | 13.04% | 115 (9.2%) | 9.25 | |
| Total | 1109 | 1400 | 889 | 479 | 3877 | 3877 | |||||
| Average | 7.701 | 9.72 | 6.17 | 3.32 | |||||||
| SD | 11.136 | 17.54 | 8.00 | 5.70 | |||||||
| % | 28.60 | 36.11 | 22.93 | 12.35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grano-Maldonado, M.I.; Moreno-Navas, J.; Rodriguez-Santiago, M.A. Transmission Strategies Used by Gyrodactylus gasterostei (Monogenea) on Its Host, the Three-Spined Stickleback Gasterosteus aculeatus. Fishes 2018, 3, 20. https://doi.org/10.3390/fishes3020020
Grano-Maldonado MI, Moreno-Navas J, Rodriguez-Santiago MA. Transmission Strategies Used by Gyrodactylus gasterostei (Monogenea) on Its Host, the Three-Spined Stickleback Gasterosteus aculeatus. Fishes. 2018; 3(2):20. https://doi.org/10.3390/fishes3020020
Chicago/Turabian StyleGrano-Maldonado, Mayra I., Juan Moreno-Navas, and Maria Amparo Rodriguez-Santiago. 2018. "Transmission Strategies Used by Gyrodactylus gasterostei (Monogenea) on Its Host, the Three-Spined Stickleback Gasterosteus aculeatus" Fishes 3, no. 2: 20. https://doi.org/10.3390/fishes3020020





