A Field Observation of Rotational Feeding by Neogobius melanostomus
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Video Methods
2.3. Video Analysis
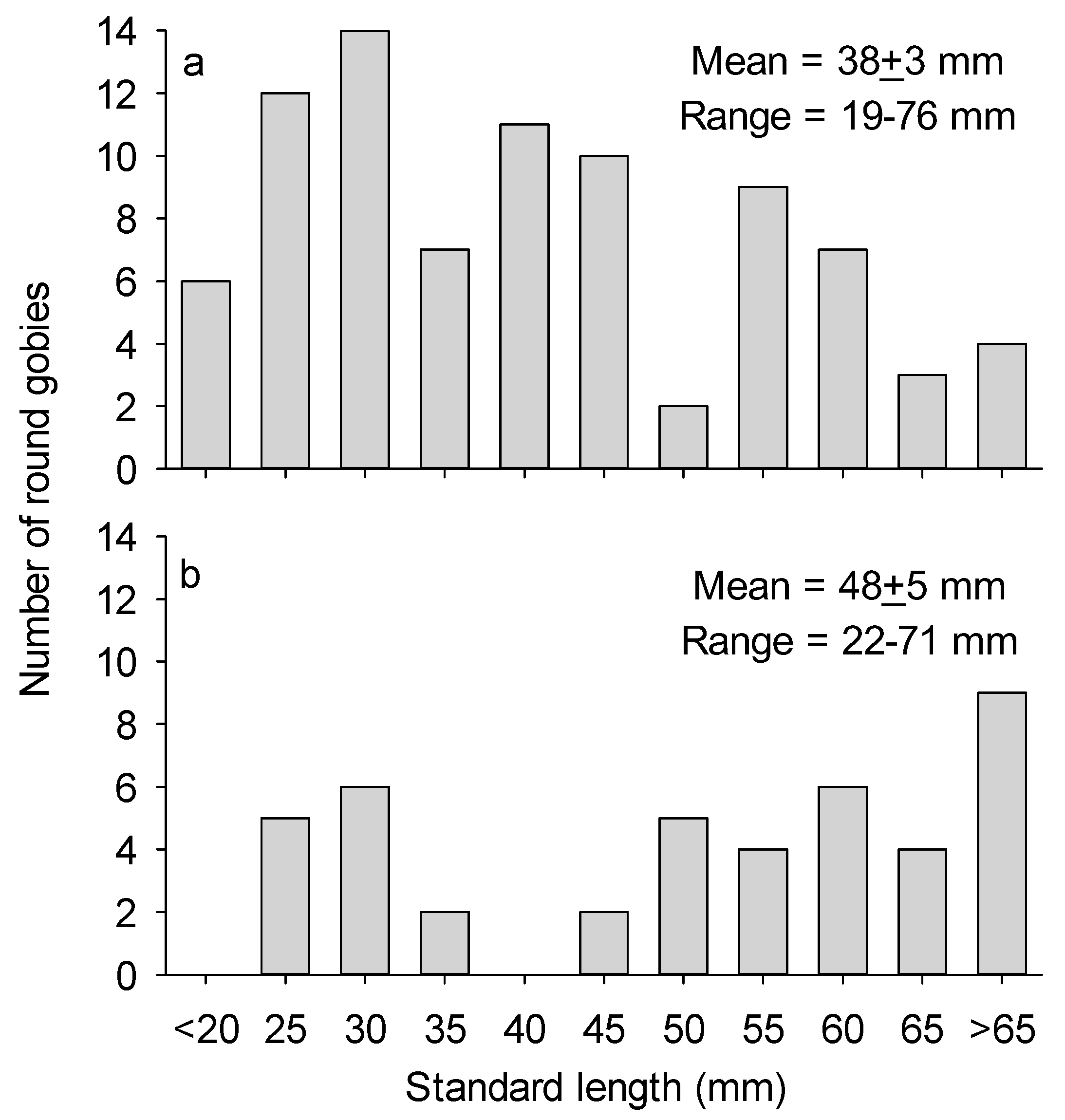
3. Results
3.1. Habitat and Dreissenid Mussels
3.2. Round Goby Size and Behavior
4. Discussion
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Helfman, G.S. Mode selection and mode switching in foraging animals. Adv. Stud. Behav. 1990, 19, 249–298. [Google Scholar]
- Taylor, M.A. How tetrapods feed in water: A functional analysis by paradigm. Zool. J. Linn. Soc. 1987, 91, 171–195. [Google Scholar] [CrossRef]
- Fish, F.E.; Bostic, S.A.; Nicastro, A.J.; Beneski, J.T. Death roll of the alligator: Mechanics of twist feeding in water. J. Exp. Biol. 2007, 210, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Measey, G.J.; Herrel, A. Rotational feeding in caecilians: Putting a spin on the evolution of cranial design. Biol. Lett. 2006, 2, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Helfman, G.S.; Clark, J.B. Rotational feeding: Overcoming gape-limited foraging in anguillid eels. Copeia 1986, 3, 679–685. [Google Scholar] [CrossRef]
- Miller, T.J. Feeding behavior of Echidna nebulosa, Enchelycore pardalis, and Gymnomuraena zebra (Teleostei: Muraenidae). Copeia 1989, 3, 662–672. [Google Scholar] [CrossRef]
- Jude, D.J.; Reider, R.H.; Smith, G.R. Establishment of Gobiidae in the Great Lakes basin. Can. J. Fish. Aquat. Sci. 1992, 49, 416–421. [Google Scholar] [CrossRef]
- Kornis, M.S.; Mercado-Silva, N.; Vander Zanden, M.J. Twenty years of invasion: A review of round goby Neogobius melanostomus biology, spread and ecological implications. J. Fish Biol. 2012, 80, 235–285. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Lashbrook, G. Two New Fish Aliens in the Great Lakes; Michigan Natural Resources: Lansing, MI, USA, 1993; pp. 17–19.
- Wilson, K.A.; Howell, E.T.; Jackson, D.A. Replacement of zebra mussels by quagga mussels in the Canadian nearshore of Lake Ontario: The importance of substrate, round goby abundance, and upwelling frequency. J. Great Lakes Res. 2006, 32, 11–28. [Google Scholar] [CrossRef]
- Ghedotti, M.J.; Smihula, J.C.; Smith, G.R. Zebra mussel predation by round gobies in the laboratory. J. Great Lakes Res. 1995, 21, 665–669. [Google Scholar] [CrossRef]
- French, J.R., III; Black, M.G. Maximum length and age of round gobies (Apollonia melanostomus) in Lake Huron. J. Freshw. Ecol. 2009, 24, 173–175. [Google Scholar] [CrossRef]
- Charlebois, P.M.; Marsden, J.E.; Goettel, R.G.; Wolfe, R.K.; Jude, D.J. The Round Goby, Neogobius melanostomus (Pallas): A Review of European and North American Literature; Illinois Natural History Survey Special Publication: Champaign, IL, USA, 1997. [Google Scholar]
- Ray, W.J.; Corkum, L.D. Predation of zebra mussels by round gobies, Neogobius melanostomus. Environ. Biol. Fishes 1997, 50, 267–273. [Google Scholar] [CrossRef]
- Schrandt, M.N.; Stone, L.C.; Klimek, B.; Mäkelin, S.; Heck, K.L.; Mattila, J.; Herlevi, H. A laboratory study of potential effects of the invasive round goby on nearshore fauna of the Baltic Sea. Aquat. Invasions 2016, 11, 327–335. [Google Scholar] [CrossRef]
- Andraso, G.M.; Ganger, M.T.; Adamczyk, J. Size-selective predation by round gobies (Neogobius melanostomus) on dreissenid mussels in the field. J. Great Lakes Res. 2011, 37, 298–304. [Google Scholar] [CrossRef]
- Kipp, R.; Hébert, I.; Lacharité, M.; Ricciardi, A. Impacts of predation by the Eurasian round goby (Neogobius melanostomus) on mollusks in the upper St. Lawrence River. J. Great Lakes Res. 2012, 38, 78–89. [Google Scholar] [CrossRef]
- Djuricich, P.; Janssen, J. Impact of round goby predation on zebra mussel size distribution at Calumet Harbor, Lake Michigan. J. Great Lakes Res. 2001, 27, 312–318. [Google Scholar] [CrossRef]
- Morano, J.L. Ecomorphology of Round Goby, Neogobius melanostomus, Predation on Zebra Mussels, Dreissena polymorpha. Masters’s Thesis, Loyola University, Chicago, IL, USA, 2007. [Google Scholar]
- Houghton, C.J.; Janssen, J. Variation in predator-prey interactions between round gobies and dreissenid mussels. In Quagga and Zebra Mussels Biology, Impacts, and Control, 2nd ed.; Nalepa, T., Schlosser, D., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 359–367. [Google Scholar]
- Polačik, M.; Jurajda, P.; Blažek, R.; Janáč, M. Carcass feeding as a cryptic foraging mode in round goby, Neogobius melanostomus. J. Fish Biol. 2015, 87, 194–199. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angradi, T.R. A Field Observation of Rotational Feeding by Neogobius melanostomus. Fishes 2018, 3, 5. https://doi.org/10.3390/fishes3010005
Angradi TR. A Field Observation of Rotational Feeding by Neogobius melanostomus. Fishes. 2018; 3(1):5. https://doi.org/10.3390/fishes3010005
Chicago/Turabian StyleAngradi, Ted R. 2018. "A Field Observation of Rotational Feeding by Neogobius melanostomus" Fishes 3, no. 1: 5. https://doi.org/10.3390/fishes3010005



