1. Introduction
Worldwide, estuarine areas and their associated salt marsh habitats are described as highly productive and valuable aquatic ecosystems [
1]. Due to high levels of primary production, large reserves of organic matter and habitat diversity, these areas are considered biochemical hotspots that offer optimal conditions for numerous birds and aquatic species [
2]. These systems provide potential advantages for the growth and survival of young fish, namely high prey availability and refuge from predators [
1] and, consequently, support the offshore stocks of economically valuable species [
3,
4]. The Guadiana estuary is no exception to this general observation since it provides an exceptionally suitable environment for fish spawning, breeding, feeding, and growth not only for estuarine but also economically important marine species, such as sardine
Sardina pilchardus, seabream
Diplodus sp., and sole
Solea spp. [
5,
6]. In particular, the sardine is a small pelagic fish of great socio-economic importance for Portugal. Fluctuations in their productivity may cause serious issues for fisheries management and policies in the region [
7]. Since winter is the main spawning season of sardine in the study area [
8], sampling campaigns for this preliminary study were carried out between January and February. Tidal shifts in fish assemblages have been reported by several authors [
9,
10,
11] and, therefore, fish were sampled in both tidal stages.
Over the years, estuarine and coastal areas became increasingly affected by anthropogenic activities such as urbanization, industrialization, and tourism [
12]. Also, these ecosystems are facing impacts of climatic changes [
13]. Such anthropogenic and natural pressures exert a great influence on several environmental factors including temperature, salinity, and macronutrients concentration which in turn will disturb fish. They respond to environmental changes by changing their numbers and/or distributional range, in particular, larvae and juvenile fish which are highly susceptible to environmental fluctuations [
14].
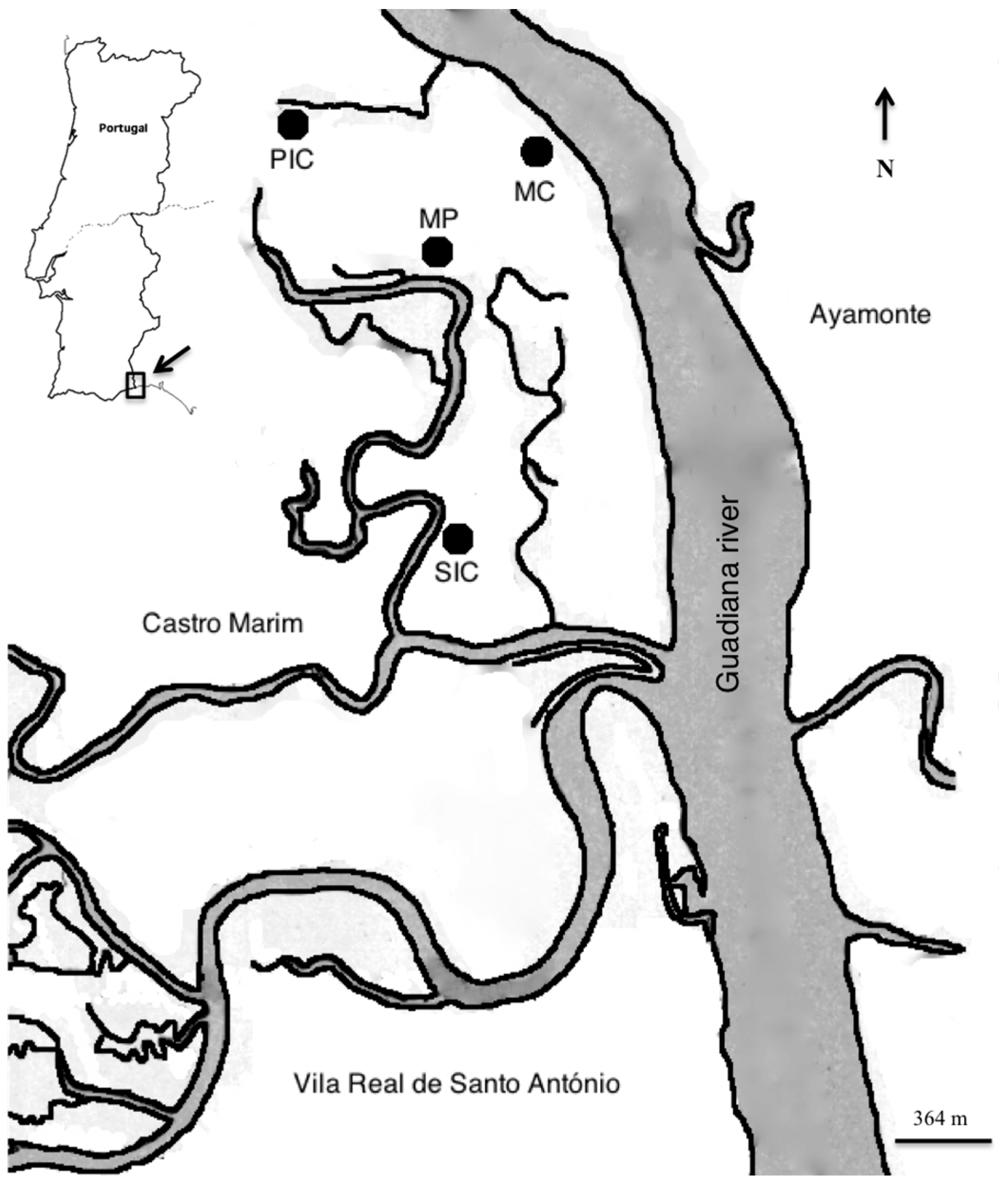
The completion of the Alqueva dam located 150 km from the Guadiana River mouth promoted a reduction in freshwater inflow leading to the degradation of salt marsh vegetation in the lower Guadiana estuary [
15]. Moreover, climate change scenarios predict an increase in temperature, length, and frequency of dry periods for this region [
13]. Predicted lower precipitation will potentiate retention of river waters and sediments by upstream dams and consequently the degradation of water quality and lack of sediment for plant accretion on salt marsh areas of the lower Guadiana estuary.
The resulting degradation observed in salt marsh habitats per se are a threat to associated fish species, but also increase the risk of invasion by exotic opportunistic species [
16]. Invasions are also facilitated by altered environmental conditions. Environmental changes encourage the establishment of non-indigenous species (NIS) which may become potential competitors for native species [
17]. In the Guadiana estuary, a community shift has already been documented, including plankton and fish [
15,
18,
19]. In addition, the first occurrences of several marine invasive species have also been recorded, with potentially detrimental effects on native biota [
13]. In 2008, the invasive
Blackfordia virginica (cnidarian) and
Palaemon macrodactylus (caridean shrimp) were first observed within the Guadiana estuary [
17]. For both species, there is the potential competition for space and resources with native fish such as
Sardina pilchardus,
Engraulis encrasicolus,
Pomatoschistus sp.,
Solea sp.,
Diplodus sp.,
Syngnathus sp. Although the sources of introduction for
B. virginica and
P. macrodactylus to the Guadiana Estuary remain unknown, previous research has shown that reduction of variability of river flow in estuaries has facilitated the establishment of NIS species [
20].
Mummichog is a native species and a dominant faunal component of salt marshes in the Atlantic coast of the United States and Canada [
21]. It is described as an opportunistic species due to its high tolerance to extreme and highly variable environmental conditions, namely high salinity and a wide range of temperature [
22] which makes mummichog a potential candidate as an invasive species. It has reportedly been introduced to Hawaii, and from there to the Philippines, in the early 20th century [
23]. Later, in the 1970s, mummichog arrived in the southwestern Iberian Peninsula apparently due to cross-contamination of ballast water of ships coming from the United States [
24]. Occurrences of the species remain poorly documented in the Guadiana [
5,
6]. There are doubts as to the outcomes of introductions but for now there are no records of native species disappearance where mummichog has been introduced [
25].
The objectives of this work are: (a) to characterize winter fish communities in salt marsh areas of Guadiana estuary and their occurrence variability according to tide regime; (b) to investigate the effect of environmental factors on fish species distribution; and (c) to discuss the potential risks of habitat dominance by mummichog, an NIS species.
3. Discussion
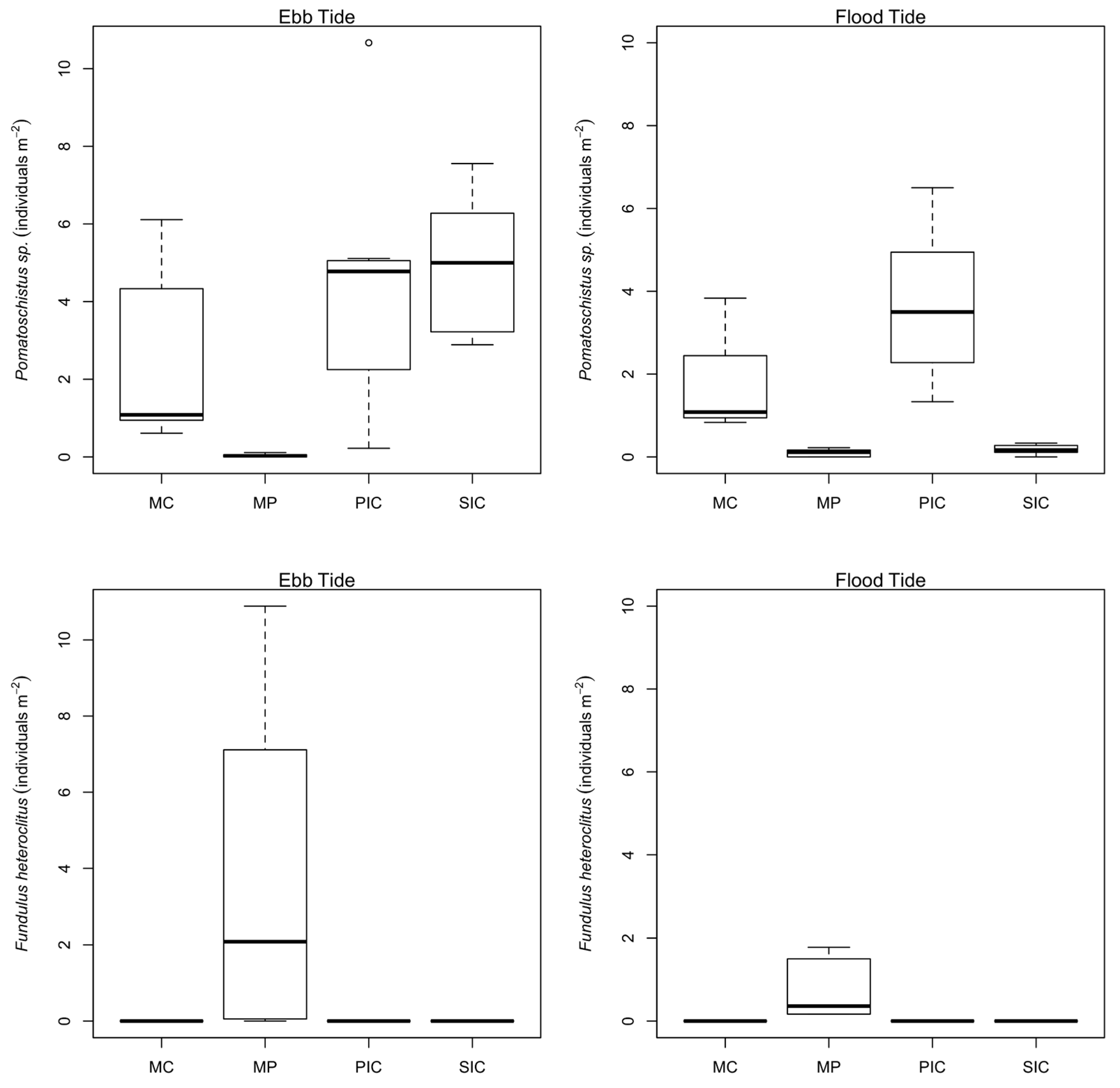
For the first time, the occurrence of mummichog in such high densities is reported in South Portugal and their potential risk for native species discussed. The present study was performed during winter months and thus, fish density and diversity registered herein were relatively low, as expected for this season and region [
5,
6] and in other salt marsh areas of Portugal [
11], North Europe [
26,
27] and United States [
28,
29]. All the seven fish genera captured had been previously observed in the study area [
5,
6] although mummichog in very low densities [
5]. Goby and mummichog, two estuarine residents, the first being native and the latter NIS, were the two dominant species. Overall, the degree of habitat overlap between these two species was very low in both tidal stages, as shown by the low values of Pianka’s index. This indicates that the two species occupy different habitats with the NIS mummichog being exclusively present in an isolated habitat (MP). Gobies are among the most abundant genera all over European salt marshes [
6,
27,
30], and are particularly successful in temperate estuarine environments [
31]. There are records of mummichog in southwest Spain [
22]; however, occurrences of mummichog in Portugal are poorly documented. Veiga recorded their presence between 2001 and 2002 in Guadiana salt marsh subtidal creeks but as low as one individual in the total catch [
5].
In the salt marshes, marine species were mainly caught at larval stages, while estuarine residents occurred at both larval and juvenile stages. These results indicate that marine species use salt marshes mainly as a nursery area while estuarine species depend on salt marshes during their entire life cycle [
32].
Tides affected goby density in MC, PIC and SIC habitats in a similar way, with higher densities registered during ebb. The authors of [
33] developed a hydrodynamic model for Guadiana estuary. Results indicate water surface velocities of 5.3 cms
−1 (SIC) and 12.9 cms
−1 (MC) during the ebb and 8.8 cms
−1 (SIC) and 15.8 cms
−1 (MC) during the flood. Lower velocities in the ebb may facilitate fish spread throughout the sampled salt marsh habitats especially for the youngest. In fact, changes in fish density according to the tidal stages occur due to the movement of fish from permanently inundated areas towards the inundated intertidal areas with the flood tide [
11]. Differences in fish assemblages may also be influenced by marsh plain availability in the proximity to each sampling site. However, this does not seem to be the case if marsh plain area and vegetation type is very similar among habitats. It is worth mentioning that the presence of estuarine fish larvae in all the sampled sites indicate an active habitat selection capacity from an early life stage. Our data do not support the same assumption for marine species due to the small number of marine larvae caught. Nevertheless, previous studies performed in some of the fish genera caught during this investigation reported larvae swimming speeds (
Table 5) in the range of simulated current velocities by [
33]. It should be noted that although the model developed by [
33] was calibrated with data recorded after the construction of the Alqueva dam, it is not guaranteed that similar velocities occurred during samplings. Results show that despite inter- and subtidal salt marsh creeks not being continuously available habitats, they play a major role in both estuarine and marine species, and for both larvae and juvenile fish.
MP was the most dissimilar habitat, both in terms of fish composition and environmental parameters, presenting the highest temperatures and salinities and the lowest macronutrients concentration, namely nitrates and phosphates. Extreme abiotic conditions registered were caused by the lack of water renewal since the MP is only partially flushed during high tides. Mummichog inhabits a wide range of salinities but prefers the most saline sites, usually above 25 [
22,
25,
37]. They present a great euryhaline range, covering 0 to 128 [
38]. Altogether, with their wide thermic acclimation range, this species is able to colonize new habitats with great success [
25]. MP was also the habitat with the lowest concentration of macronutrients, in particular phosphates and nitrates. According to [
39], phosphate and nitrate concentrations registered at MP indicate a low impacted area, in comparison to the other three habitats (SIC, PIC, MC) that presented typical values of moderate to high eutrophic sites. Nutrient enrichment is known to stimulate primary production causing a bottom-up enrichment of the food web, fostering increased fish biomass and body size [
39]. Particularly, nitrogen and phosphorus enrichment stimulates benthic algae [
40], which in turn stimulates infauna and epibenthic invertebrates [
41]. Benthic algae, infauna and epibenthic invertebrates all serve as food resources for most estuarine dependent fish species [
39]. However, a nutrient over-enrichment can have deleterious consequences, namely a decrease in dissolved oxygen leading to a reduction in fish growth rates [
39]. Fish such as gobies, that make use of eutrophic environments, are not likely to stay long enough to experience the negative effect of hypoxia on their growth. As observed by [
42], they invade the salt marshes through tidal creeks, forage there for up to a few hours and swim back at the ebb. Such habitats are available for a limited time dependent on tides. Instead, for short periods, they colonize salt marsh creeks and main channel edge and benefit from the high availability of food which in turn are probably influenced by the high concentration of macronutrients [
41]. Accounting for the lower concentration of macronutrients observed in MP, also a lower stimulation of primary production and consequently less food availability may be expected for this habitat which might thus explain the lower occurrence of gobies.
Gobies are described as opportunistic carnivores feeding on prey according to its availability. Most important prey items in their diet are polychaetes, mysids, isopods and decapods [
31]. Mummichog is also an opportunistic species but omnivore. Their diet is mainly based on amphipods, isopods, and snails [
39]. There is some overlap on feeding preferences of both species but mummichog is highly flexible, easily adapting to a more herbivory diet (plant tissues) in case of animal prey reduction [
39].
Mummichog growth is quite fast, being able to reproduce within the first year of life. Their eggs are resistant to desiccation, their development is fast and thus, the post-hatched larvae start with great advantages due to their size [
37]. Such characteristics provide this fish species an opportunistic life-history strategy effectively adapting to habitats with extreme environmental conditions as observed in MP. Although goby is described as a widespread species, relatively tolerant to fluctuations in environmental conditions [
43], the establishment of mummichog in MP suggests that this habitat was not attractive to goby as the remain three sampled habitats [
22]. In fact, the majority of the studies observed that mummichog is occupying extreme habitats (empty niches) not previously used by native fish species [
25,
37,
44] as it seems to be the current scenario in Guadiana salt marshes. This accounts for the low degree of habitat overlap (Pianka’s index) between gobies and mummichog. However, a species with such an expansion capacity, along with its productivity, must have a great influence on the local fish populations.
At least 35 NIS fish species have been introduced into the Iberian Peninsula in the last century and, although not all of them prospered, most are now widespread in this area especially linked to degraded environments [
45]. Extensive urban development has occurred in the Guadiana River basin over the past century: the consequent reduction in river flow contributed to decreases in water quality [
46]. The presence of mummichog does not necessarily imply that a successful invasion has occurred. We did, however, find specimens over a wide range of sizes (1.6–52.0 mm) and development stages that imply local reproduction. As such, mummichog must be classified as an NIS species in Guadiana salt marsh area, i.e., a species introduced outside its natural distribution that might survive and subsequently reproduce. Not all NIS species turn into invasive species, defined as species with the potential to cause native species extinction, modify ecosystem processes and act as disease vectors [
47]. However, some species out of their natural habitats lose their natural predators or control agents. As a result, they are able to increase to levels which are potentially detrimental to the native environment [
47].
Mummichog establishment was recorded in an isolated and low attractive area for native species due to extreme environmental conditions registered. However, similar to what is happening in areas relatively close in South Spain, Guadiana Estuary salt marsh habitats are facing the threat of an expansion of this NIS species to close areas of great value for native species. More sampling effort at reference and impact sites in the study area will be needed to verify the potential effects of mummichog on native communities. Various measures are being taken to improve management of water bodies in the Guadiana river basin under the Water Framework Directive in conjunction with the new European Marine Strategy Framework Directive. The new strategic plan which came into force in 2016 and will be in action until 2021 provides for several measures that will potentially mitigate the risk of mummichog expansion, among them: (a) reduce or eliminate discharges of pollutants; (b) define and implement ecological flows; and (c) increase monitoring and supervising plans [
48]. The authors recommend that future monitoring studies should be carried out in the study area to evaluate the effectiveness of the new measures implemented in control not only of this but other NIS species.