1. Introduction
Drug dissolution testing is essential in medicinal product development and quality control as it is a predictor of in vivo drug behaviour and, thus, ultimately therapeutic results. Dissolution testing was first applied for immediate release solid oral dosage forms and later broadened to controlled/modified release solid oral pharmaceuticals [
1]. However, over the span of years the usage of dissolution testing has extended to a range of dosage forms for instance, suspensions, orally disintegrating tablets, chewable tablets, chewing gums, transdermal patches, semisolid topical preparations, suppositories, implants, and injectable microparticulate formulations and liposomes [
1,
2]. As these formulations have become more prevalent, revised testing methods have also emerged [
1,
2]. For immediate release oral solid drug products, drug release studies are commonly referred to as ‘dissolution’ tests, as the intent of these formulations is to lead to rapid drug dissolution in the target medium. In the case of topical dosage forms, the test is preferably called a ‘drug release’ or ‘in vitro release’ test [
3]. The overall concepts of drug dissolution tests for solid oral dosage forms should also be applicable to several in vitro dissolution/release tests for ‘novel’ or ‘special’ dosage forms [
3,
4,
5,
6]. However, as the above mentioned ‘novel’ or ‘special’ dosage forms reveal important variations in formulation design, which ultimately result in diverse physicochemical and release characteristics, it is challenging to develop a single test system which can be applied to study the drug release profile of all special dosage forms [
3,
4]. Various apparatus, processes, and methods are applied on a case-by-case basis, and the adopted method may be explicit to the dosage form category, the formulation type, or even to a particular individual product [
4,
5].
Table 1 shows the official dissolution methods for different dosage forms. The United States Pharmacopeia (USP) includes several apparatuses for transdermal systems (TDSs) such as Apparatus 5 (paddle over disc), Apparatus 6 (cylinder), and Apparatus 7 (reciprocating holder). However, there is no stated method for determining the dissolution/release of natural-product-based formulations like honey. The capture of the in vitro release profile of multiple compounds from dosage forms incorporating complex natural products, like honey, is particularly challenging as a suitable, convenient analytical method is required to account for often small concentrations of different compounds in the release medium.
As listed in
Table 1, the Franz diffusion cell, which is the official testing apparatus for semisolid dosage forms, might offer a suitable setup for the release study of active constituents from natural-product-based topical semisolid formulations. However, there are some limitations, most notably the relatively large volume of the receptor chamber (
Figure 1), which necessitates relatively large quantities of samples to be tested in order to detect various bioactive compounds that are released from the sample’s complex phytochemical constituent profile. In the case of honey, for example, non-sugar constituents, which are associated with many of its bioactivities, for example its antioxidant effects [
7], only comprise about 3% of the total weight [
8,
9,
10]. Nonetheless, it is the release of these minor constituents that is of interest and, hence, requires adequate monitoring in release studies on honey and formulations incorporating honey as its active pharmaceutical ingredient (API). Furthermore, the diffusion area created by the dialysis membrane used in the Franz cell is relatively small and, therefore, application of an adequate amount of the semisolid products is challenging. In particular, the introduction of air bubbles while spreading a sticky sample like honey onto the membrane needs to be avoided as they might lead to inconsistent and inaccurate results. This is true for both the commercial and also the customised Franz cells. However, because the surface area of the commercial cell is small, any error associated with the presence of air bubbles is magnified compared to the customised cell, where the area of sample application is larger. A larger membrane surface area, as presented in the customised Franz-type cell developed as part of this study, assists with easier sample application.
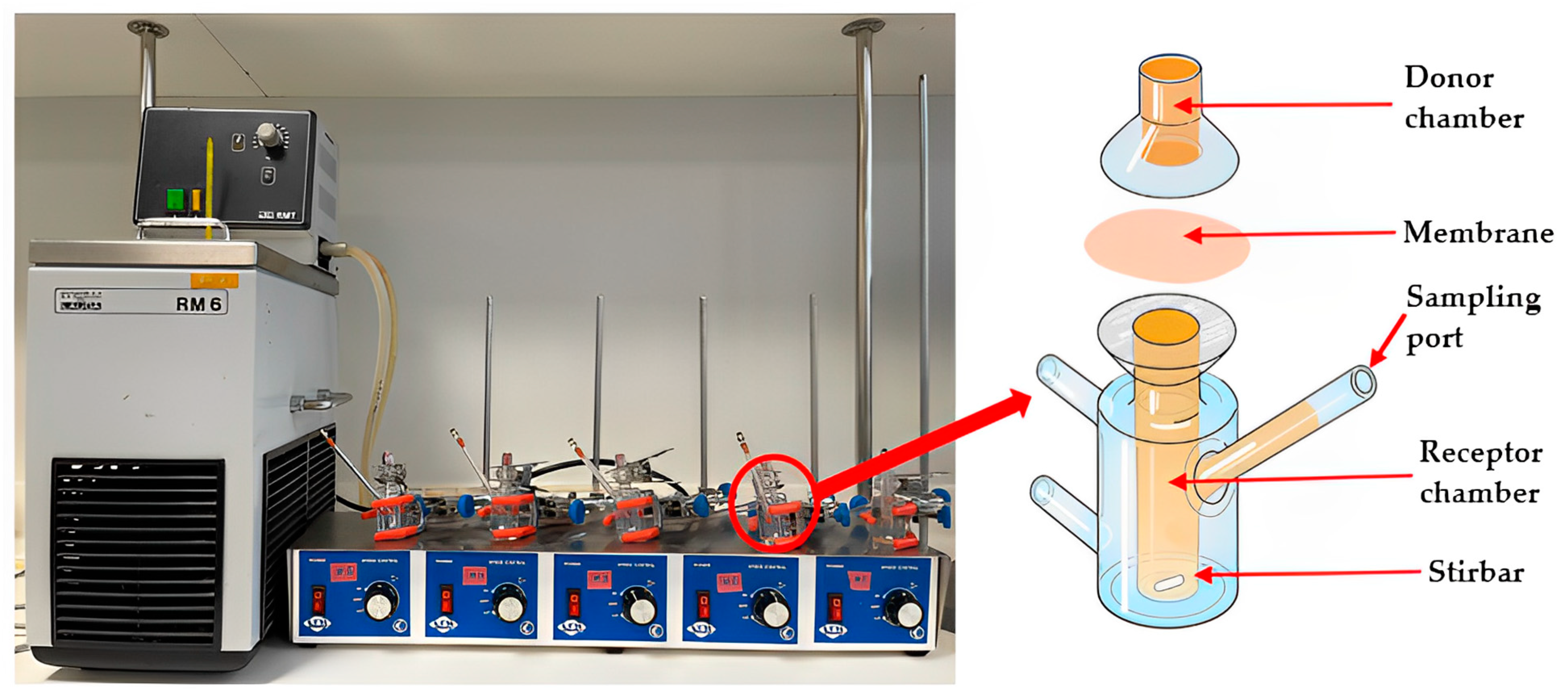
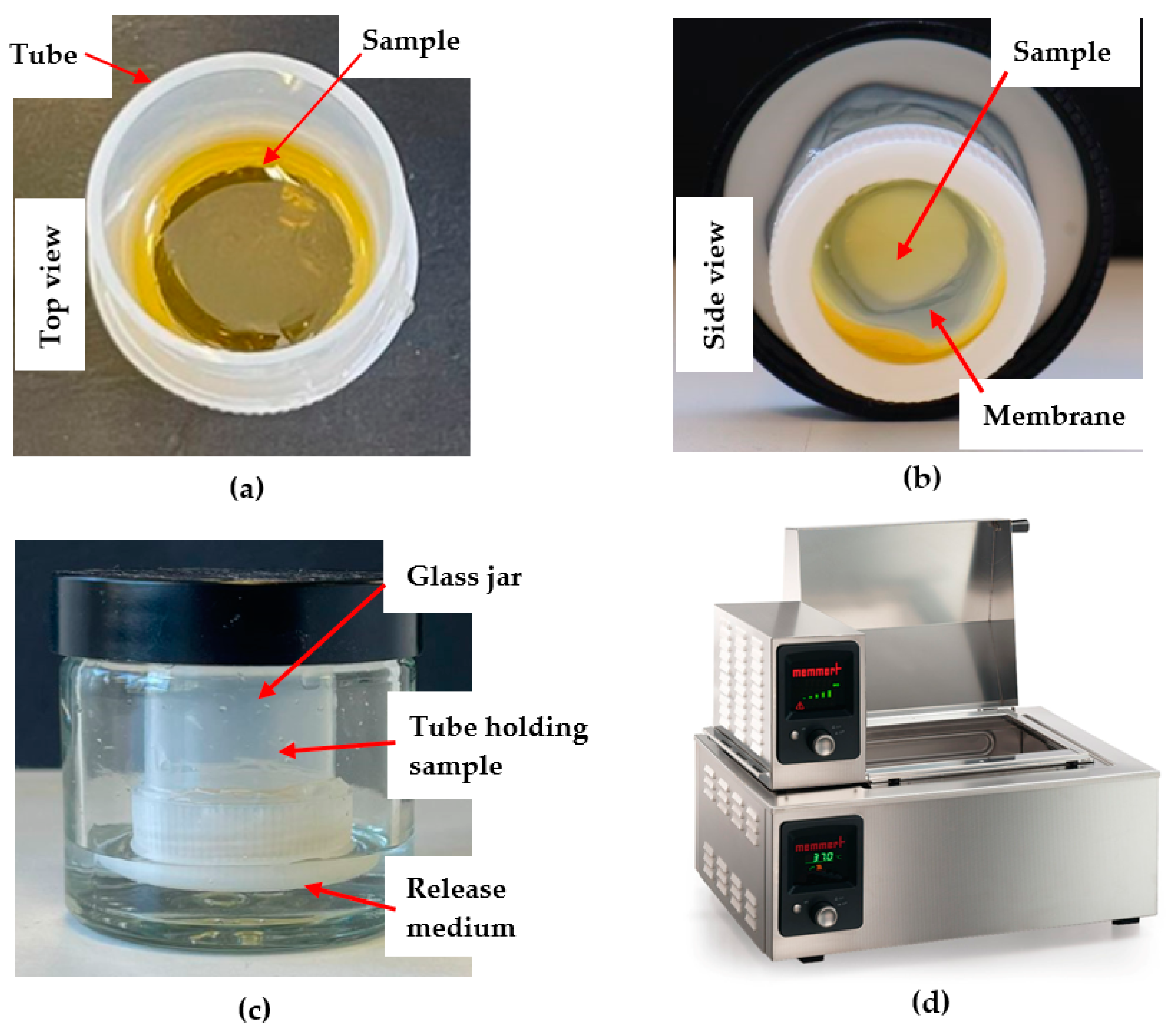
The custom-designed Franz-type diffusion cell (
Figure 2) adopts essential components of the Franz cell as it also features a donor and a receptor compartment separated by a dialysis membrane, which mimics a natural barrier such as a mucous membrane. In vivo motion simulated in the Franz cell apparatus by a magnetic stirrer is incorporated in the customised Franz-type cell setup by placing the glass jars holding the sample tubes in a shaking water bath. Like the Franz cell, the customised Franz-type cell setup also allows to mimic body temperature as the water bath temperature is set to 37 °C. An advantage of the customised Franz-type cell setup is the ability to test a large number of samples simultaneously. In this study, fifteen samples were run simultaneously, but the size of the shaking water bath could accommodate up to twenty samples, whereas the Franz cell only allows the running of five samples in parallel. Another difference, already stated earlier, is the ability to use a smaller receptor compartment in the customised Franz-type cell setup, which translates into smaller volumes of receptor fluid and, thus, higher concentrations of APIs for analysis, which is of particular importance when analysing formulations incorporating complex natural products, in this study honey and honey-based formulations, which might contain multiple bioactive compounds present at low concentrations. The customised Franz-type cell setup can also be customised in terms of glass jar size, which will impact on the volume of the receptor compartment as well as the diffusion surface area, and, thus, allows for adaption to specific analysis requirements. The essential features of Franz cell and customised Franz-type cell setup are presented in
Table 2.
An additional consideration is the type of instrumentation used to detect and quantify compounds released from complex natural-product-based samples that might contain a multitude of APIs into the receptor compartment.
High-Performance Thin-Layer Chromatography (HPTLC) is a widely employed semiautomated technique for the chromatographic analysis of pharmaceuticals, natural products, clinical samples, and foodstuffs. It is an enhanced and sophisticated form of thin-layer chromatography consisting of semiautomated sample application, development, visualisation, and data analysis. The usage of HPTLC plates and the capability to regulate and automate critical steps (e.g., sample application, development, and derivatisation) along with entirely automated image analysis allows for qualitative and also quantitative analyses [
11,
12]. Moreover, HPTLC analyses generate a range of datasets, such as images taken under different light conditions, R
F and RGB values of individual bands, and their peak height and peak area. If a TLC Scanner is included in the instrumental setup, UV-Vis and fluorescence spectra of individual bands can also be generated. With this, HPTLC analysis is an ideal approach to monitoring the simultaneous release of multiple constituents, even if their chemical identity is not yet established, as is demonstrated in this study for pure honey and honey-based formulations.
2. Materials and Methods
2.1. Chemicals and Reagents
4,5,7-Trihydroxyflavanone was obtained from Alfa Aesar, England, UK; Anhydrous sodium sulphate and dichloromethane were purchased from Merck KGaA, Darmstadt, Germany. Spectra/Por® Dialysis Membrane (molecular weight cut-off (MWCO): 3500 Da) was sourced from Repligen, Waltham, MA, USA. Methanol, toluene, ethyl acetate, and formic acid were obtained from Ajax Finechem Pvt Ltd., Sydney, NSW, Australia. NaCl and KCl were sourced from ChemSupply Pty Ltd., Gillman, South Australia and Na2HPO4 and KH2PO4 were purchased from Ajax Finechem, New South Wales, Australia. Blu Tack© was obtained from Officeworks, Perth, Australia.
2.2. Honey and Honey-Based Formulations
Honey-based formulations were prepared according to a previously published protocol [
13]. The honeys used in this study were two Western Australian (WA) Manuka honeys (
Leptospermum sp.), a WA Coastal Peppermint (
Agonis flexuosa) and a WA Jarrah honey (
Eucalyptus marginata), and a New Zealand Manuka honey (
Leptospermum scoparium).
The botanical origin, supplier name, and year of the five honeys are shown in
Table 3. The identification of the nectar source was based on beekeeper information taking into account the availability of flowering nectar, the honeys’ organoleptic characteristics, and the location of the respective apiaries/hives. In brief, honey loaded gel solutions were prepared by incorporating pure honey into sodium alginate solution. Firstly, using a 100 mL volumetric flask, 2 g (or 3 g in the case of Coastal Peppermint honey preparations) of alginate were dissolved in 60 mL sterile water, followed by the addition of 70 g honey and sterile water to a final volume of 100 mL.
2.3. Commercial Franz Cell Diffusion Apparatus
The release study of honey and honey-based products was conducted in a commercial Franz diffusion cell (Scientific Equipment Manufacturers (S.E.M) (SA) Pty. Ltd., Magill, South Australia) following a methodology described by Hossain et al. [
14]. The dialysis membrane to be used in the experiment was cut into pieces of 4.5 cm
2 resulting, once fitted into the apparatus, in a diffusion area of 0.78 cm
2. The membrane pieces were incubated in phosphate buffered saline (PBS) for 15 min before being mounted between the donor and the receptor chambers of the five Franz cells, all of which were maintained at 37 °C using a temperature-controlled water bath. A total of 5 mL sonicated PBS were added to the receptor chambers and stirred constantly using a magnetic bar (RPM 500). After applying 200 mg of the test samples (pure honeys or the corresponding honey-loaded formulations) on the membrane surfaces, 300 μL samples were withdrawn from the receptor chamber at 15 min, 30 min, 1 h, 3 h, 6 h, and 12 h. After each sample withdrawal, the volume taken from the receptor chamber (300 µL) was replaced with the equal volume of new PBS buffer.
2.4. Customised Franz-Type Cell Setup on Shaking Water Bath
The newly developed customised Franz-type cell setup (
Figure 2) consisted of a 50 × 27 mm polycarbonate-based transparent tube with screw cap closure (Thermo Fisher Scientific, Waltham, MA, USA) as donor compartment, a glass jar (50 mL) with a plastic screw cap as the receptor compartment and a temperature controlled shaking water bath (Memmert, GmbH+Co.KG, Schwabach, Germany). The dialysis membrane (diffusion area 3.80 cm
2) was attached to the lower part of the plastic tube using the screw cap in which a circular opening of 22 mm in diameter had been cut (
Figure 2b). To allow for a direct comparison of release data obtained from this customised setup with data generated by using the commercial Franz cell, the same sample quantities and solution volumes were used. Thus, 200 mg of the pure honeys and their respective formulations were loaded directly onto the membrane in the donor tube (
Figure 2a). The donor compartment was then attached to the cap of the glass jar cap using a putty-like pressure-sensitive adhesive (Blu Tack©). The tube holding the sample was immersed into the glass jar filled with 5 mL of PBS buffer as the release medium (
Figure 2c). The container was placed in the shaking water bath (
Figure 2d) at a temperature of 37 °C and its shaking motion was set to Level 5 (150 strokes/min) (
Figure 2). A total of 300 µL of sample were collected at 15 min, 30 min, 1 h, 3 h, 6 h, and 12 h. After each sample withdrawal (300 µL), the volume taken from the receptor chamber was replaced with the same volume of fresh PBS buffer.
2.5. Preanalysis Sample Preparation
A solution of 4,5,7-trihydroxyflavanone in methanol was prepared (0.5 mg/mL) and used as a reference solution for HPTLC analysis. The respective baseline samples for all honeys and their corresponding formulations (t = 0 min) were prepared as follows: 200 mg pure honey/honey-loaded formulations were dissolved in 1 mL of deionised water followed by three extractions with 5 mL each of a mixture of dichloromethane and acetonitrile (50:50
v/
v) [
13]. The extraction efficiency for this approach was determined by spiking artificial honey (made from 1.5 g sucrose, 7.5 g maltose, 40.5 g fructose, and 33.5 g glucose in 17 mL of sterile distilled water) [
14] with 0.3 mg of 4,5,7-trihydroxyflavanone and was found to be 99.55%. After the addition of MgCl
2 anhydrous (approximately 500 mg) to the combined organic extracts and filtration, the solvent was evaporated to dryness using compressed air. The dried extracts were stored at 4 °C. Prior to HPTLC analysis, they were reconstituted in 100 µL methanol. Sample aliquots (300 µL) collected at different time points from the release medium of both release apparatus were extracted in the same way as described above.
2.6. HPTLC Analysis of Released Honey Constituents
A total of 4 µL of the reference solution and 7 µL of each sample were applied onto silica gel 60 F
254 HPTLC glass plates using the semiautomated sample application device (Linomat 5; CAMAG, Muttenz, Switzerland). The chromatographic separation was performed in an automated development chamber (ADC2, CAMAG) using a mixture of toluene: ethyl acetate: formic acid, 1:6:1 (
v/
v) as the mobile phase. The obtained chromatographic results were documented using an HPTLC imaging device (TLC Visualizer, CAMAG) at the wavelengths 254 nm and 366 nm, respectively, followed by automated digital processing and analysis of the obtained chromatographic images using a specialized HPTLC software (visionCATS, CAMAG) [
13].
2.7. Statistical Analysis
All tests were accomplished in triplicate, and the results were analysed using a one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (TukeyHSD) test. The level of significance was set at 0.05 and a p-value of less than 0.05 was reasoned statistically significant. All statistical analyses were accomplished using Microsoft Office 365, GraphPad Prism 9.4.1 (GraphPad Software, San Diego, CA, USA).
4. Discussion
The in vitro drug release profile is considered an important indicator of in vivo product behaviour and, hence, therapeutic action [
15,
16]. For all dosage forms, product quality and performance may be measured through numerous in vivo and/or in vitro experiments [
17,
18]. Because of this, it is greatly desired to measure the release of APIs from pharmaceutical formulations, including semisolid dosage forms [
19]. Because of the cost, time, labour, and need for human subjects/animals associated with in vivo pharmacokinetic studies, the collation of in vitro drug release data is a popular surrogate measure for in vivo product performance. The choice of an appropriate release testing method depends on the type of formulation. For example, according to the USP, oral solid dosage forms can be analysed through a dissolution apparatus fitted either with a basket or a paddle stirrer as well as through a reciprocating cylinder or flow-through cell apparatus.
Honey is a super saturated sugary natural substance produced by bees mainly from the nectar of flowers [
8,
9]. Honey is composed of sugars (approximately 80%), water (about 17%), and ‘other’ constituents (approximately 3%) [
8,
9,
10]. These minor components are believed to be significant in affecting not only the organoleptic characteristics of honeys but also their bioactivity profiles. To date, more than 400 compounds have been reported in honey [
13]. When honey is used in its pure form or as a therapeutic agent in a formulation, it is desirable to quantify the release of each of the numerous phytochemicals present in the honey, even though often their chemical identity is not yet known. In this light, the release studies of honey and also honey-loaded formulations are more challenging compared to formulations which contain only a single or a few well defined APIs.
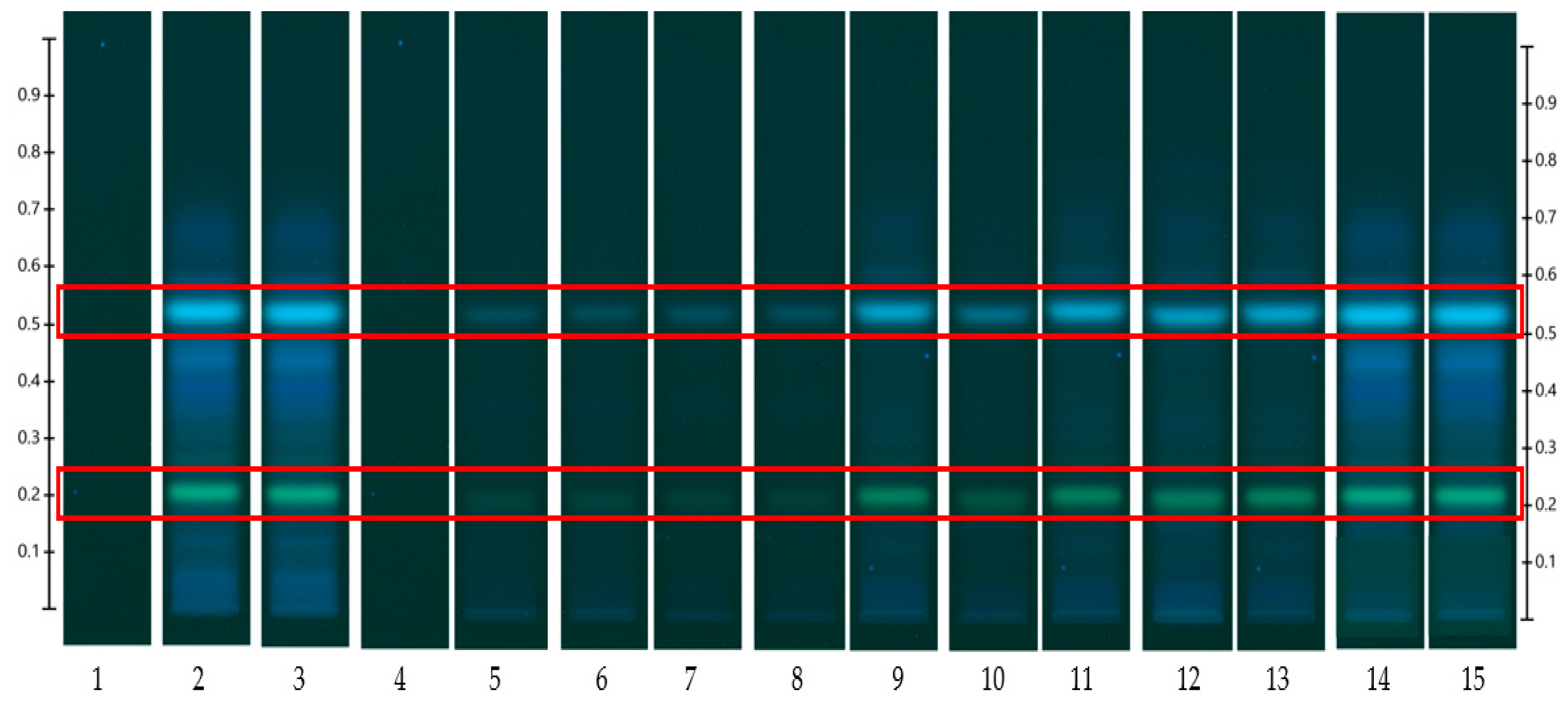
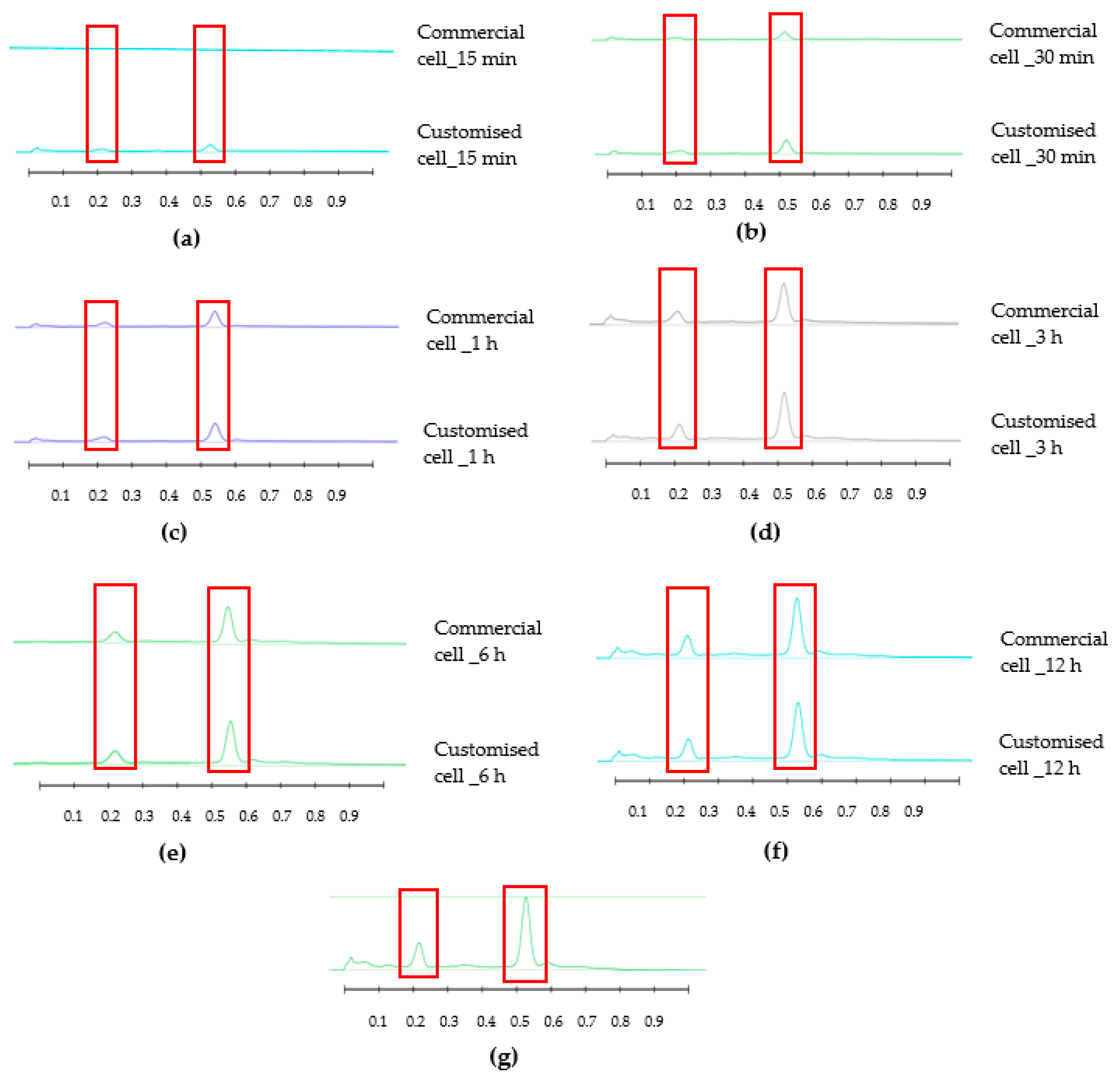
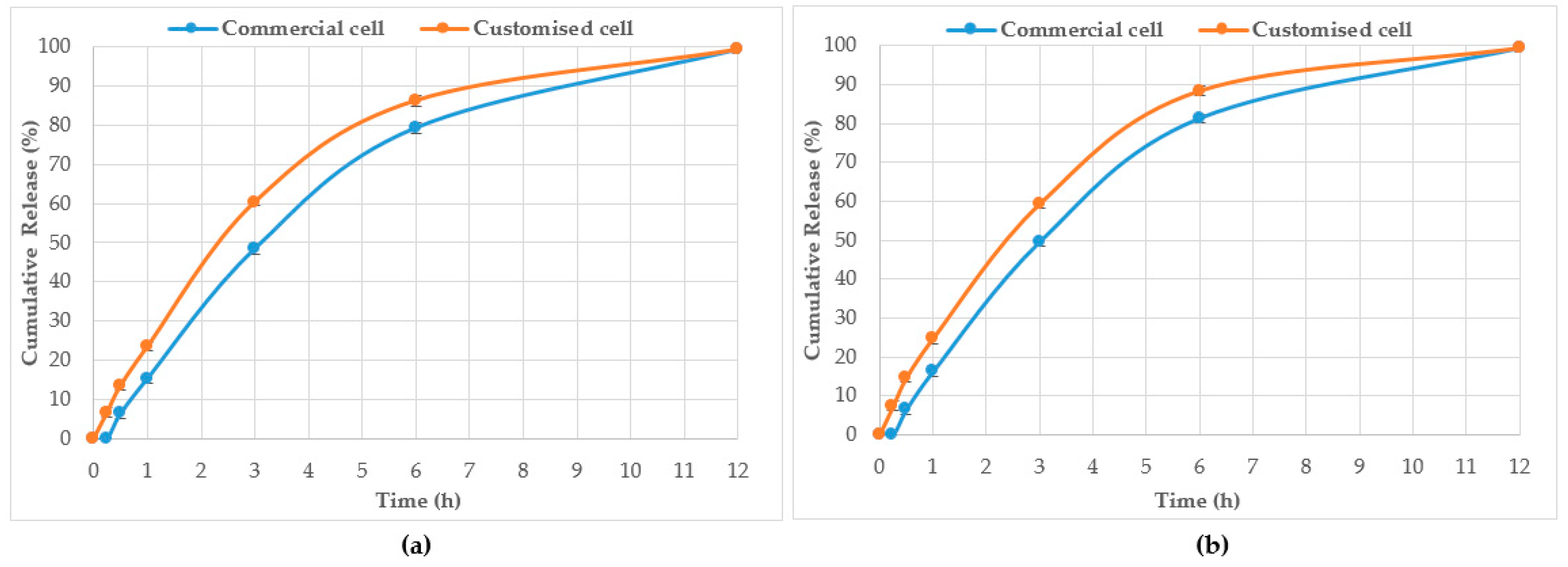
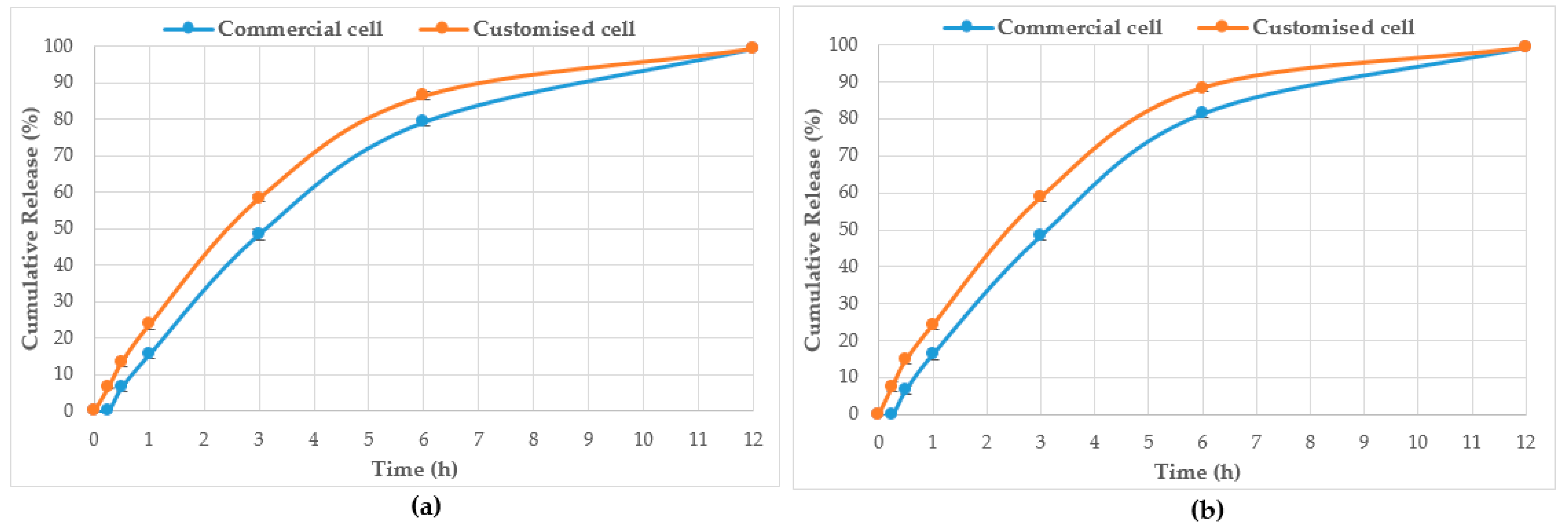
HPTLC is an ideal analytical approach to monitor the individual constituents from a complex natural mixture like honey as it allows us to visualise the constituents even if they are not yet chemically identified. In this study, using the commercial Franz cell to determine the release of constituents from pure honey and the honey gel solution, it took 30 min for any bands of compounds to be detectable in the sample collected from the receptor compartment (
Figure 3,
Figure 4,
Figure 5 and
Figure 6). On the other hand, using the customised Franz-type cell setup developed as part of this study, bands of compounds of interest could be detected as early as 15 min in the case of pure Jarrah honey as well as its gel formulations (
Figure 3,
Figure 4,
Figure 5 and
Figure 6). A similar trend can be seen when the % release of a particular compound (R
F 0.21) from pure Jarrah honey is considered, with 48.4% detectable at 3 h in the Franz cell system but 60.3% in the customised Franz-type cell setup. The prolongation of incubation time tended to minimize the difference in the release rate, with the % release of that compound reaching more than 99% at 12 h using both methods. This illustrates that the customised Franz-type cell setup allows for a faster release of constituents compared to the Franz cell. This might be due to its larger surface area for release. When the % release was normalised against the surface area available for release (
Table 5), it was noticed that, except for the first time point of sample collection (15 min), the percentage release of the monitored honey constituents per unit area of the dialysis membrane was higher at all time points in the commercial Franz cell compared to the customised Franz cell setup. This might be due to the faster stirring rate of magnetic stirring in the commercial cell compared to the motion created in the shaker bath employed for the customised cell, and also, possibly, due to the higher concentration gradient across the release area in the commercial Franz cell given its smaller surface area (0.78 cm
2 vs. 3.80 cm
2). Similar trends were seen when comparing the other investigated honeys and their respective pre-gel formulations (see
Supplementary Files). These findings suggest that the customised Franz-type cell coupled with HPTLC analysis is effective in monitoring the release pattern of honey constituents and might also be useful for release studies of other formulations incorporating complex natural products as APIs.
The newly developed setup offers several advantages over the traditional Franz cell system. As mentioned earlier, the Franz diffusion cell allows us to analyse five samples in a single run, whereas the customised Franz-type cell setup permits running 20 samples simultaneously and can be customised to specific analysis requirements. Moreover, it offers a larger surface area for diffusion which makes sample application, assuming the same level of competency when using both apparatuses, much easier and assists in avoiding air bubble formation, which otherwise might interfere with the diffusion leading to inaccurate results [
20,
21,
22,
23,
24,
25]. An additional benefit is related to accessibility because any lab could devise the customised Franz cell whereas not every lab has access to the commercial Franz cell setup. Moreover, while this study was conducted using a complex natural product like honey, it can be assumed that the monitoring of the release of constituents from the modified setup, in particular when coupled with HPTLC analysis, might also be applicable to other natural products incorporating multiple constituents, even at a low concentration.