Three-Dimensional Radiographic Evaluation of the Malar Bone Engagement Available for Ideal Zygomatic Implant Placement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phase 1
2.2. Phase 2
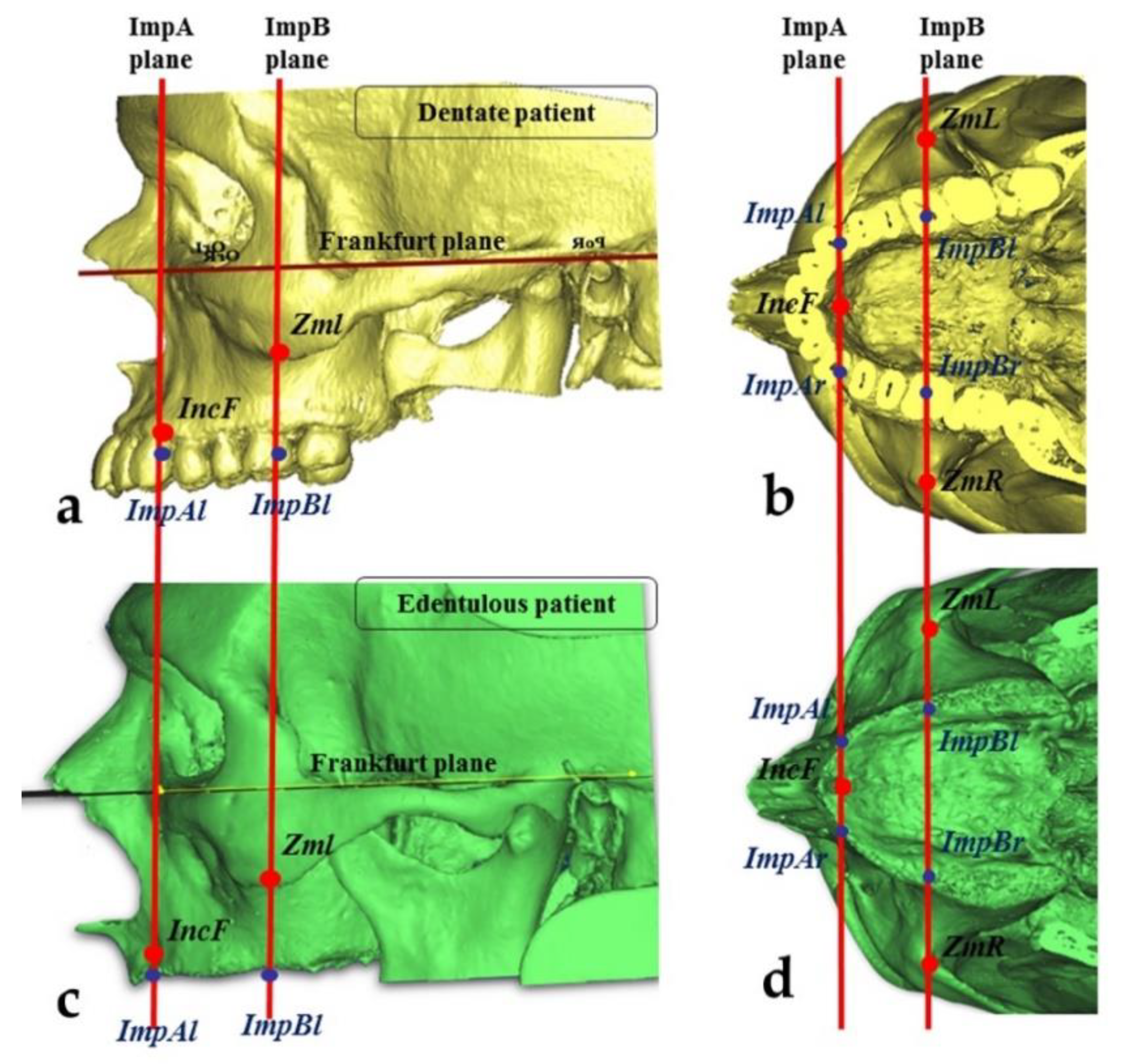
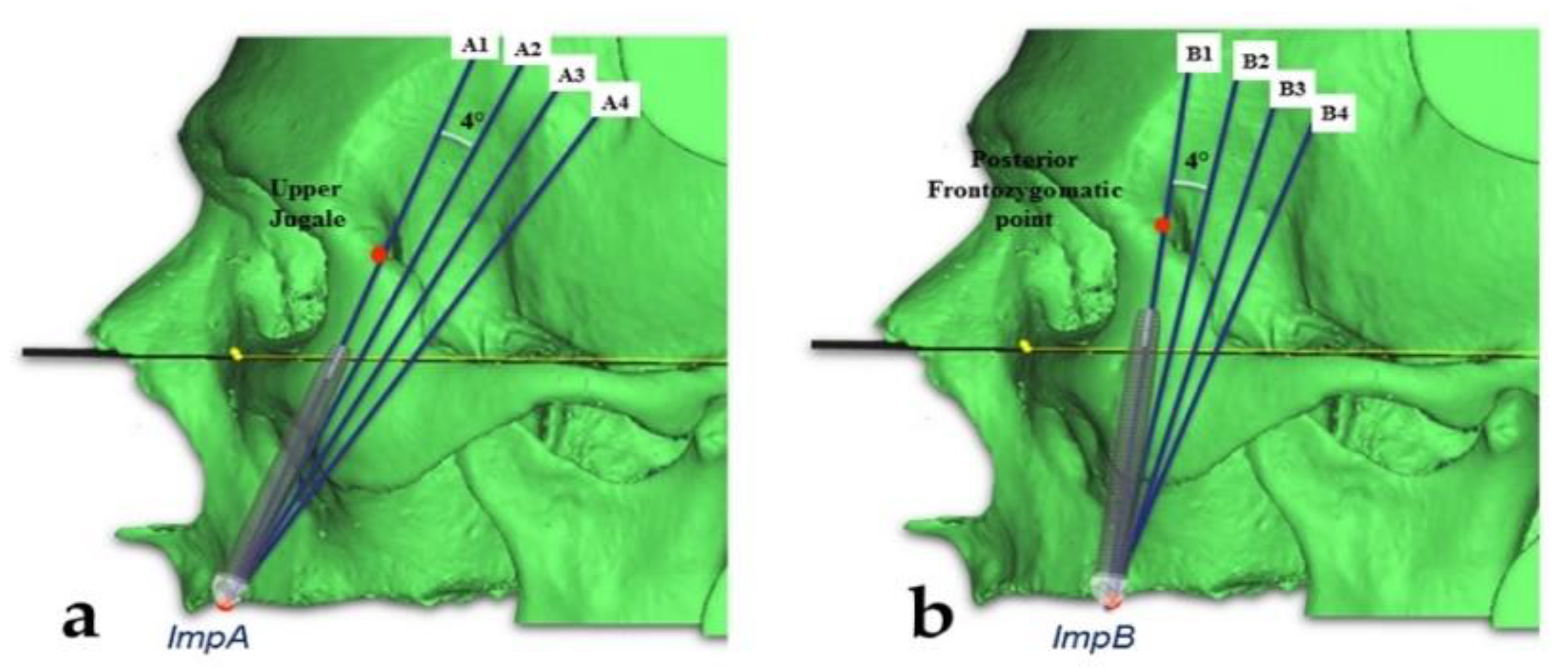
2.3. Measurements of the Zygomatic Bone
- The “upper jugale”: the highest point of the posterior frontal process eminence of the zygomatic bone
- The posterior point of the frontozygomatic suture.Two planes were drawn using the “CAD Objects” tool:
- -
- The “zygomatic anterior” plane, showing the ideal emergence points of prosthetic anterior implants; these were ImpAr/ImpAl and the upper jugale.
- -
- The “zygomatic posterior” plane, showing the ideal emergence points of prosthetic posterior implants; these were ImpBr/ImpBl and the posterior point of the frontozygomatic suture.
3. Results
3.1. Phase 1
3.2. Phase 2
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lombardi, T.; Stacchi, C.; Berton, F.; Traini, T.; Torelli, L.; Di Lenarda, R. Influence of Maxillary Sinus Width on New Bone Formation After Transcrestal Sinus Floor Elevation. Implant. Dent. 2017, 26, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S.; Froum, S.J. Effect of Maxillary Sinus Augmentation on the Survival of Endosseous Dental Implants. A Systematic Review. Ann. Periodontol. 2003, 8, 328–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrino, G.; Lizio, G.; Corinaldesi, G.; Marchetti, C. Titanium Mesh Technique in Rehabilitation of Totally Edentulous Atrophic Maxillae: A Retrospective Case Series. J. Periodontol. 2016, 87, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Pedrosa, A.R.; Custódio, A.L.N. Zygomatic implants: A critical review of the surgical techniques. Oral Maxillofac. Surg. 2012, 17, 1–9. [Google Scholar] [CrossRef]
- Fernandez, H.; Gómez-Delgado, A.; Trujillo-Saldarriaga, S.; Varón-Cardona, D.; Castro-Núñez, J. Zygomatic Implants for the Management of the Severely Atrophied Maxilla: A Retrospective Analysis of 244 Implants. J. Oral Maxillofac. Surg. 2014, 72, 887–891. [Google Scholar] [CrossRef]
- Malevez, C.; Abarca, M.; Durdu, F.; Daelemans, P. Clinical outcome of 103 consecutive zygomatic implants: A 6-48 months follow-up study. Clin. Oral Implant. Res. 2004, 15, 18–22. [Google Scholar] [CrossRef]
- Brånemark, P.-I.; Gröndahl, K.; Ohrnell, L.-O.; Nilsson, P.; Petruson, B.; Svensson, B.; Engstrand, P.; Nannmark, U. Zygoma fixture in the management of advanced atrophy of the maxilla: Technique and long-term results. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2004, 38, 70–85. [Google Scholar] [CrossRef]
- Sartori, E.M.; Padovan, L.E.M.; Sartori, I.A.D.M.; Ribeiro, P.D.; Carvalho, A.C.G.D.S.; Goiato, M.C. Evaluation of Satisfaction of Patients Rehabilitated With Zygomatic Fixtures. J. Oral Maxillofac. Surg. 2012, 70, 314–319. [Google Scholar] [CrossRef]
- Bothur, S.; Garsten, M. Initial speech problems in patients treated with multiple zygomatic implants. Int. J. Oral Maxillofac. Implant. 2010, 25, 379–384. [Google Scholar]
- Aparicio, C.; Manresa, C.; Francisco, K.; Claros, P.; Alández, J.; Gonzalez-Martin, O.; Albrektsson, T. Zygomatic implants: Indications, techniques and outcomes, and the zygomatic success code. Periodontology 2000 2014, 66, 41–58. [Google Scholar] [CrossRef]
- Stella, J.P.; Warner, M.R. Sinus slot technique for simplification and improved orientation of zygomaticus dental implants: A technical note. Int. J. Oral Maxillofac. Implant. 2001, 15, 889–893. [Google Scholar]
- Maló, P.; De Nobre, M.; Lopes, A.; Francischone, C.; Rigolizzo, M. Three-year outcome of a retrospective cohort study on the rehabilitation of completely edentulous atrophic maxillae with immediately loaded extra-maxillary zygomatic implants. Eur. J. Oral Implant. 2012, 5, 37–46. [Google Scholar]
- Migliorança, R.M.; Coppedê, A.; Rezende, R.C.L.D.; De Mayo, T. Restoration of the edentulous maxilla using extrasinus zygomatic implants combined with anterior conventional implants: A retrospective study. Int. J. Oral Maxillofac. Implant. 2011, 26, 665–672. [Google Scholar]
- Aparicio, C.; Ouazzani, W.; Aparicio, A.; Fortes, V.; Muela, R.; Pascual, A.; Codesal, M.; Barluenga, N.; Manresa, C.; Franch, M. Extrasinus Zygomatic Implants: Three Year Experience from a New Surgical Approach for Patients with Pronounced Buccal Concavities in the Edentulous Maxilla. Clin. Implant. Dent. Relat. Res. 2010, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C. A proposed classification for zygomatic implant patient based on the zygoma anatomy guided approach (ZAGA): A cross-sectional survey. Eur. J. Oral Implant. 2011, 4, 269–275. [Google Scholar]
- Tallarico, M.; Esposito, M.; Xhanari, E.; Caneva, M.; Meloni, S.M. Computer-guided vs freehand placement of immediately loaded dental implants: 5-year postloading results of a randomised controlled trial. Eur. J. Oral Implant. 2018, 11, 203–213. [Google Scholar]
- Pellegrino, G.; Tarsitano, A.; Taraschi, V.; Vercellotti, T.; Marchetti, C. Simplifying Zygomatic Implant Site Preparation Using Ultrasonic Navigation: A Technical Note. Int. J. Oral Maxillofac. Implant. 2018, 33, e67–e71. [Google Scholar] [CrossRef] [Green Version]
- Van Steenberghe, D.; Malevez, C.; Van Cleynenbreugel, J.; Serhal, C.B.; Dhoore, E.; Schutyser, F.; Suetens, P.; Jacobs, R. Accuracy of drilling guides for transfer from three-dimensional CT-based planning to placement of zygoma implants in human cadavers. Clin. Oral Implant. Res. 2003, 14, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Bothur, S.; Jonsson, G.; Sandahl, L. Modified technique using multiple zygomatic implants in reconstruction of the atrophic maxilla: A technical note. Int. J. Oral Maxillofac. Implant. 2003, 18, 902–904. [Google Scholar]
- Hung, K.; Wang, F.; Wang, H.-W.; Zhou, W.; Huang, W.; Wu, Y. Accuracy of a real-time surgical navigation system for the placement of quad zygomatic implants in the severe atrophic maxilla: A pilot clinical study. Clin. Implant. Dent. Relat. Res. 2017, 19, 458–465. [Google Scholar] [CrossRef]
- Uchida, Y.; Goto, M.; Katsuki, T.; Akiyoshi, T. Measurement of the maxilla and zygoma as an aid in installing zygomatic implants. J. Oral Maxillofac. Surg. 2001, 59, 1193–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, K.-F.; Ai, Q.-Y.; Fan, S.-C.; Wang, F.; Huang, W.; Wu, Y.-Q. Measurement of the zygomatic region for the optimal placement of quad zygomatic implants. Clin. Implant. Dent. Relat. Res. 2017, 19, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Duarte, L.R.; Mendonça, R.; Fernandes, A.C.D.S. Anatomical Bases for the Insertion of Zygomatic Implants. Clin. Implant. Dent. Relat. Res. 2008, 10, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Triplett, R.G.; Schow, S.R.; Laskin, D.M. Oral and maxillofacial surgery advances in implant dentistry. Int. J. Oral Maxillofac. Implant. 2000, 15, 47–55. [Google Scholar]
- Xu, X.; Zhao, S.; Liu, H.; Sun, Z.; Wang, J.; Zhang, W. An Anatomical Study of Maxillary-Zygomatic Complex Using Three-Dimensional Computerized Tomography-Based Zygomatic Implantation. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Quílez, J.B.; Guijarro-Martínez, R.; Centenero, S.A.-H.; Hernández-Alfaro, F. Virtual quad zygoma implant placement using cone beam computed tomography: Sufficiency of malar bone volume, intraosseous implant length, and relationship to the sinus according to the degree of alveolar bone atrophy. Int. J. Oral Maxillofac. Surg. 2017, 47, 252–261. [Google Scholar] [CrossRef]
- Nkenke, E.; Hahn, M.; Lell, M.; Wiltfang, J.; Schultze-Mosgau, S.; Stech, B.; Radespiel-Troeger, M.; Neukam, F.W. Anatomic site evaluation of the zygomatic bone for dental implant placement. Clin. Oral Implant. Res. 2003, 14, 72–79. [Google Scholar] [CrossRef]
- Takamaru, N.; Nagai, H.; Ohe, G.; Tamatani, T.; Sumida, K.; Kitamura, S.; Miyamoto, Y. Measurement of the zygomatic bone and pilot hole technique for safer insertion of zygomaticus implants. Int. J. Oral Maxillofac. Surg. 2016, 45, 104–109. [Google Scholar] [CrossRef]
- Balshi, T.J.; Wolfinger, G.J.; Shuscavage, N.J.; Balshi, S.F. Zygomatic Bone-to-Implant Contact in 77 Patients With Partially or Completely Edentulous Maxillas. J. Oral Maxillofac. Surg. 2012, 70, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Corvello, P.C.; Montagner, A.; Batista, F.C.; Smidt, R.; Shinkai, R.S.A. Length of the drilling holes of zygomatic implants inserted with the standard technique or a revised method: A comparative study in dry skulls. J. Cranio Maxillofac. Surg. 2011, 39, 119–123. [Google Scholar] [CrossRef] [Green Version]
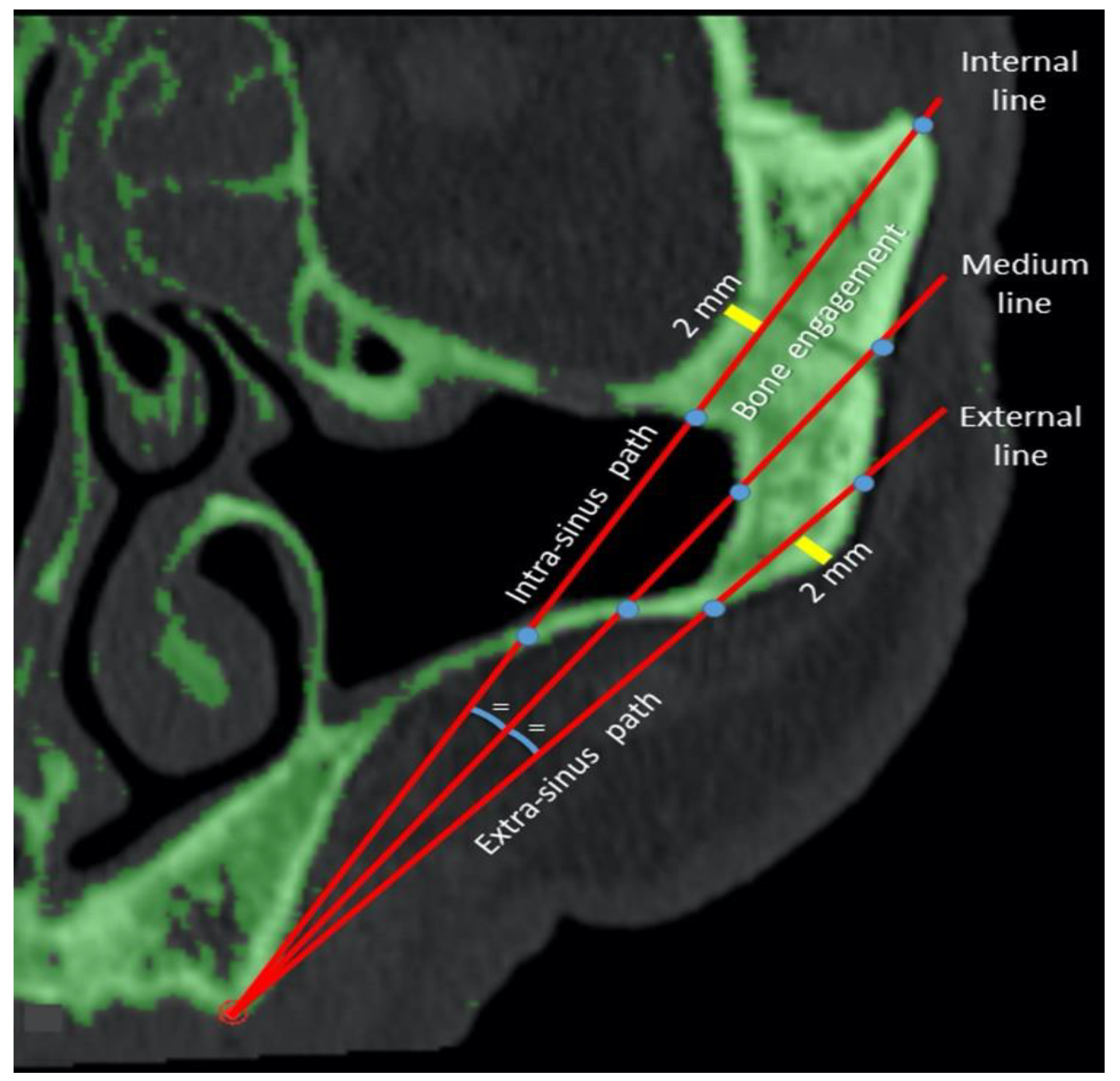
| Line | Mean (mm) | SD (mm) | |
|---|---|---|---|
| Total length | 1 | 61.82 a | 6.95 |
| 2 | 53.14 b | 6.61 | |
| 3 | 46.36 c | 7.01 | |
| Total | 53.77 | 9.33 | |
| Bone engagement | 1 | 20.03 a | 8.06 |
| 2 | 19.00 b | 6.30 | |
| 3 | 14.72 c | 3.35 | |
| Total | 17.92 | 6.62 | |
| Intra-sinus path | 1 | 13.28 a | 10.16 |
| 2 | 4.65 b | 6.66 | |
| 3 | 0.03 c | 0.46 | |
| Total | 5.99 | 8.91 | |
| Extra-sinus path | 1 | 28.51 a | 10.86 |
| 2 | 29.48 b | 7.86 | |
| 3 | 31.60 c | 6.29 | |
| Total | 29.86 | 8.64 |
| Plane (°) | Mean (mm) | SD (mm) | |
|---|---|---|---|
| Total length | 0 | 52.60a | 9.53 |
| 4 | 55.01b | 9.99 | |
| 8 | 54.27c | 9.07 | |
| 12 | 53.22d | 8.50 | |
| Total | 53.77 | 9.33 | |
| Bone engagement | 0 | 18.60 a | 6.18 |
| 4 | 19.43 b | 6.44 | |
| 8 | 17.55 c | 6.64 | |
| 12 | 16.09d | 6.74 | |
| Total | 17.92 | 6.62 | |
| Intra-sinus path | 0 | 6.32a | 9.50 |
| 4 | 7.69b | 9.96 | |
| 8 | 6.14a | 8.73 | |
| 12 | 3.80c | 6.65 | |
| Total | 5.99 | 8.91 | |
| Extra-sinus path | 0 | 27.66a | 9.10 |
| 4 | 27.89a | 9.16 | |
| 8 | 30.58b | 7.79 | |
| 12 | 33.32c | 7.11 | |
| Total | 29.86 | 8.64 |
| Implant Emergence | Plane (°) | Line | Bone Engagement (mm) | Intra-Sinus Path (mm) |
|---|---|---|---|---|
| Anterior | 0 | 1 | 24.26 ± 5.84 19.02 ± 4.93 14.41 ± 2.92 19.23 ± 6.20 | 7.45 ± 7.61 3.28 ± 5.10 0.03 ± 0.44 3.59 ± 6.10 |
| 2 | ||||
| 3 | ||||
| Total | ||||
| 4 | 1 | 24.09 ± 6.36 20.18 ± 5.67 16.02 ± 2.98 20.10 ± 6.16 | 11.64 ± 7.89 5.08 ± 6.08 0.07 ± 0.68 5.60 ± 7.45 | |
| 2 | ||||
| 3 | ||||
| Total | ||||
| 8 | 1 | 21.40 ± 6.91 20.84 ± 5.87 16.67 ± 2.61 19.63 ± 5.83 | 11.59 ± 7.62 4.52 ± 5.77 0.00 ± 0.00 5.37 ± 7.29 | |
| 2 | ||||
| 3 | ||||
| Total | ||||
| 12 | 1 | 20.00 ± 7.46 21.29 ± 5.26 16.45 ± 2.43 19.25 ± 5.81 | 10.05 ± 7.07 3.51 ± 5.16 0.03 ± 0.38 4.53 ± 6.54 | |
| 2 | ||||
| 3 | ||||
| Total | ||||
| Posterior | 0 | 1 | 21.31 ± 6.38 17.98 ± 6.33 14.65 ± 3.04 17.98 ± 6.10 | 19.94 ± 10.91 7.19 ± 8.01 0.03 ± 0.43 9.05 ± 11.35 |
| 2 | ||||
| 3 | ||||
| Total | ||||
| 4 | 1 | 23.08 ± 6.92 18.23 ± 6.58 14.90 ± 2.92 18.74 ± 6.66 | 21.08 ± 10.30 8.24 ± 8.45 0.04 ± 0.55 9.79 ± 11.58 | |
| 2 | ||||
| 3 | ||||
| Total | ||||
| 8 | 1 | 14.58 ± 7.88 18.40 ± 7.10 13.40 ± 3.31 15.46 ± 6.75 | 16.32 ± 10.27 4.32 ± 6.93 0.06 ± 0.61 6.90 ± 9.92 | |
| 2 | ||||
| 3 | ||||
| Total | ||||
| 12 | 1 | 11.48 ± 6.54 15.99 ± 6.78 11.29 ± 3.04 12.92 ± 6.10 | 8.16 ± 8.98 1.07 ± 3.86 0.00 ± 0.00 3.07 ± 6.70 | |
| 2 | ||||
| 3 | ||||
| Total |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, G.; Grande, F.; Ferri, A.; Pisi, P.; Gandolfi, M.G.; Marchetti, C. Three-Dimensional Radiographic Evaluation of the Malar Bone Engagement Available for Ideal Zygomatic Implant Placement. Methods Protoc. 2020, 3, 52. https://doi.org/10.3390/mps3030052
Pellegrino G, Grande F, Ferri A, Pisi P, Gandolfi MG, Marchetti C. Three-Dimensional Radiographic Evaluation of the Malar Bone Engagement Available for Ideal Zygomatic Implant Placement. Methods and Protocols. 2020; 3(3):52. https://doi.org/10.3390/mps3030052
Chicago/Turabian StylePellegrino, Gerardo, Francesco Grande, Agnese Ferri, Paolo Pisi, Maria Giovanna Gandolfi, and Claudio Marchetti. 2020. "Three-Dimensional Radiographic Evaluation of the Malar Bone Engagement Available for Ideal Zygomatic Implant Placement" Methods and Protocols 3, no. 3: 52. https://doi.org/10.3390/mps3030052







