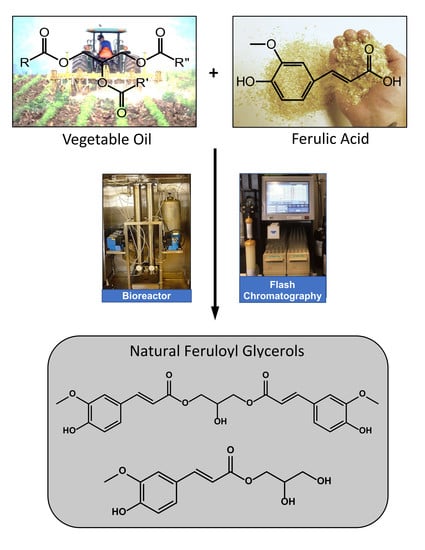
Enzymatic Synthesis and Flash Chromatography Separation of 1,3-Diferuloyl-sn-Glycerol and 1-Feruloyl-sn-Glycerol †
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
2.2. Equipment
- Model OPF-10 cooking oil filter (Chard, Two Rivers, WI, USA);
- Whatman54 filter paper (Fisher Scientific, Pittsburgh, PA, USA);
- CombiFlash Rf 200i flash chromatography system with UV detection (Teledyne ISCO, Inc. Lincoln, NE, USA);
- Shimadzu HPLC system, consisting of a LC-30AD pump, SIL-20AXR Prominence autosampler, CBM-20A communications bus module, and SPD-M20A Prominence photodiode array detector (Kyoto, Japan);
- Eppendorf CH-500 column heater (Hauppauge, NY, USA);
- Phenomenex Luna Phenyl–Hexyl column (5 µm, 250 × 4.6 mm) (Torrance, CA, USA);
- Bruker AVANCE 500 NMR spectrometer (500 MHz 1H/125.77 MHz 13C) using a 5 mm broadband inverse (BBI) probe (Billerica, MA, USA);
- Shimadzu UV-1280 UV–Vis spectrophotometer (Shimadzu Scientific Instruments, Inc., Addison, IL, USA);
- Rainin pipettes (Sigma-Aldrich, St. Louis, MO, USA);
- Q2000 Differential Scanning Calorimetry (TA instruments, New Castle, DE).
3. Procedure
3.1. Synthesis of Feruloylated Soy Glycerides
3.2. Flash Chromatography Purification of 1-Feruloyl-sn-Glycerol (FG) and 1,3-Diferuloyl-sn-Glycerol (F2G)
3.3. HPLC
3.4. LC–ESI–Mass Spectroscopy
3.5. NMR Spectroscopy
3.6. UV Spectroscopy
3.7. Differential Scanning Calorimetry
4. Results and Discussion
4.1. Synthesis of F2G and FG
4.2. Flash Chromatography Separation and Isolation of F2G and FG
4.3. Physicochemical Properties of F2G and FG
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Singh, V.; Nuñez, A.; Hicks, K.B. Phytosterols in the aleurone layer of corn kernels. Biochem. Soc. Trans. 2000, 28, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Chang, S.W.; Lee, K.R. Feruloyl sucrose derivatives from Bistorta manshuriensis. Can. J. Chem. 2010, 88, 519–523. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.K.; Kremling, K.A.; Ding, Y.; Bennett, J.S.; Bae, J.S.; Kim, D.K.; Ackerman, H.H.; Kolomiets, M.V.; Schmelz, E.A.; et al. Ethylene signaling regulates natural variation in the abundance of antifungal acetylated diferuloylsucroses and Fusarium graminearum resistance in maize seedling roots. New Phytol. 2019, 221, 2096–2111. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.; Kong, L. Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chem. 2012, 131, 1056–1062. [Google Scholar] [CrossRef]
- Graca, J.; Pereira, H. Suberin structure in potato periderm: Glycerol, long-chain monomers, and glyceryl and feruloyl dimers. J. Agric. Food Chem. 2000, 48, 5476–5483. [Google Scholar] [CrossRef]
- Cooper, R.; Gottlieb, H.; Lavie, D. New phenolic diglycerides from Aegilops ovata. Phytochemistry 1978, 17, 1673–1675. [Google Scholar] [CrossRef]
- Compton, D.L.; Goodell, J.R.; Evans, K.O.; Palmquist, D.E. Ultraviolet absorbing efficacy and photostability of feruloylated soybean oil. J. Am. Oil Chem. Soc. 2018, 95, 421–431. [Google Scholar] [CrossRef]
- Ruhland, C.T.; Day, T.A. Effects of ultraviolet-B radiation on leaf elongation, production and phenylpropanoid concentrations of Deschampsia antarctica and Colobanthus quitensis in Antarctica. Physiol. Plant. 2000, 109, 244–251. [Google Scholar] [CrossRef]
- Jiaa, Y.; Hea, Y.; Lua, F. The structure-antioxidant activity relationship of dehydrodiferulates. Food Chem. 2018, 269, 480–485. [Google Scholar] [CrossRef]
- Rozema, J.; Noordijk, A.J.; Broekman, R.A.; van Beem, A.; Meijkamp, B.M.; de Bakker, N.V.J.; van de Staaij, J.W.M.; Stroetenga, M.; Bohncke, S.J.P.; Konert, M.; et al. (Poly)phenolic compounds in pollen and spores of Antarctic plants as indicators of solar UV-B: A new proxy for the reconstruction of past solar UV-B. Plant Ecol. 2001, 154, 9–26. [Google Scholar] [CrossRef]
- Evans, K.O.; Compton, D.L.; Laszlo, J.A.; Appell, M. Feruloyl glycerol and 1,3-diferuloyl glycerol antioxidant behavior in phospholipid vesicles. Chem. Phys. Lipids 2016, 195, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dowd, P.F.; Berhow, M.A.; Johnson, E.T. Enhanced pest resistance and increased phenolic production in maize callus transgenically expressing a maize chalcone isomerase-3 like gene. Plant Gene 2018, 13, 50–55. [Google Scholar] [CrossRef]
- Batovska, D.I.; Kishimoto, T.; Bankova, V.S.; Kamenarska, Z.G.; Ubukata, M. Synthesis of some phenylpropanoid monoglycerides via the Mitsunobu protocol. Molecules 2005, 10, 552–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holser, R. Kinetics of Cinnamoyl Glycerol Formation. J. Am. Oil Chem. Soc. 2008, 85, 221–225. [Google Scholar] [CrossRef]
- Holser, R.; Mitchell, T.; Harry-O’kuru, R.; Vaughn, S.; Walter, E.; Himmelsbach, D. Preparation and characterization of 4-methoxy cinnamoyl glycerol. J. Am. Oil Chem. Soc. 2008, 85, 347–351. [Google Scholar] [CrossRef]
- Sun, S.; Chen, X.; Bi, Y.; Chen, J.; Yang, G.; Liu, W. Functionalized ionic liquid-catalyzed 1-feruloyl-sn-glycerol synthesis. J. Am. Oil Chem. Soc. 2014, 91, 759–765. [Google Scholar] [CrossRef]
- Sun, S.; Song, F.; Bi, Y.; Yang, G.; Liu, W. Solvent-free enzymatic transesterification of ethyl ferulate and monostearin: Optimized by response surface methodology. J. Biotechnol. 2012, 164, 340–345. [Google Scholar] [CrossRef]
- Sun, S.; Chen, X. Kinetics of enzymatic synthesis of monoferuloyl glycerol and diferuloyl glycerol by transesterification in [BMIM] PF6. Biochem. Eng. J. 2015, 97, 25–31. [Google Scholar] [CrossRef]
- Sun, S.; Shan, L.; Jin, Q.; Liu, Y.; Wang, X. Solvent-free synthesis of glyceryl ferulate using a commercial microbial lipase. Biotechnol. Lett. 2007, 29, 945–949. [Google Scholar] [CrossRef]
- Tsuchiyama, M.; Sakamoto, T.; Fujita, T.; Murata, S.; Kawasaki, H. Esterification of ferulic acid with polyols using a ferulic acid esterase from Aspergillus niger. Biochim. Biophys. Acta 2006, 1760, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Compton, D.L.; Goodell, J.R.; Grall, S.; Evans, K.O.; Cermak, S.C. Continuous, packed-bed, enzymatic bioreactor production and stability of feruloyl soy glycerides. Ind. Crop. Prod. 2015, 77, 787–794. [Google Scholar] [CrossRef]
- Compton, D.L.; Goodell, J.R.; Berhow, M.A.; Kenar, J.A.; Cermak, S.C.; Evans, K.O. Feruloylated products from coconut oil and shea butter. J. Am. Oil Chem. Soc. 2017, 94, 397–411. [Google Scholar] [CrossRef]
- Compton, D.L.; Laszlo, J.A. 1,3-Diferuloyl-sn-glycerol from the biocatalytic transesterification of ethyl 4-hydroxy-3-methoxy cinnamic acid (ethyl ferulate) and soybean oil. Biotechnol. Lett. 2009, 31, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Compton, D.L.; Evans, K.O.; Appell, M. Experimental and theoretical study of the influence of water on hydrolyzed product formation during the feruloylation of vegetable oil. J. Sci. Food Agric. 2017, 97, 3022–3029. [Google Scholar] [CrossRef]
- Compton, D.L.; Laszlo, J.A.; Berhow, M.A. Identification and quantification of feruloylated mono-, di-, and triacylglycerols from vegetable oils. J. Am. Oil Chem. Soc. 2006, 83, 753–758. [Google Scholar] [CrossRef]
- Ingredients.com, Oil, Cooking and Salad, ENOVA, 80% Diglycerides. Available online: http://ingredients.com/ingredients/oil-cooking-and-salad-enova-80-diglycerides (accessed on 22 July 2019).
- Laszlo, J.A.; Compton, D.L.; Eller, F.J.; Taylor, S.L.; Isbell, T.A. Packed-bed bioreactor synthesis of feruloylated monoacyl- and diacylglycerols: Clean production of a “green” sunscreen. Green Chem. 2003, 5, 382–386. [Google Scholar] [CrossRef]
- Compton, D.L.; Laszlo, J.A.; Evans, K.O. Antioxidant properties of feruloyl glycerol derivatives. Ind. Crop. Prod. 2012, 36, 217–221. [Google Scholar] [CrossRef]
- Laszlo, J.A.; Compton, D.L.; Vermillion, K.E. Acyl migration kinetics of vegetable oil 1,2-diacylglycerols. J. Am. Oil Chem. Soc. 2008, 85, 307–312. [Google Scholar] [CrossRef]
- Kodali, D.R.; Tercyak, A.; Fahey, D.A.; Small, D.M. Acyl migration in 1,2-dipalmitoyl-sn-glycerol. Chem. Phys. Lipids 1990, 52, 163–170. [Google Scholar] [CrossRef]
- Fureby, A.M.; Virto, C.; Adlercreutz, P.; Mattiasson, B. Acyl group migrations in 2-monoolein. Biocatal. Biotransform. 1996, 14, 89–111. [Google Scholar] [CrossRef]
- Lyubachevskaya, G.; Boyle-Roden, E. Kinetics of 2-monoacylglycerol acyl migration in model chylomicra. Lipids 2000, 35, 1353–1358. [Google Scholar] [CrossRef]
- Compton, D.L.; Vermillion, K.E.; Laszlo, J.A. Acyl migration kinetics of 2-monoacylglycerols from soybean oil via 1H NMR. J. Am. Oil Chem. Soc. 2007, 84, 343–348. [Google Scholar] [CrossRef]
- Boswinkel, G.; Derksen, J.; van’t Riet, K.; Cuperus, F. Kinetics of acyl migration in monoglycerides and dependence on acyl chainlength. J. Am. Oil Chem. Soc. 1996, 73, 707–711. [Google Scholar] [CrossRef]
- Mu, H.; Kurvinenb, J.-P.; Kalliob, H.; Xua, X.; Høya, C.-E. Quantitation of acyl migration during lipase-catalyzed acidolysis, and of the regioisomers of structure triacylglycerols formed. J. Am. Oil Chem. Soc. 2001, 78, 959–964. [Google Scholar] [CrossRef]
- Cervera, M.Á.R.; Venegas, E.V.; Bueno, R.R.; García, I.R.; Guil-Guerrero, J.L. Acyl migration evaluation in monoacylglycerols from Echium plantagineum seed oil and marinol. J. Biosci. Bioeng. 2013, 115, 518–522. [Google Scholar] [CrossRef]
- Li, W.; Du, W.; Li, Q.; Sun, T.; Liu, D. Study on acyl migration kinetics of partial glycerides: Dependence on temperature and water activity. J. Mol. Catal. B Enzym. 2010, 63, 17–22. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Warner, K.; Laszlo, J. Addition of ferulic acid, ethyl ferulate, and feruloylated monoacyl- and diacylglycerols to salad oils and frying oils. J. Am. Oil Chem. Soc. 2005, 82, 647–652. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Laszlo, J.A.; Evans, K.O.; Vermillion, K.E.; Appell, M. Feruloyl dioleoylglycerol antioxidant capacity in phospholipid vesicles. J. Agric. Food Chem. 2010, 58, 5842–5850. [Google Scholar] [CrossRef]
- Shaath, N.A. Evolution of Modern Sunscreen Chemicals. In Suncreens: Development, Evaluation, and Regulatory Aspects, 2nd ed.; Lowe, N.J., Shaath, N.A., Pathak, M.A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 3–34. [Google Scholar]
- USFDA. Sunscreen Drug Products for Over-The-Counter Human Use; Final Rules and Proposed Rules; U.S. Department of Health and Human Services-Food and Drug Administration, Ed.; U.S. Federal Register: Washington, DC, USA, 2011; Volume 76, pp. 35620–35665.
- Compton, D.L.; Evans, K.O.; Appell, M.; Goodell, J.R. Protection of antioxidants, vitamins E and C, from ultraviolet degradation using feruloylated vegetable oil. J. Am. Oil Chem. Soc. 2019, 96, 999–1009. [Google Scholar] [CrossRef]
- Agrapidis-Paloympis, L.E.; Nash, R.A.; Shaath, N.A. The effect of solvents on the ultraviolet absorbance of sunscreens. J. Soc. Cosmet. Chem. 1987, 38, 209–221. [Google Scholar]
- Vallejo, J.J.; Mesa, M.; Gallardo, C. Evaluation of the avobenzone photostability in solvents used in cosmetic formulations. Vitae 2011, 18, 63–71. [Google Scholar]





| Size | Column Volume | Flow Rate | Default Slope-Based Peak Width Detection | Max. Pressure | Sample Loading a |
|---|---|---|---|---|---|
| (g) | (mL) | (mL/min) | (min) | (psi | kPa) | (g) |
| 4 | 4.8 | 18 | 0.5 | 600 | 4137 | 0.020–0.40 |
| 12 | 16.8 | 30 | 1 | 400 | 2758 | 0.060–1.2 |
| 24 | 35.9 | 35 | 1 | 350 | 2413 | 0.12–2.4 |
| 120 | 192.0 | 85 | 4 | 225 | 1551 | 0.60–12.0 |
| 220 | 243.0 | 150 | 4 | 150 | 1034 | 1.10–22.0 |
| 330 | 443.0 | 200 | 4 | 150 | 1034 | 1.65–33.0 |
| Column Size a | Sample Load b | Product Yield c | Run Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Run | Load | Separation | Solute | Solvent | F2G | FG | Total | Flow Rate | Length d | Solvent |
| (#) | (g) | (g) | (g) | (mL) | (g | %) | (g | %) | (%) | (mL/min) | (CV | min) | (L) |
| 1 | 4 | 24 | 0.100 | 1.0 | - | - | - | 35 | 16 | 33 g | 1.2 |
| 2 e | 4 | 24 | 0.100 | 1.0 | 0.038 ± 0.0025 | 38 ± 2.5 | 0.0094 ± 0.0002 | 9.4 ± 0.2 | 47.4 ± 2.4 | 20 | 16 | 33 g | 0.7 |
| 3 | 12 | 120 | 1.000 | 5.0 | 0.383 | 38.3 | 0.088 | 8.8 | 47.1 | 85 | 10 | 41 h | 3.5 |
| 4 | 12 | 120 | 1.000 | 5.0 | 0.400 | 40.0 | 0.090 | 9.0 | 49.0 | 57 | 10 | 52 h | 3.0 |
| 5 f | 12 | 120 | 3.000 | 10.0 | 1.188 ± 0.052 | 39.6 ± 1.7 | 0.313 ± 0.038 | 10.4 ± 1.3 | 50.0 ± 2.5 | 57 | 10 | 52 h | 3.0 |
| 6 | 12 | 120 | 5.000 | 10.0 | - | - | - | 57 | 10 | 52 h | 3.0 |
| 7 | 12 | 120 | 5.000 | 15.0 | 1.730 | 34.6 | 0.433 | 8.7 | 43.3 | 57 | 15 | 74 i | 4.2 |
| 8 | 24 | 120 | 7.000 | 15.0 | 2.639 | 37.7 | 0.627 | 9.0 | 46.7 | 57 | 15 | 74 i | 4.2 |
| 9 | 24 | 120 | 10.00 | 22.0 | - | - | - | 57 | 15 | 74 i | 4.2 |
| 10 | 24 | 330 | 5.000 | 15.0 | 1.760 | 35.2 | 0.414 | 8.3 | 43.5 | 134 | 15 | 100 i | 13.4 |
| 11 | 24 | 330 | 7.000 | 17.0 | 2.615 | 37.4 | 0.602 | 8.6 | 46.0 | 134 | 15 | 100 i | 13.4 |
| 12 | 24 | 330 | 10.00 | 22.0 | 2.457 | 24.6 | 0.727 | 7.3 | 31.9 | 134 | 15 | 100 i | 13.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compton, D.L.; Appell, M.; Kenar, J.A.; Evans, K.O. Enzymatic Synthesis and Flash Chromatography Separation of 1,3-Diferuloyl-sn-Glycerol and 1-Feruloyl-sn-Glycerol. Methods Protoc. 2020, 3, 8. https://doi.org/10.3390/mps3010008
Compton DL, Appell M, Kenar JA, Evans KO. Enzymatic Synthesis and Flash Chromatography Separation of 1,3-Diferuloyl-sn-Glycerol and 1-Feruloyl-sn-Glycerol. Methods and Protocols. 2020; 3(1):8. https://doi.org/10.3390/mps3010008
Chicago/Turabian StyleCompton, David L., Michael Appell, James A. Kenar, and Kervin O. Evans. 2020. "Enzymatic Synthesis and Flash Chromatography Separation of 1,3-Diferuloyl-sn-Glycerol and 1-Feruloyl-sn-Glycerol" Methods and Protocols 3, no. 1: 8. https://doi.org/10.3390/mps3010008