Cholesterol-Enriched Hybrid Lipid Bilayer Formation on Inverse Phosphocholine Lipid-Functionalized Titanium Oxide Surfaces
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. DOCP SAM Formation
3.2. Chol-Containing Upper Leaflet Formation
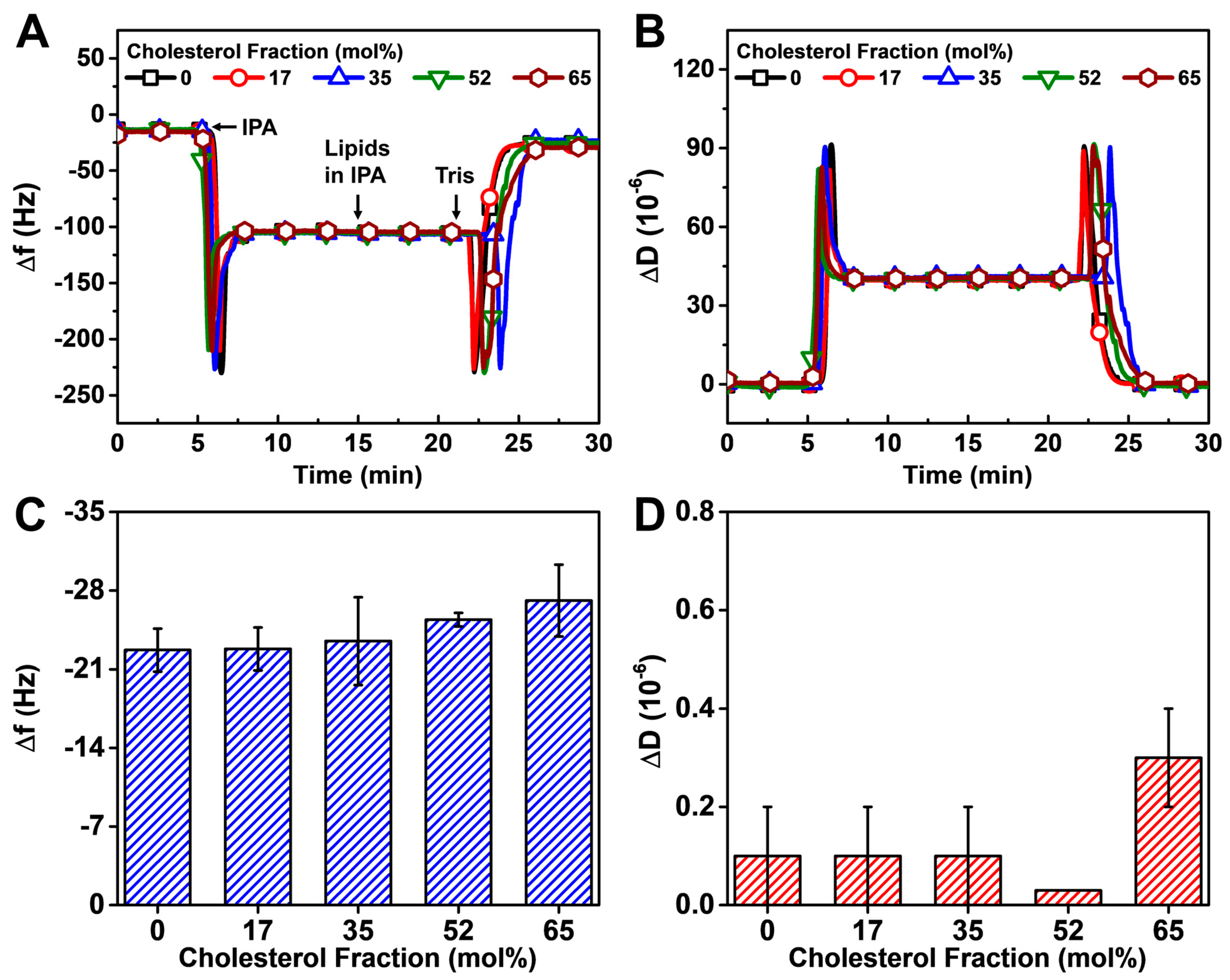
3.2.1. Solvent Exchange
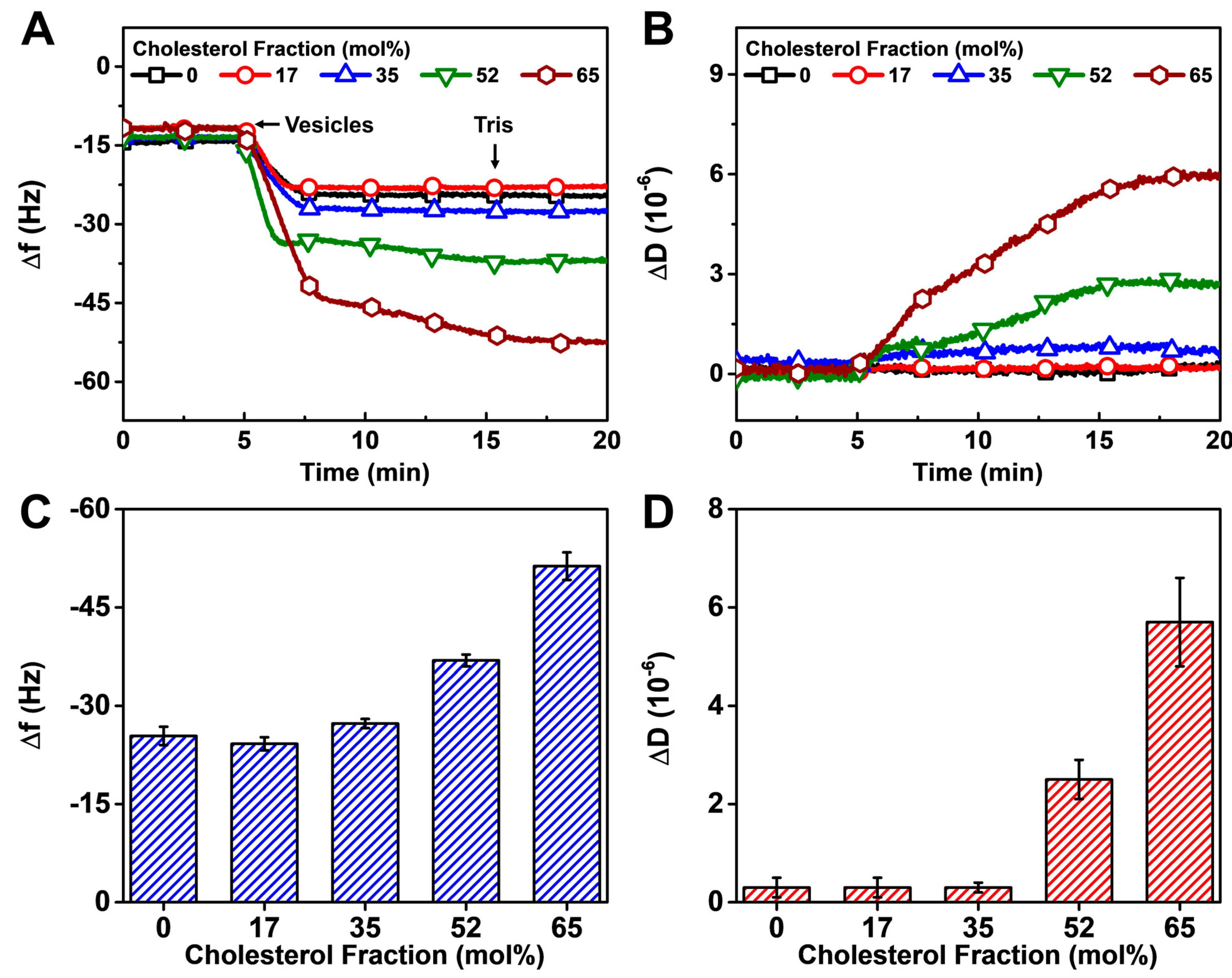
3.2.2. Vesicle Fusion
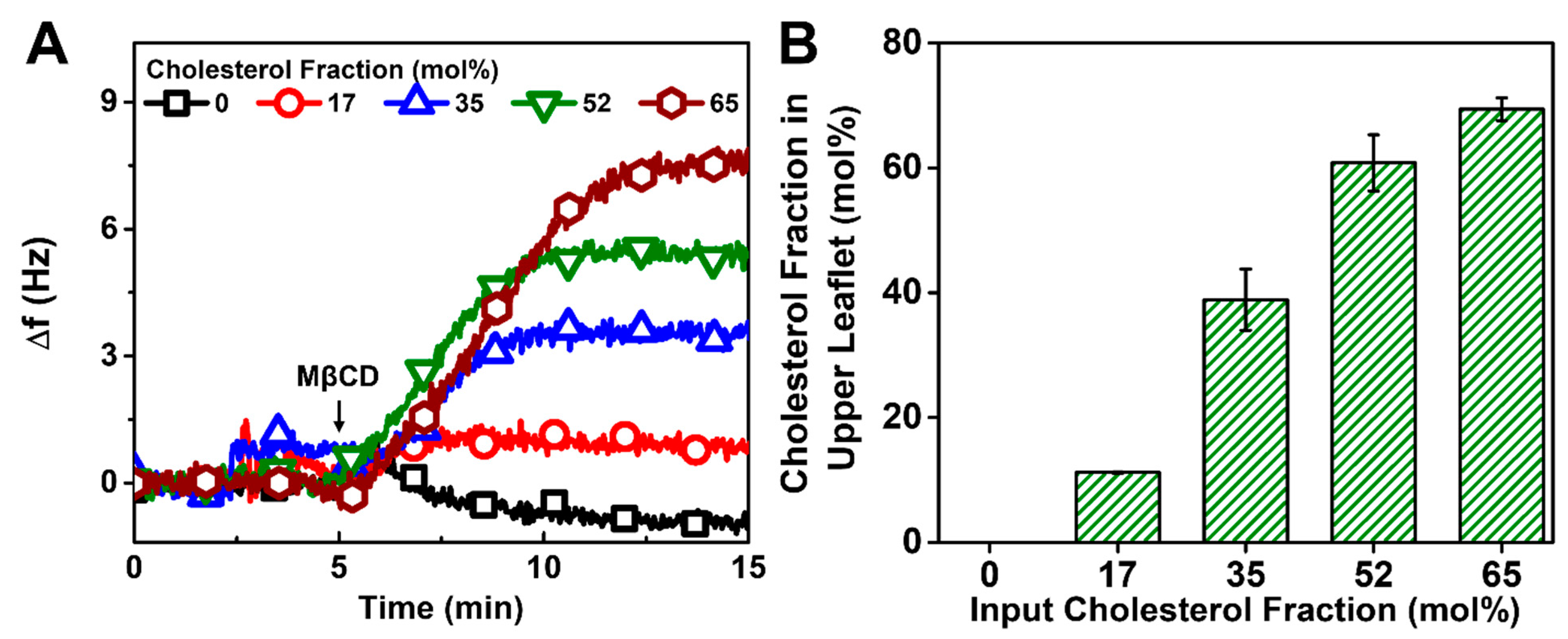
3.3. Estimation of Chol Amount in HLB Upper Leaflet
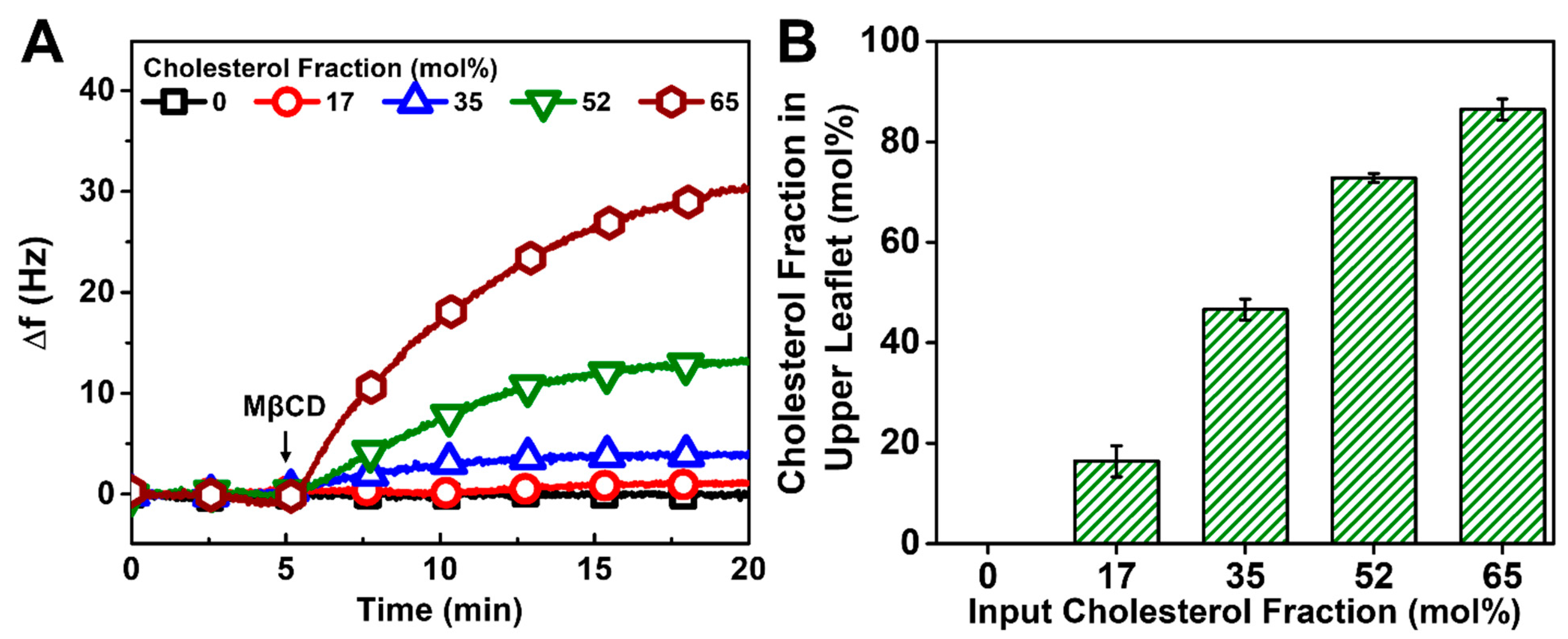
3.4. Comparison of Chol Incorporation Effects by Different Fabrication Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Luchini, A.; Vitiello, G. Understanding the nano-bio interfaces: Lipid-coatings for inorganic nanoparticles as promising strategy for biomedical applications. Front. Chem. 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Willumeit, R.; Schuster, A.; Iliev, P.; Linser, S.; Feyerabend, F. Phospholipids as implant coatings. J. Mater. Sci. Mater. Med. 2007, 18, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Brian, A.A.; McConnell, H.M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 6159–6163. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, A.; Mashaghi, S.; Reviakine, I.; Heeren, R.M.; Sandoghdar, V.; Bonn, M. Label-free characterization of biomembranes: From structure to dynamics. Chem. Soc. Rev. 2014, 43, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E. Supported membranes: Scientific and practical applications. Science 1996, 271, 43–48. [Google Scholar] [CrossRef]
- Reviakine, I.; Bergsma-Schutter, W.; Brisson, A. Growth of protein 2-D crystals on supported planar lipid bilayers imaged in Situ by AFM. J. Struct. Biol. 1998, 121, 356–362. [Google Scholar] [CrossRef]
- Reviakine, I.; Brisson, A. Streptavidin 2D crystals on supported phospholipid bilayers: Toward constructing anchored phospholipid bilayers. Langmuir 2001, 17, 8293–8299. [Google Scholar] [CrossRef]
- Richter, R.P.; Him, J.L.K.; Brisson, A. Supported lipid membranes. Mater. Today 2003, 6, 32–37. [Google Scholar] [CrossRef]
- Sut, T.N.; Yoon, B.K.; Jeon, W.-Y.; Jackman, J.A.; Cho, N.-J. Supported lipid bilayer coatings: Fabrication, bioconjugation, and diagnostic applications. Appl. Mater. Today 2021, 25, 101183. [Google Scholar] [CrossRef]
- Richter, R.P.; Bérat, R.; Brisson, A.R. Formation of solid-supported lipid bilayers: An integrated view. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Zeineldin, R.; Last, J.A.; Slade, A.L.; Ista, L.K.; Bisong, P.; O’Brien, M.J.; Brueck, S.; Sasaki, D.Y.; Lopez, G.P. Using bicellar mixtures to form supported and suspended lipid bilayers on silicon chips. Langmuir 2006, 22, 8163–8168. [Google Scholar] [CrossRef] [PubMed]
- Csúcs, G.; Ramsden, J.J. Solubilization of planar bilayers with detergent. Biochim. Biophys. Acta (BBA)-Biomembr. 1998, 1369, 304–308. [Google Scholar] [CrossRef]
- Marquês, J.T.; Viana, A.S.; De Almeida, R.F. Ethanol effects on binary and ternary supported lipid bilayers with gel/fluid domains and lipid rafts. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 405–414. [Google Scholar] [CrossRef]
- Plant, A.L. Supported hybrid bilayer membranes as rugged cell membrane mimics. Langmuir 1999, 15, 5128–5135. [Google Scholar] [CrossRef]
- Favero, G.; Campanella, L.; Cavallo, S.; D’Annibale, A.; Perrella, M.; Mattei, E.; Ferri, T. Glutamate receptor incorporated in a mixed hybrid bilayer lipid membrane array, as a sensing element of a biosensor working under flowing conditions. J. Am. Chem. Soc. 2005, 127, 8103–8111. [Google Scholar] [CrossRef] [PubMed]
- Suraniti, E.; Tumolo, T.; Baptista, M.S.; Livache, T.; Calemczuk, R. Construction of hybrid bilayer membrane (HBM) biochips and characterization of the cooperative binding between Cytochrome-C and HBM. Langmuir 2007, 23, 6835–6842. [Google Scholar] [CrossRef] [PubMed]
- Meuse, C.W.; Krueger, S.; Majkrzak, C.F.; Dura, J.A.; Fu, J.; Connor, J.T.; Plant, A.L. Hybrid bilayer membranes in air and water: Infrared spectroscopy and neutron reflectivity studies. Biophys. J. 1998, 74, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Oberts, B.; Blanchard, G. Formation of air-stable supported lipid monolayers and bilayers. Langmuir 2009, 25, 2962–2970. [Google Scholar] [CrossRef]
- Sut, T.N.; Ferhan, A.R.; Park, S.; Koo, D.J.; Yoon, B.K.; Jackman, J.A.; Cho, N.-J. Modulating noncovalent and covalent forces to control inverse phosphocholine lipid self-assembly on inorganic surfaces: Nanoarchitectonic design principles. Appl. Mater. Today 2022, 29, 101618. [Google Scholar] [CrossRef]
- Sabirovas, T.; Valiūnienė, A.; Valincius, G. Mechanically polished titanium surface for immobilization of hybrid bilayer membrane. J. Electrochem. Soc. 2018, 165, G109. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F.; Kasemo, B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: Influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir 2003, 19, 1681–1691. [Google Scholar] [CrossRef]
- Reviakine, I.; Rossetti, F.F.; Morozov, A.N.; Textor, M. Investigating the properties of supported vesicular layers on titanium dioxide by quartz crystal microbalance with dissipation measurements. J. Chem. Phys. 2005, 122, 204711. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E.; Höök, F. Design of surface modifications for nanoscale sensor applications. Sensors 2015, 15, 1635–1675. [Google Scholar] [CrossRef] [PubMed]
- Perttu, E.K.; Kohli, A.G.; Szoka, F.C., Jr. Inverse-phosphocholine lipids: A remix of a common phospholipid. J. Am. Chem. Soc. 2012, 134, 4485–4488. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J. A stable lipid/TiO2 interface with headgroup-inversed phosphocholine and a comparison with SiO2. J. Am. Chem. Soc. 2015, 137, 11736–11742. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Liu, Y.; Lin, Z.Y.; Liu, B.; Liu, J. Profiling metal oxides with lipids: Magnetic liposomal nanoparticles displaying DNA and proteins. Angew. Chem. Int. Ed. 2016, 55, 12063–12067. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Wang, H.; Zhang, X.; Zhang, L.; Wang, F.; Liu, J. Charge and coordination directed liposome fusion onto SiO2 and TiO2 nanoparticles. Langmuir 2018, 35, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.; Zuilhof, H. Covalent surface modification of oxide surfaces. Angew. Chem. Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef]
- Sut, T.N.; Meker, S.; Koo, D.J.; Jackman, J.A.; Cho, N.-J. Interfacial approach to fabricate covalently and noncovalently attached inverse-phosphocholine supported lipid bilayers on TiO2 and SiO2 surfaces. J. Ind. Eng. Chem. 2023, 128, 235–244. [Google Scholar] [CrossRef]
- Meker, S.; Halevi, O.; Chin, H.; Sut, T.N.; Jackman, J.A.; Tan, E.-L.; Potroz, M.G.; Cho, N.-J. Inkjet-printed phospholipid bilayers on titanium oxide surfaces: Towards functional membrane biointerfaces. Membranes 2022, 12, 361. [Google Scholar] [CrossRef]
- Sut, T.N.; Tan, S.W.; Jeon, W.-Y.; Yoon, B.K.; Cho, N.-J.; Jackman, J.A. Streamlined fabrication of hybrid lipid bilayer membranes on titanium oxide surfaces: A comparison of one-and two-tail sam molecules. Nanomaterials 2022, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Bar, L.; Villanueva, M.E.; Martín, C.; Ramirez, A.V.; Goole, J.; Renner, F.U.; Losada-Pérez, P. Stability of supported hybrid lipid bilayers on chemically and topographically-modified surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131125. [Google Scholar] [CrossRef]
- Silin, V.I.; Wieder, H.; Woodward, J.T.; Valincius, G.; Offenhausser, A.; Plant, A.L. The role of surface free energy on the formation of hybrid bilayer membranes. J. Am. Chem. Soc. 2002, 124, 14676–14683. [Google Scholar] [CrossRef] [PubMed]
- Płachta, Ł.; Mach, M.; Kowalska, M.; Wydro, P. The effect of trans-resveratrol on the physicochemical properties of lipid membranes with different cholesterol content. Biochim. Biophys. Acta (BBA)-Biomembr. 2024, 1866, 184212. [Google Scholar] [CrossRef] [PubMed]
- Nasri, Z.; Ahmadi, M.; Striesow, J.; Ravandeh, M.; von Woedtke, T.; Wende, K. Insight into the impact of oxidative stress on the barrier properties of lipid bilayer models. Int. J. Mol. Sci. 2022, 23, 5932. [Google Scholar] [CrossRef] [PubMed]
- Ravandeh, M.; Coliva, G.; Kahlert, H.; Azinfar, A.; Helm, C.A.; Fedorova, M.; Wende, K. Protective role of sphingomyelin in eye lens cell membrane model against oxidative stress. Biomolecules 2021, 11, 276. [Google Scholar] [CrossRef]
- Mielke, S.; Sorkin, R.; Klein, J. Effect of cholesterol on the mechanical stability of gel-phase phospholipid bilayers studied by AFM force spectroscopy. Eur. Phys. J. E 2023, 46, 77. [Google Scholar] [CrossRef]
- Valiūnienė, A.; Petrulionienė, T.; Balevičiūtė, I.; Mikoliūnaitė, L.; Valinčius, G. Formation of hybrid bilayers on silanized thin-film Ti electrode. Chem. Phys. Lipids 2017, 202, 62–68. [Google Scholar] [CrossRef]
- Sabirovas, T.; Valiūnienė, A.; Gabriunaite, I.; Valincius, G. Mixed hybrid bilayer lipid membranes on mechanically polished titanium surface. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183232. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Jackman, J.A.; Kim, S.-O.; Liedberg, B.; Knoll, W.; Parikh, A.N.; Cho, N.-J. Formation of cholesterol-rich supported membranes using solvent-assisted lipid self-assembly. Langmuir 2014, 30, 13345–13352. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Jackman, J.A.; Liedberg, B.; Parikh, A.N.; Cho, N.J. Observation of stripe superstructure in the beta-two-phase coexistence region of cholesterol-phospholipid mixtures in supported membranes. J. Am. Chem. Soc. 2014, 136, 16962–16965. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.M.; Takeshita, K.; Subbarao, N.K.; Hu, L.-R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 1991, 1061, 297–303. [Google Scholar] [CrossRef]
- Cho, N.-J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Pegueroles Neyra, M. Interactions between Titanium Surfaces and Biological Components; Universitat Politècnica de Catalunya: Barcelona, Spain, 2009. [Google Scholar]
- Jackman, J.A.; Zan, G.H.; Zhao, Z.; Cho, N.-J. Contribution of the hydration force to vesicle adhesion on titanium oxide. Langmuir 2014, 30, 5368–5372. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.A.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Rodahl, M.; Höök, F.; Krozer, A.; Brzezinski, P.; Kasemo, B. Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum. 1995, 66, 3924–3930. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Fredriksson, C.; Keller, C.A.; Krozer, A.; Brzezinski, P.; Voinova, M.; Kasemo, B. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss. 1997, 107, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Glasmästar, K.; Zhdanov, V.; Kasemo, B. Formation of supported membranes from vesicles. Phys. Rev. Lett. 2000, 84, 5443. [Google Scholar] [CrossRef]
- Jackman, J.A. Quartz crystal microbalance-dissipation technique for tracking dynamic biomacromolecular interactions. In Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Zhi, Z.; Hasan, I.Y.; Mechler, A. Formation of alkanethiol supported hybrid membranes revisited. Biotechnol. J. 2018, 13, 1800101. [Google Scholar] [CrossRef]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of lipid vesicle deposition on solid surfaces: A combined QCM-D and AFM study. Biophys. J. 2003, 85, 3035–3047. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Yoon, B.K.; Park, S.; Sut, T.N.; Chin, H.; Park, J.H.; Jackman, J.A.; Cho, N.-J. Solvent-assisted preparation of supported lipid bilayers. Nat. Protoc. 2019, 14, 2091–2118. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, S.R.; Jackman, J.A.; Kim, S.-O.; Zhdanov, V.P.; Cho, N.-J. Solvent-assisted lipid self-assembly at hydrophilic surfaces: Factors influencing the formation of supported membranes. Langmuir 2015, 31, 3125–3134. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, L.M.; Yoon, B.K.; Jackman, J.A.; Knoll, W.; Weiss, P.S.; Cho, N.-J. Understanding how sterols regulate membrane remodeling in supported lipid bilayers. Langmuir 2017, 33, 14756–14765. [Google Scholar] [CrossRef] [PubMed]
- Alwarawrah, M.; Dai, J.; Huang, J. A molecular view of the cholesterol condensing effect in DOPC lipid bilayers. J. Phys. Chem. B 2010, 114, 7516–7523. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Rand, R.P. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 1997, 73, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Meleard, P.; Gerbeaud, C.; Pott, T.; Fernandez-Puente, L.; Bivas, I.; Mitov, M.D.; Dufourcq, J.; Bothorel, P. Bending elasticities of model membranes: Influences of temperature and sterol content. Biophys. J. 1997, 72, 2616–2629. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Buboltz, J.T.; Feigenson, G.W. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 1999, 1417, 89–100. [Google Scholar] [CrossRef]
- Beseničar, M.P.; Bavdek, A.; Kladnik, A.; Maček, P.; Anderluh, G. Kinetics of cholesterol extraction from lipid membranes by methyl-β-cyclodextrin—A surface plasmon resonance approach. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 175–184. [Google Scholar] [CrossRef]
- Al-Husseini, J.K.; Fong, E.M.; Wang, C.; Ha, J.H.; Upreti, M.; Chiarelli, P.A.; Johal, M.S. Ex vivo drug screening assay with artificial membranes: Characterizing cholesterol desorbing competencies of beta-cyclodextrins. Langmuir 2023, 39, 12590–12598. [Google Scholar] [CrossRef]
- Bint E Naser, S.F.; Liu, H.-Y.; Su, H.; Kouloumpis, A.; Carten, J.D.; Daniel, S. An impedance-based approach for sensing cyclodextrin-mediated modulation of membrane cholesterol. Langmuir 2023, 39, 9831–9840. [Google Scholar] [CrossRef]
- Sauerbrey, G. Use of quartz crystal units for weighing thin films and microweighing. Mag. Phys. 1959, 155, 206–222. [Google Scholar]
- Kalb, E.; Frey, S.; Tamm, L.K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim. Biophys. Acta (BBA)-Biomembr. 1992, 1103, 307–316. [Google Scholar] [CrossRef]
- Rodahl, M.; Kasemo, B. On the measurement of thin liquid overlayers with the quartz-crystal microbalance. Sens. Actuators A Phys. 1996, 54, 448–456. [Google Scholar] [CrossRef]
- Schmidt, C.; Heym, F. Improved methodology for absorption measurements in ionic liquids with a quartz crystal microbalance. Chem. Eng. Technol. 2017, 40, 1638–1643. [Google Scholar] [CrossRef]
- Ibarguren, M.; Alonso, A.; Tenchov, B.G.; Goñi, F.M. Quantitation of cholesterol incorporation into extruded lipid bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Hohner, A.O.; David, M.P.C.; Rädler, J.O. Controlled solvent-exchange deposition of phospholipid membranes onto solid surfaces. Biointerphases 2010, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Szoka, F.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar] [CrossRef] [PubMed]
- Feigenson, G.W. Phase diagrams and lipid domains in multicomponent lipid bilayer mixtures. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 47–52. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—Properties and fabrication: A review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Sut, T.N.; Park, S.; Jackman, J.A.; Cho, N.-J. Controlling molecular self-assembly of inverse-phosphocholine lipids at oxide interfaces with divalent cations. Appl. Mater. Today 2023, 35, 101953. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sut, T.N.; Jackman, J.A.; Cho, N.-J. Cholesterol-Enriched Hybrid Lipid Bilayer Formation on Inverse Phosphocholine Lipid-Functionalized Titanium Oxide Surfaces. Biomimetics 2023, 8, 588. https://doi.org/10.3390/biomimetics8080588
Sut TN, Jackman JA, Cho N-J. Cholesterol-Enriched Hybrid Lipid Bilayer Formation on Inverse Phosphocholine Lipid-Functionalized Titanium Oxide Surfaces. Biomimetics. 2023; 8(8):588. https://doi.org/10.3390/biomimetics8080588
Chicago/Turabian StyleSut, Tun Naw, Joshua A. Jackman, and Nam-Joon Cho. 2023. "Cholesterol-Enriched Hybrid Lipid Bilayer Formation on Inverse Phosphocholine Lipid-Functionalized Titanium Oxide Surfaces" Biomimetics 8, no. 8: 588. https://doi.org/10.3390/biomimetics8080588





