Porous Polylactide Microparticles as Effective Fillers for Hydrogels
Abstract
:1. Introduction
- The development of composite hydrogels based on a natural polymer filled by a biocompatible filler containing active biomolecules. For example, a novel injectable nanocomposite was designed as a sustained-release platform for cartilage regeneration through the integration of a chitosan-based hydrogel, articular cartilage stem cells (ACSCs) and mesoporous SiO2 nanoparticles loaded with anhydroicaritin (AHI) [9].
1.1. Collagen and Collagen Gels
1.2. Chitosan and Chitosan Gels
1.3. Composite Gels with Porous Microparticles
1.4. C-Phycocyanin
2. Materials and Methods
2.1. Materials
2.1.1. Porous Polylactide Microparticles
2.1.2. Highly Concentrated Collagen Gel
2.1.3. Chitosan–Genipin Hydrogels
2.1.4. Composite Hydrogels
2.1.5. C-Phycocyanin
2.2. Experimental
2.2.1. Scanning Electron Microscope
2.2.2. Optical Microscopy
2.2.3. Ultrasound Investigation
2.2.4. Mechanical Properties
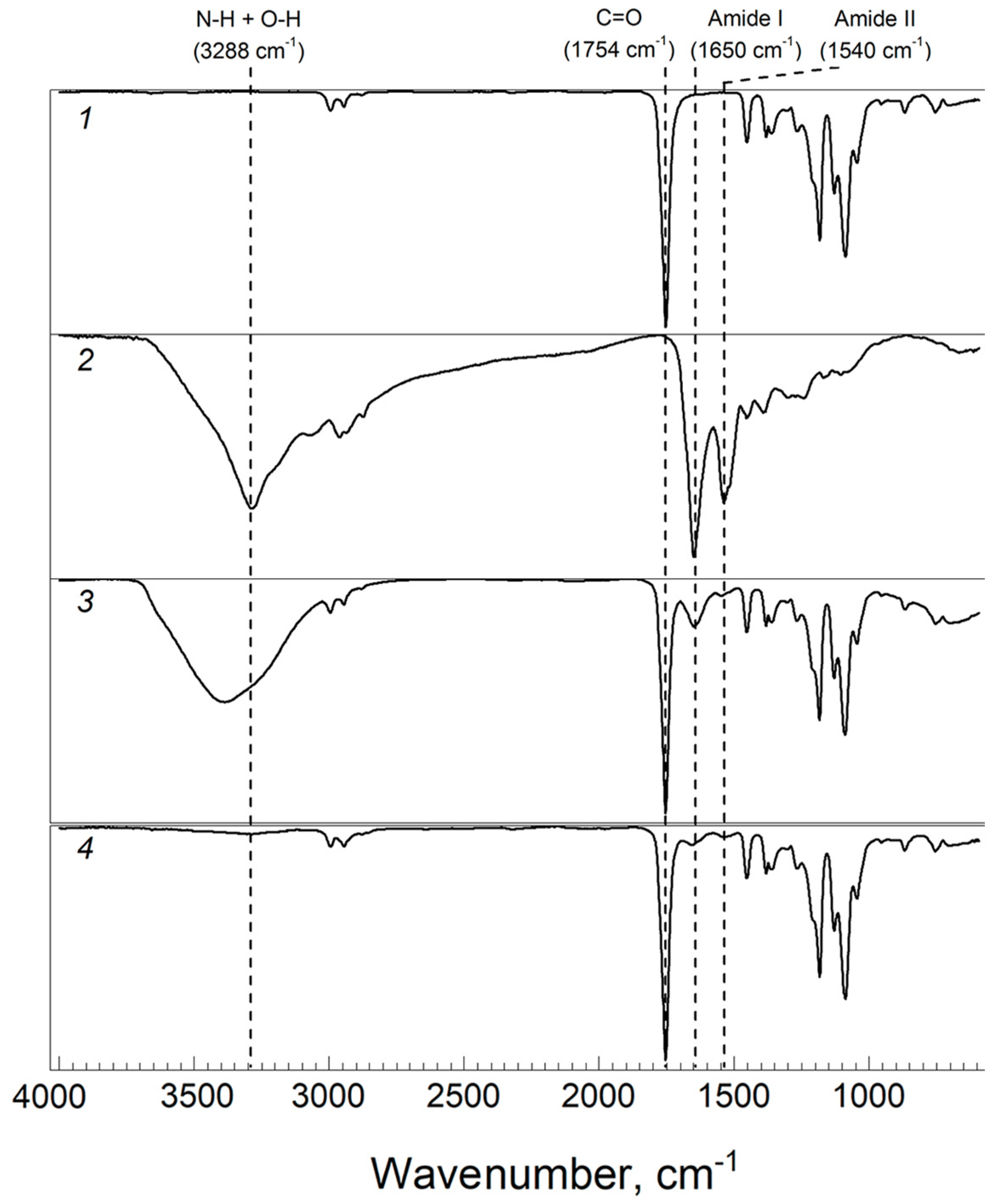
2.2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Statistical Analysis
3. Results and Discussion
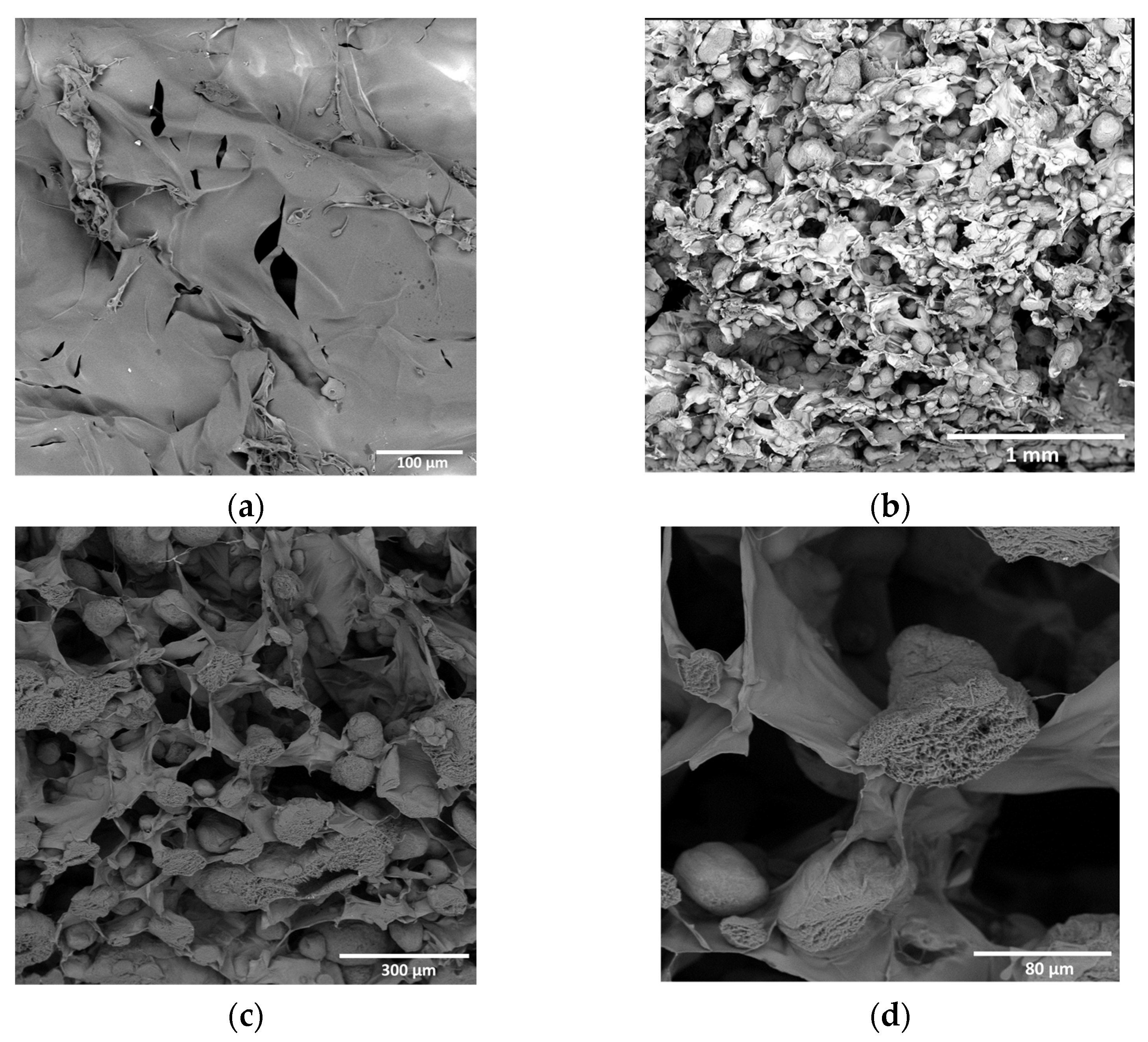
3.1. Structure Polylactide Particles
3.2. C-PC-Loaded Polylactide Particles
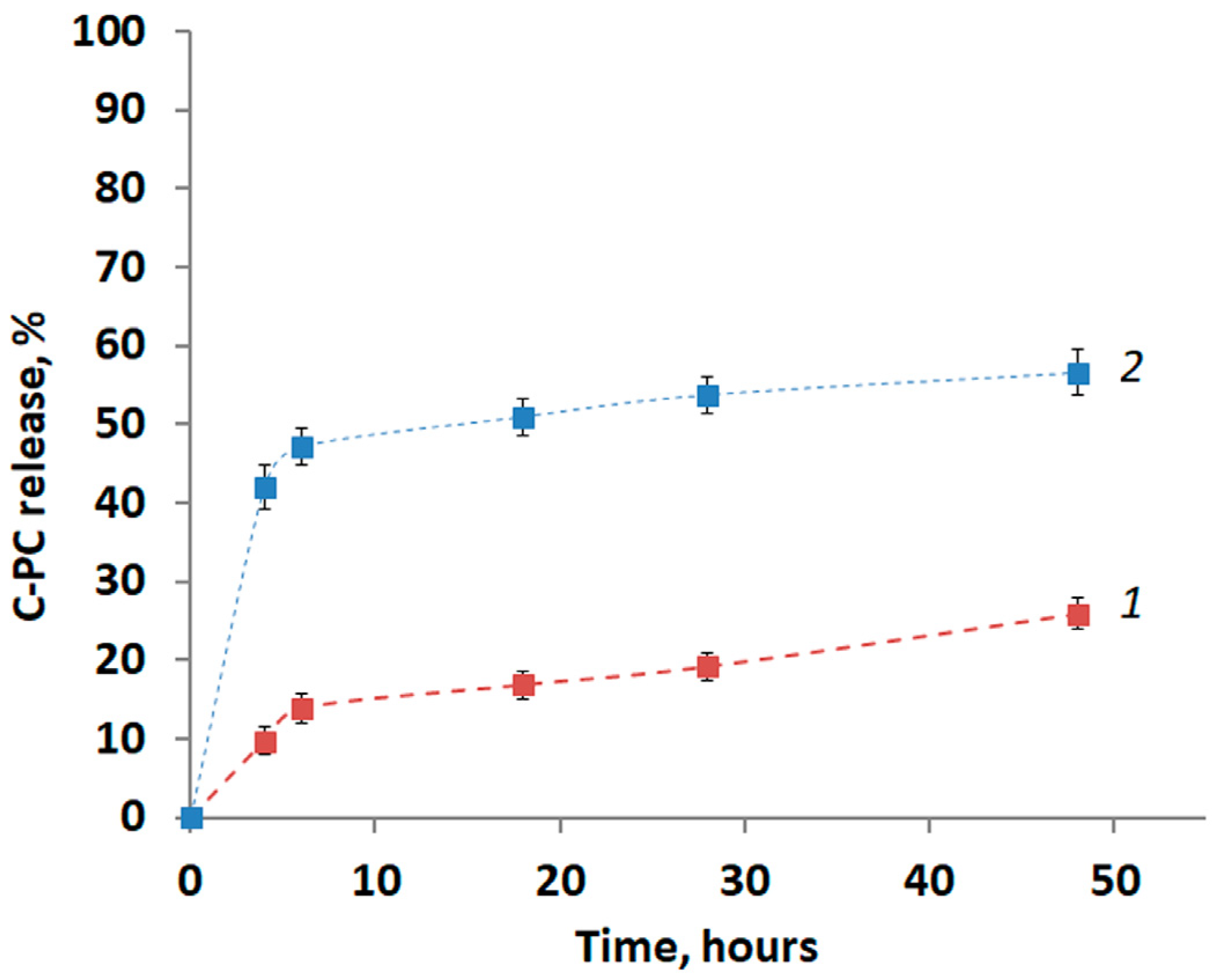
3.3. C-PC Release from the PLA Particles
3.4. Structure of Composite Hydrogels
3.4.1. Collagen–PLA Microparticle Composite Hydrogels
3.4.2. Chitosan–PLA Microparticle Composite Hydrogels
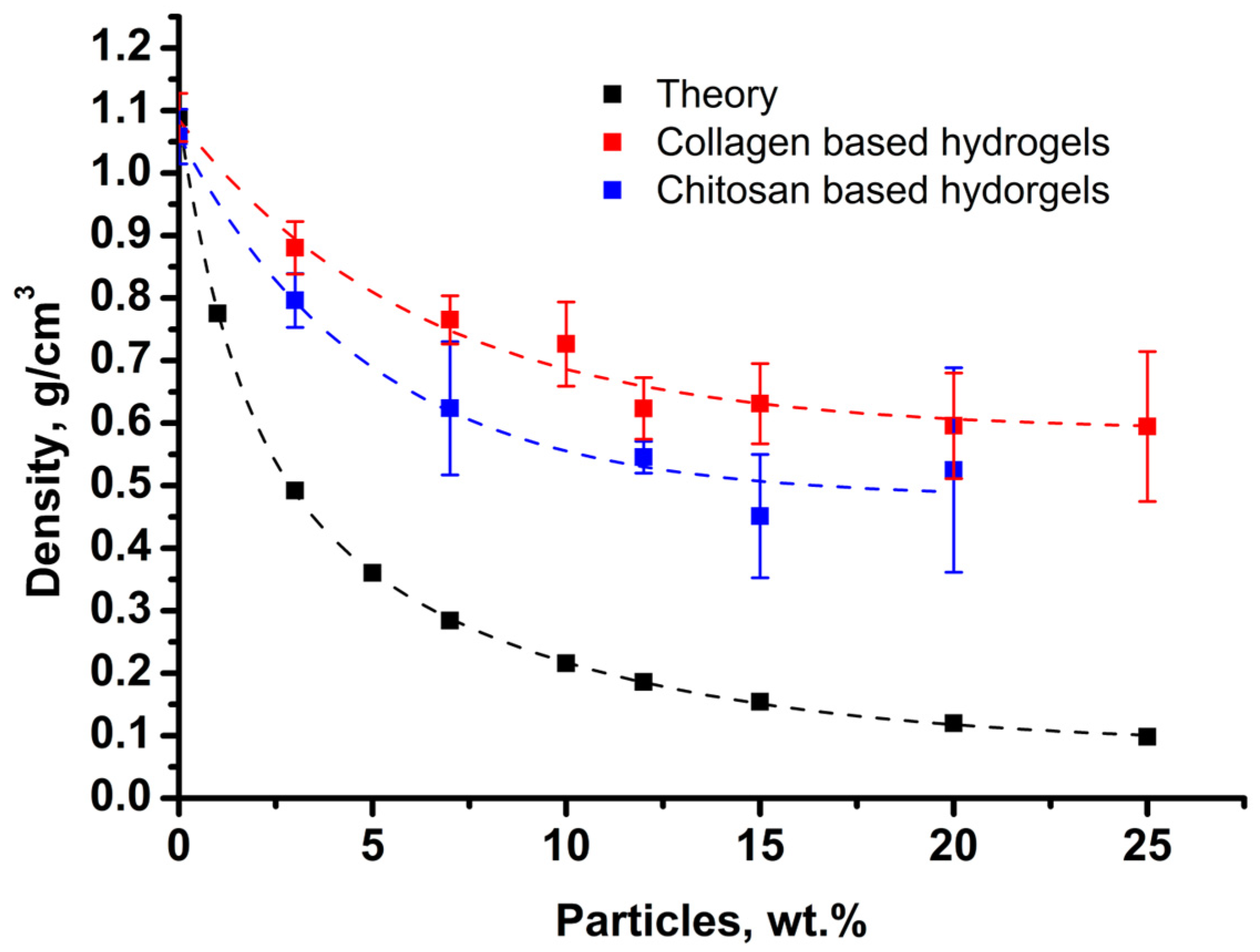
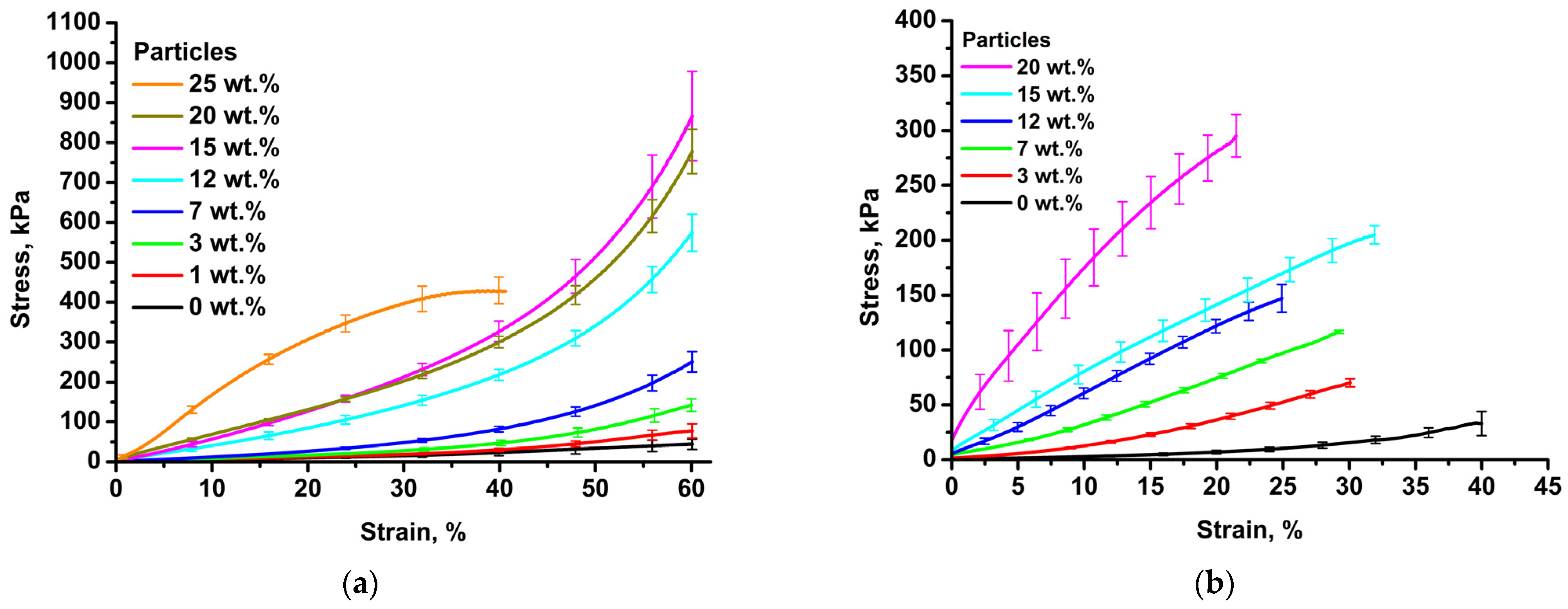
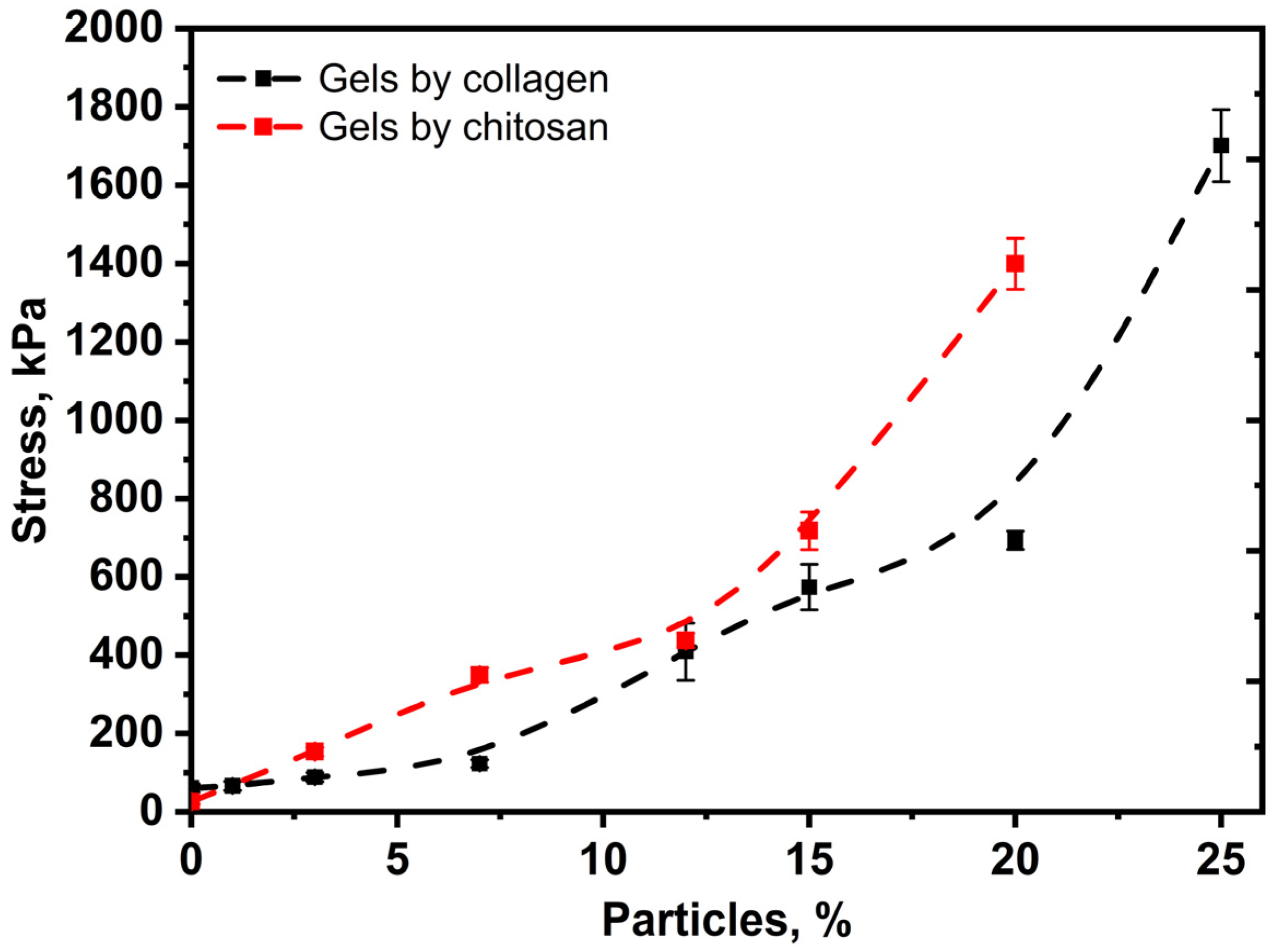
3.5. Mechanical Properties of Collagen and Chitosan Hydrogels Filled with Porous Lactide Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khanmohammadi, M.; Jalessi, M.; Asghari, A. Biomimetic Hydrogel Scaffolds via Enzymatic Reaction for Cartilage Tissue Engineering. BMC Res. Notes 2022, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Ngadimin, K.D.; Stokes, A.; Gentile, P.; Ferreira, A.M. Biomimetic Hydrogels Designed for Cartilage Tissue Engineering. Biomater. Sci. 2021, 9, 4246–4259. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, J.; Marchant, R.E. Biomimetic Hydrogels as Scaffolds for Tissue-Engineering Applications. In Biomimetic Biomaterials: Structure and Applications; Woodhead Publishing: Sawston, UK, 2013; pp. 238–275. ISBN 9780857094162. [Google Scholar]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef] [PubMed]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A Tough Act to Follow: Collagen Hydrogel Modifications to Improve Mechanical and Growth Factor Loading Capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef] [PubMed]
- Ollier, R.C.; Xiang, Y.; Yacovelli, A.M.; Webber, M.J. Biomimetic Strain-Stiffening in Fully Synthetic Dynamic-Covalent Hydrogel Networks. Chem. Sci. 2023, 14, 4796–4805. [Google Scholar] [CrossRef]
- Vashahi, F.; Martinez, M.R.; Dashtimoghadam, E.; Fahimipour, F.; Keith, A.N.; Bersenev, E.A.; Ivanov, D.A.; Zhulina, E.B.; Popryadukhin, P.; Matyjaszewski, K.; et al. Injectable Bottlebrush Hydrogels with Tissue-Mimetic Mechanical Properties. Sci. Adv. 2022, 8, eabm2469. [Google Scholar] [CrossRef]
- Cui, P.; Pan, P.; Qin, L.; Wang, X.; Chen, X.; Deng, Y.; Zhang, X. Nanoengineered Hydrogels as 3D Biomimetic Extracellular Matrix with Injectable and Sustained Delivery Capability for Cartilage Regeneration. Bioact. Mater. 2023, 19, 487–498. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Kumar Verma, A. Collagen-Based Biomaterial as Drug Delivery Module. In Collagen Biomaterials; IntechOpen: London, UK, 2022. [Google Scholar]
- Kumari, S.; Kishor, R. Chitin and Chitosan: Origin, Properties, and Applications. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Liu, R.-R.; Shi, Q.-Q.; Meng, Y.-F.; Zhou, Y.; Mao, L.-B.; Yu, S.-H. Biomimetic Chitin Hydrogel via Chemical Transformation. Nano Res. 2023. [Google Scholar] [CrossRef]
- Fang, J.; Liao, J.; Zhong, C.; Lu, X.; Ren, F. High-Strength, Biomimetic Functional Chitosan-Based Hydrogels for Full-Thickness Osteochondral Defect Repair. ACS Biomater. Sci. Eng. 2022, 8, 4449–4461. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Genipin-Crosslinked Chitosan Hydrogels as Biomedical and Pharmaceutical Aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Vo, N.T.N.; Huang, L.; Lemos, H.; Mellor, A.L.; Novakovic, K. Genipin-crosslinked Chitosan Hydrogels: Preliminary Evaluation of the in Vitro Biocompatibility and Biodegradation. J. Appl. Polym. Sci. 2021, 138, 50848. [Google Scholar] [CrossRef]
- Pomari, A.A.D.N.; Montanheiro, T.L.D.A.; de Siqueira, C.P.; Silva, R.S.; Tada, D.B.; Lemes, A.P. Chitosan Hydrogels Crosslinked by Genipin and Reinforced with Cellulose Nanocrystals: Production and Characterization. J. Compos. Sci. 2019, 3, 84. [Google Scholar] [CrossRef]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Osidak, E.O.; Grigoriev, T.E.; Zagoskin, Y.D.; Nedorubova, I.A.; Domogatsky, S.P.; Babichenko, I.I.; Zorina, O.A.; et al. Osteoinductive Moldable and Curable Bone Substitutes Based on Collagen, Bmp-2 and Highly Porous Polylactide Granules, or a Mix of Hap/β-Tcp. Polymers 2021, 13, 3974. [Google Scholar] [CrossRef]
- Bukharova, T.B.; Nedorubova, I.A.; Mokrousova, V.O.; Meglei, A.Y.; Basina, V.P.; Nedorubov, A.A.; Vasilyev, A.V.; Grigoriev, T.E.; Zagoskin, Y.D.; Chvalun, S.N.; et al. Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery. Cells 2023, 12, 1762. [Google Scholar] [CrossRef]
- Phycocyanin Market to Be Worth $279.6 Million by 2030|Meticulous Market Research Pvt. Ltd. Available online: https://www.meticulousresearch.com/pressrelease/30/phycocyanin-market-2030 (accessed on 9 August 2023).
- Liu, R.; Qin, S.; Li, W. Phycocyanin: Anti-Inflammatory Effect and Mechanism. Biomed. Pharmacother. 2022, 153, 113362. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A Potential Drug for Cancer Treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef]
- Fernandes e Silva, E.; da Figueira, F.S.; Lettnin, A.P.; Carrett-Dias, M.; Filgueira, D.M.V.B.; Kalil, S.; Trindade, G.S.; de Votto, A.P.S. C-Phycocyanin: Cellular Targets, Mechanisms of Action and Multi Drug Resistance in Cancer. Pharmacol. Rep. 2018, 70, 75–80. [Google Scholar] [CrossRef]
- Ziyaei, K.; Abdi, F.; Mokhtari, M.; Daneshmehr, M.A.; Ataie, Z. Phycocyanin as a Nature-Inspired Antidiabetic Agent: A Systematic Review. Phytomedicine 2023, 119, 154964. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, C.H.; Lu, X.; Pang, Y.Y.; Jiang, H.R.; Liu, X.L. Stability and Fluorescence Characteristics of C-Phycocyanin in Spirulina Platensis. Modern Food Sci. Technol. 2020, 36, 89–96. [Google Scholar]
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical Degradation of Phycocyanin and Means to Improve Its Stability: A Short Review. J. Pharm. Anal. 2022, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Liang, Z.-P.; Chang, X.-Y.; Li, F.-T.; Wang, X.-Q.; Lian, X.-J. Progress of Microencapsulated Phycocyanin in Food and Pharma Industries: A Review. Molecules 2022, 27, 5854. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Nogueira, A.O.; Felipe Kokuszi, L.T.; Poester Cordeiro, A.; Ziebell Salgado, H.; Costa, J.A.V.; Santos, L.O.; de Lima, V.R. Spirulina Sp. LEB 18-Extracted Phycocyanin: Effects on Liposomes’ Physicochemical Parameters and Correlation with Antiradical/Antioxidant Properties. Chem. Phys. Lipids 2021, 236, 105064. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Bacchetta, G.; Pons, R.; Demurtas, D.; Diez-Sales, O.; Fadda, A.M.; Manconi, M. Santosomes as Natural and Efficient Carriers for the Improvement of Phycocyanin Reepithelising Ability in Vitro and in Vivo. Eur. J. Pharm. Biopharm. 2016, 103, 149–158. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Catalán-Latorre, A.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Phycocyanin-Encapsulating Hyalurosomes as Carrier for Skin Delivery and Protection from Oxidative Stress Damage. J. Mater. Sci. Mater. Med. 2016, 27, 1–10. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, C.M. C-Phycocyanin-Based Nanoplatform as a Photosensitiser for Targeted Photodynamic-Mediated Cell Death. Mater. Technol. 2022, 37, 2907–2914. [Google Scholar] [CrossRef]
- Manconi, M.; Mura, S.; Manca, M.L.; Fadda, A.M.; Dolz, M.; Hernandez, M.J.; Casanovas, A.; Díez-Sales, O. Chitosomes as Drug Delivery Systems for C-Phycocyanin: Preparation and Characterization. Int. J. Pharm. 2010, 392, 92–100. [Google Scholar] [CrossRef]
- Yan, M.; Liu, B.; Jiao, X.; Qin, S. Preparation of Phycocyanin Microcapsules and Its Properties. Food Bioprod. Process. 2014, 92, 89–97. [Google Scholar] [CrossRef]
- Li, Q.; Dong, P.; Li, L. Preparation and Characterization of Mg-Doped Calcium Phosphate-Coated Phycocyanin Nanoparticles for Improving the Thermal Stability of Phycocyanin. Foods 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.V.F.; Opretzka, L.C.F.; Almeida, L.S.; Cardoso, L.G.; da Silva, J.B.A.; de Souza, C.O.; Villarreal, C.F.; Druzian, J.I. Preparation and Characterization of C-Phycocyanin Coated with STMP/STPP Cross-Linked Starches from Different Botanical Sources. Int. J. Biol. Macromol. 2020, 159, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Mohanbhai, S.J.; Kushwaha, A.C.; Sood, A.; Sardoiwala, M.N.; Choudhury, S.R.; Karmakar, S. κ-Carrageenan-C-Phycocyanin Based Smart Injectable Hydrogels for Accelerated Wound Recovery and Real-Time Monitoring. Acta Biomater. 2020, 109, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, H.; Su, W.; Song, Y.; Zaky, A.A.; Abd El-Aty, A.M.; Tan, M. Co-Delivery of Hydrophobic Astaxanthin and Hydrophilic Phycocyanin by a pH-Sensitive Water-in-Oil-in-Water Double Emulsion-Filled Gellan Gum Hydrogel. Food Hydrocoll. 2022, 131, 107810. [Google Scholar] [CrossRef]
- Azaza, Y.B.; Li, S.; Nasri, M.; Nasri, R. Chitosan/Collagen-Based Hydrogels for Sustainable Development: Phycocyanin Controlled Release. Sustain. Chem. Pharm. 2023, 31, 100905. [Google Scholar] [CrossRef]
- Alavi, N.; Golmakani, M.T.; Hosseini, S.M.H. Fabrication and Characterization of Phycocyanin-Alginate-Pregelatinized Corn Starch Composite Gel Beads: Effects of Carriers on Kinetic Stability of Phycocyanin. Int. J. Biol. Macromol. 2022, 218, 665–678. [Google Scholar] [CrossRef]
- Alavi, N.; Golmakani, M.T.; Hosseini, S.M.H.; Niakousari, M.; Moosavi-Nasab, M. Enhancing Phycocyanin Solubility via Complexation with Fucoidan or κ-Carrageenan and Improving Phycocyanin Color Stability by Encapsulation in Alginate-Pregelatinized Corn Starch Composite Gel Beads. Int. J. Biol. Macromol. 2023, 242, 124762. [Google Scholar] [CrossRef]
- Kim, S.-C.; Heo, S.-Y.; Oh, G.-W.; Chandika, P.; Park, W.S.; Choi, I.-W.; Jung, W.-K. Hierarchically Structured Phycocyanin-Loaded Micro/Nanofibrous Membrane For Guided Bone Regeneration. Mater. Today Commun. 2023, 36, 106852. [Google Scholar] [CrossRef]
- Aiba, S.; Ogawa, T. Assessment of Growth Yield of a Blue-Green Alga, Spirulina Platensis, in Axenic and Continuous Culture. J. Gen. Microbiol. 1977, 102, 179–182. [Google Scholar] [CrossRef]
- Sergeeva, Y.E.; Zakharevich, A.A.; Sukhinov, D.V.; Koshkalda, A.I.; Kryukova, M.V.; Malakhov, S.N.; Antipova, C.G.; Klein, O.I.; Gotovtsev, P.M.; Grigoriev, T.E. Chitosan Sponges for Efficient Accumulation and Controlled Release of C-Phycocyanin. BioTech 2023, 12, 55. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Richmond, A.E. Isolation and Characterization of Phycocyanins from the Blue-Green Alga Spirulina Platensis. Arch. Microbiol. 1979, 120, 155–159. [Google Scholar] [CrossRef]
- Morokov, E.; Khramtsova, E.; Kuevda, E.; Gubareva, E.; Grigoriev, T.; Lukanina, K.; Levin, V. Noninvasive Ultrasound Imaging for Assessment of Intact Microstructure of Extracellular Matrix in Tissue Engineering. Artif. Organs 2019, 43, 1104–1110. [Google Scholar] [CrossRef]
- Adli, S.A.; Ali, F.; Azmi, A.S.; Anuar, H.; Nasir, N.A.M.; Hasham, R.; Idris, M.K.H. Development of Biodegradable Cosmetic Patch Using a Polylactic Acid/Phycocyanin-Alginate Composite. Polymers 2020, 12, 1669. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Liang, Z.; Zhu, B.; Dong, T.; Inoue, Y. Roles of Physical Aging on Crystallization Kinetics and Induction Period of Poly(L-lactide). Macromolecules 2008, 41, 8011–8019. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Gumilar, G.G.; Alifia, C.R.; Marthania, M.; Stellasary, B.; Yuliani, G.; Wulandari, A.P.; Kurniawan, I.; Hidayat, R.; Ningrum, A.; et al. Photostabilization of Phycocyanin from Spirulina platensis Modified by Gormaldehyde. Process Biochem. 2020, 94, 297–304. [Google Scholar] [CrossRef]
- Singh, G.; Chanda, A. Mechanical Properties of Whole-Body Soft Human Tissues: A Review. Biomed. Mater. 2021, 16, 062004. [Google Scholar] [CrossRef]

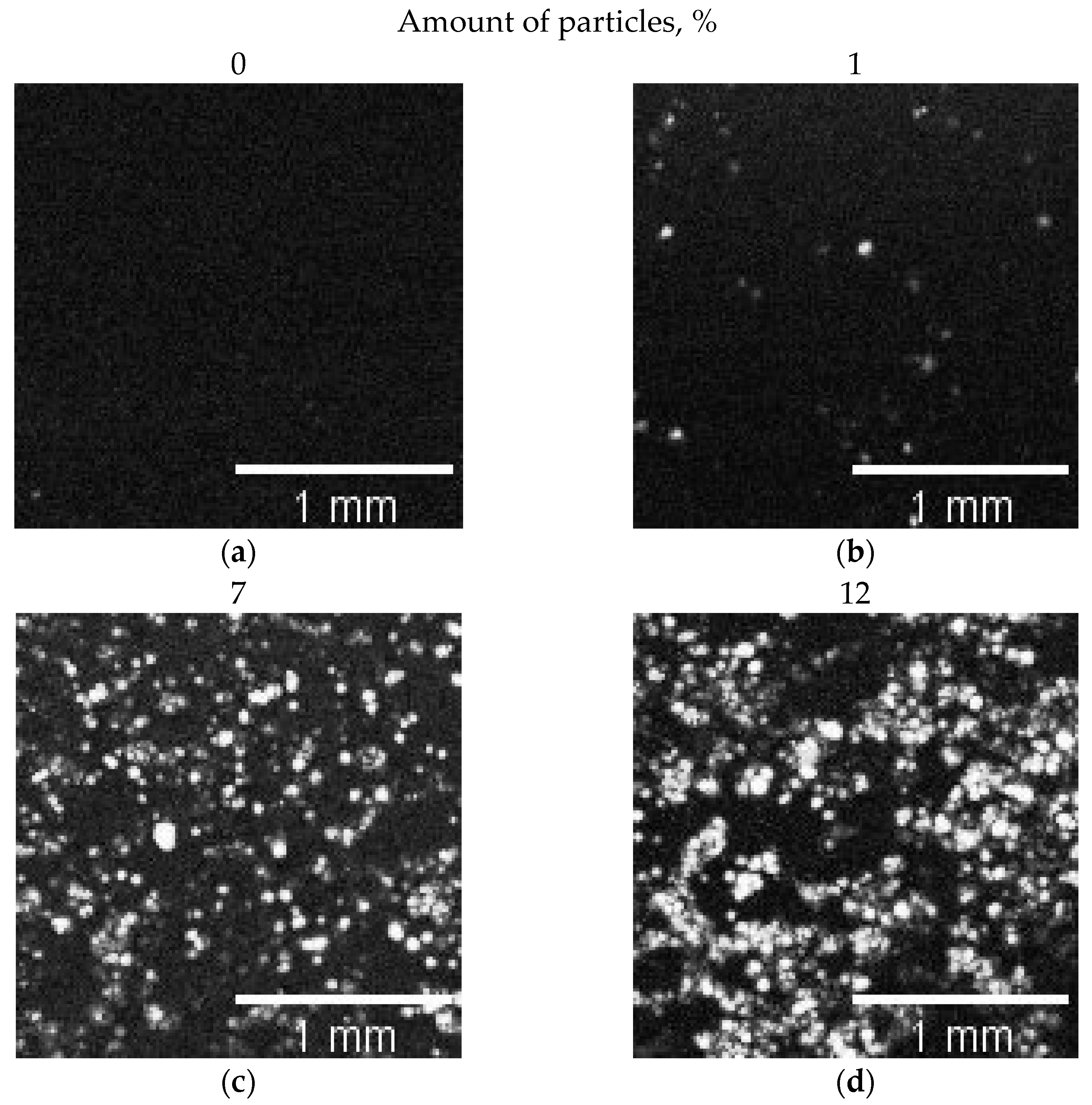
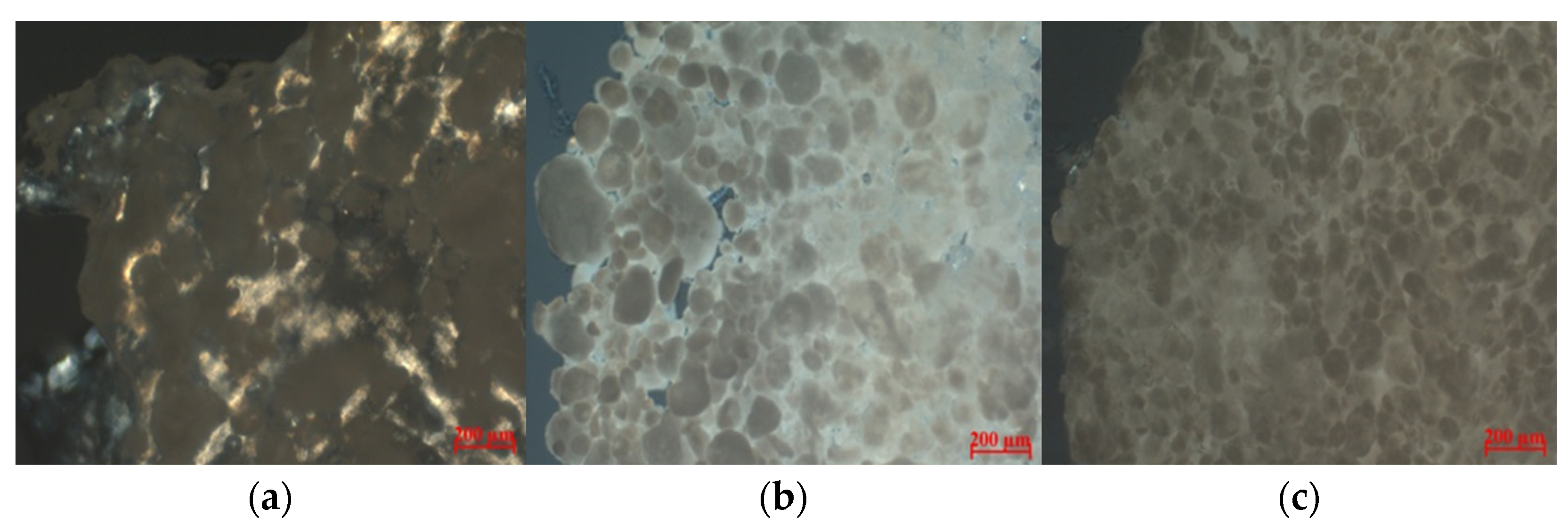
| % of Particles in Composite | % of Image Filled with Particles |
|---|---|
| 0 | 0 |
| 1 | 1 ± 0.3 |
| 7 | 22 ± 0.2 |
| 12 | 36 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagoskin, Y.D.; Sergeeva, Y.E.; Fomina, Y.S.; Sukhinov, D.V.; Malakhov, S.N.; Osidak, E.O.; Khramtsova, E.A.; Gotovtsev, P.M.; Chvalun, S.N.; Grigoriev, T.E. Porous Polylactide Microparticles as Effective Fillers for Hydrogels. Biomimetics 2023, 8, 565. https://doi.org/10.3390/biomimetics8080565
Zagoskin YD, Sergeeva YE, Fomina YS, Sukhinov DV, Malakhov SN, Osidak EO, Khramtsova EA, Gotovtsev PM, Chvalun SN, Grigoriev TE. Porous Polylactide Microparticles as Effective Fillers for Hydrogels. Biomimetics. 2023; 8(8):565. https://doi.org/10.3390/biomimetics8080565
Chicago/Turabian StyleZagoskin, Yuriy D., Yana E. Sergeeva, Yuliya S. Fomina, Daniil V. Sukhinov, Sergey N. Malakhov, Egor O. Osidak, Elena A. Khramtsova, Pavel M. Gotovtsev, Sergei N. Chvalun, and Timofei E. Grigoriev. 2023. "Porous Polylactide Microparticles as Effective Fillers for Hydrogels" Biomimetics 8, no. 8: 565. https://doi.org/10.3390/biomimetics8080565






