Bio-Inspired Molecularly Imprinted Polymer Electrochemical Sensor for Cortisol Detection Based on O-Phenylenediamine Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fabrication of the Molecularly Imprinted Polymer(MIP) Cortisol Sensors
2.3. Electrochemical Apparatus and Measurements
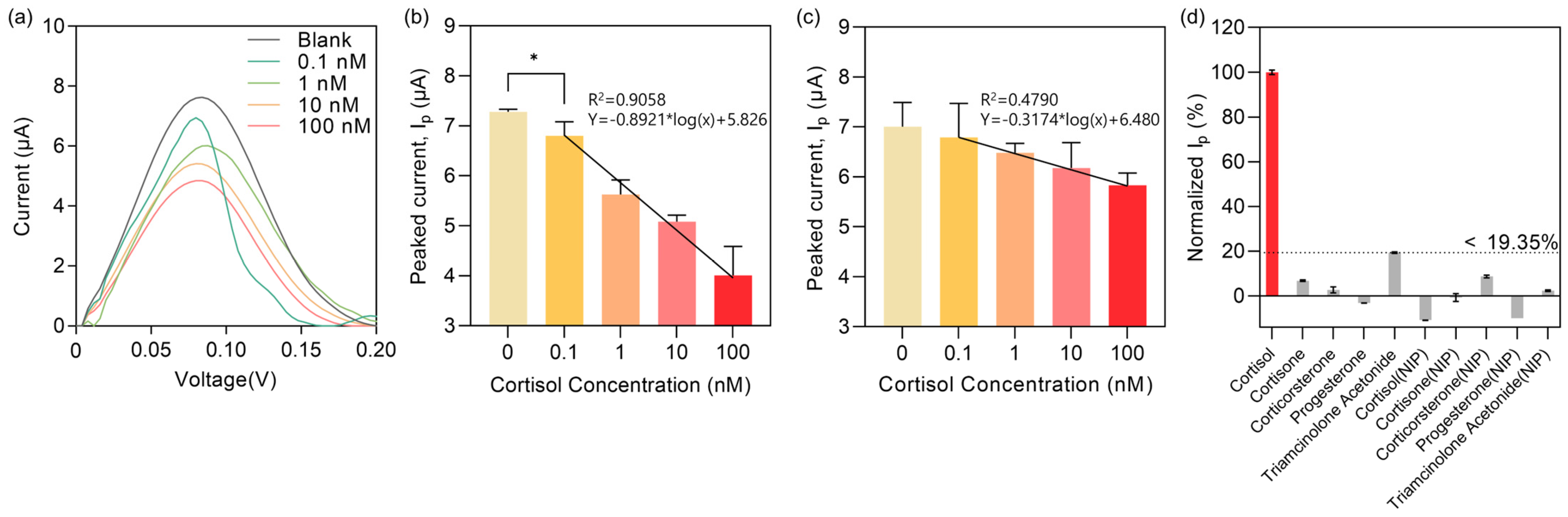
3. Results and Discussion
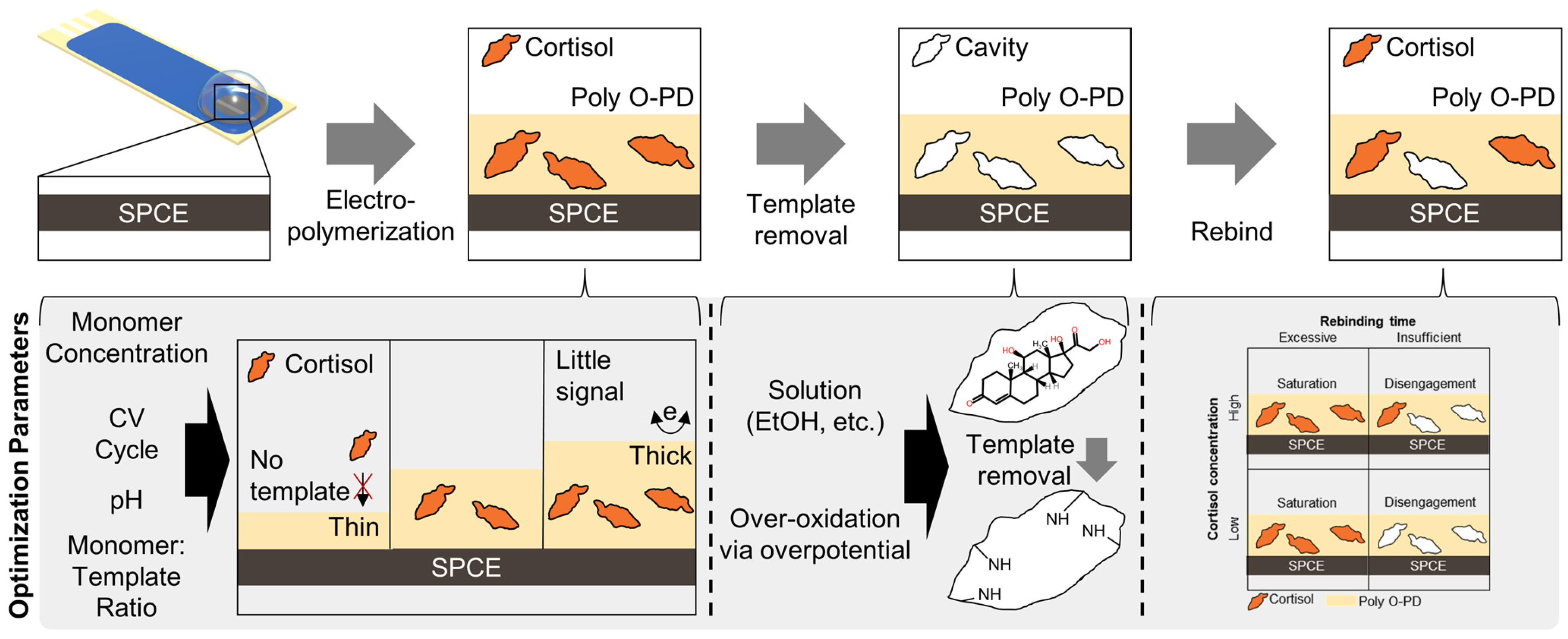
3.1. Cortisol Detection Strategy
| Template | Monomer (mM) | pH | Scan Rate (mV/s) | # of Cycle | Voltage Range (V) | Extraction Solution | Extraction Time (min) | Rebind Time (min) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 M Triclosan | 6 (O-PD) | 5.2 | 50 | 20 | 0–0.8 | 0.1 M NaOH | 10 | 15 | [34] |
| 1 mM ATZ | 5 (O-PD) | 7.4 | 50 | 15 | 0–0.8 | MeOH/Acetic acid (9:1 v/v) | N.A. | 8 | [30] |
| 0.4 mM PMX | 5 (O-PD) | 5.2 | 50 | 20 | −0.2–0.75 | 0.1 M NaOH | 1 | 5 | [35] |
| 1 mM PFOs | 10 (O-PD) | 5.8 | 50 | 25 | 0–1 | 50% MeOH | 20 | 26 | [29] |
| 20 mM Sorbitol | 5 (O-PD) | 5.2 | 50 | 30 | 0–0.8 | DI | N.A. | 10 | [28] |
| 0.4 mM 2,4-DCP | 2 (O-PD) | 5.2 | 50 | 10 | 0–1 | EtOH | 8 | 6 | [36] |
| 20 mM Glucose | 5 (O-PD) | 5.2 | 50 | 20 | 0–0.8 | pH 5.2 Acet. Buffer /10 mM glucose | Short time | N.A. | [27] |
| 0.12 M Aniline | 3 (O-PD) | 3 | 50 | 25 | −0.1–1 | DI/MeOH (6:4 v/v) | 10 | N.A. | [37] |
| 10 mM GSH | 5 (O-PD) | 6.98 | 50 | 6 | 0–0.8 | 0.1 M NaOH | 30 | N.A. | [26] |
| 0.5 mM Cortisol | 5 (O-PD) | 4 | 50 | 30 | 0–1 | EtOH | 40 | N.A. | [38] |
| 1–20 mM Cortisol | 0.03–1 M (Pyrrole) | 7.4 | 100 | 10 | −0.2–0.9 | PBS (overoxidation) | 5–40 cycles | 15 | [25] |
| 0.5 mM Cortisol | 3.5 mM (O-PD) | 5.2 | 50 | 30 | 0–1 | PBS (overoxidation) | 25 cycles | 10 | This work |
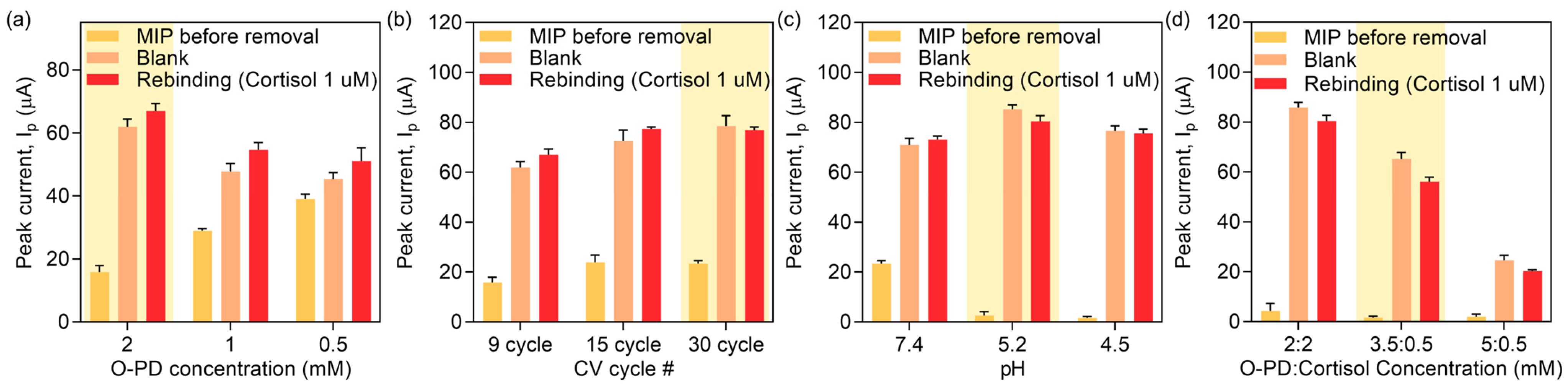
3.2. Electropolymerization Parameters Optimization
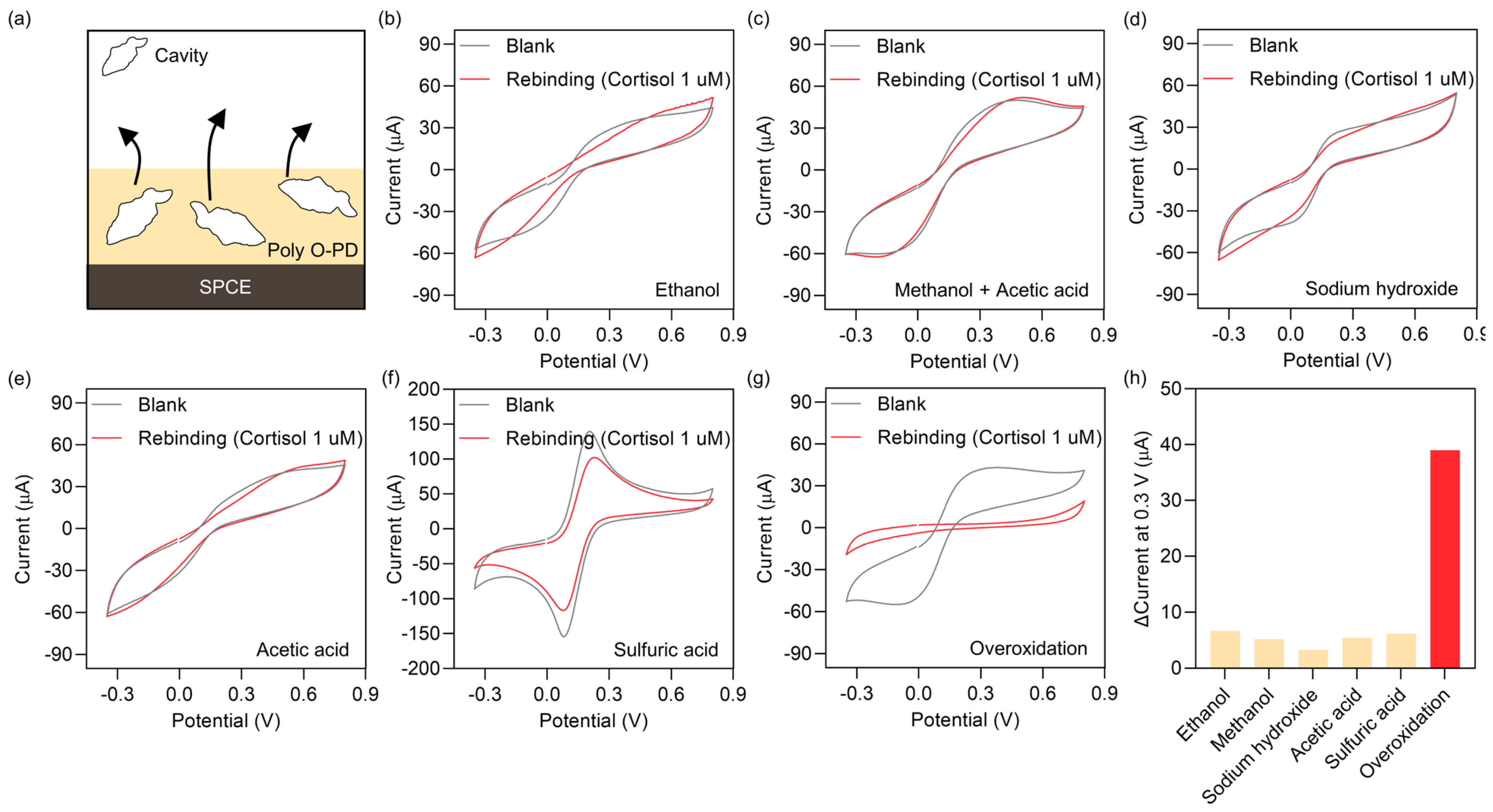
3.3. Template Removal Parameters Optimization
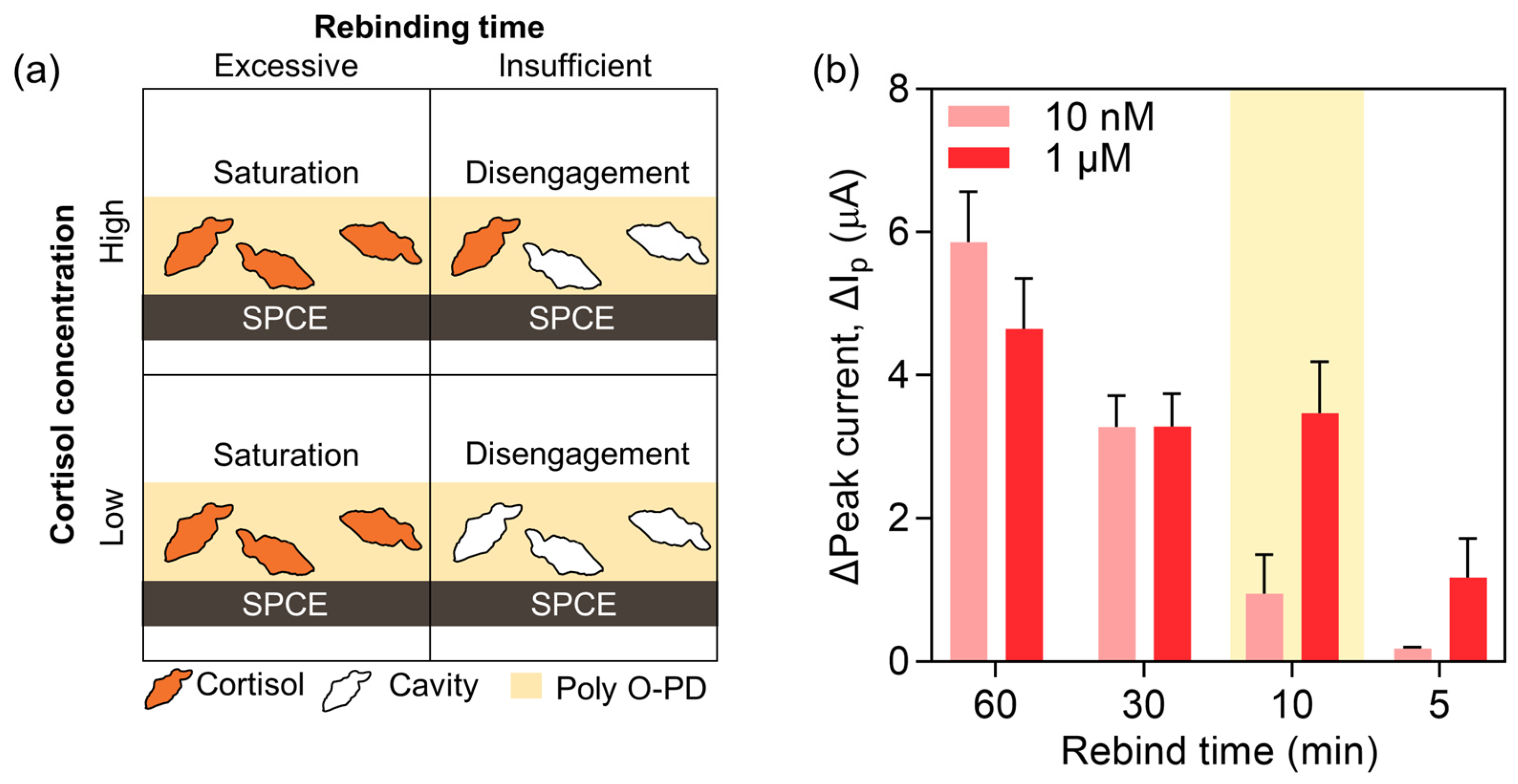
3.4. Rebinding Time Optimization
3.5. Detection of Cortisol Using the MIP Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kar, N.; Kar, B.; Kar, S. Stress and coping during COVID-19 pandemic: Result of an online survey. Psychiatry Res. 2021, 295, 113598. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E. Chronic stress and immunosenescence: A review. Neuroimmunomodulation 2008, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Frey, F.J.; Odermatt, A.; Frey, B.M. Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr. Opin. Nephrol. Hypertens. 2004, 13, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Saiah, E. The role of 11beta-hydroxysteroid dehydrogenase in metabolic disease and therapeutic potential of 11beta-hsd1 inhibitors. Curr. Med. Chem. 2008, 15, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.B.; O’Callaghan, J.P. Neuroendocrine aspects of the response to stress. Metab. Clin. Exp. 2002, 51, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355. [Google Scholar] [CrossRef]
- Ruder, H.J.; Guy, R.L.; Lipsett, M.B. A radioimmunoassay for cortisol in plasma and urine. J. Clin. Endocrinol. Metab. 1972, 35, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Elder, P. An enzyme-linked immunosorbent assay (ELISA) for plasma cortisol. J. Steroid Biochem. 1985, 22, 673–676. [Google Scholar] [CrossRef]
- Fiore, L.; Mazzaracchio, V.; Serani, A.; Fabiani, G.; Fabiani, L.; Volpe, G.; Moscone, D.; Bianco, G.M.; Occhiuzzi, C.; Marrocco, G. Microfluidic paper-based wearable electrochemical biosensor for reliable cortisol detection in sweat. Sens. Actuators B Chem. 2023, 379, 133258. [Google Scholar] [CrossRef]
- Taylor, R.L.; Machacek, D.; Singh, R.J. Validation of a high-throughput liquid chromatography–tandem mass spectrometry method for urinary cortisol and cortisone. Clin. Chem. 2002, 48, 1511–1519. [Google Scholar] [CrossRef]
- Decker Soares, D.R.; Antunes, M.V.; Linden, R. Determination of cortisol in hair using liquid chromatography-tandem mass spectrometry: A short review. Bioanalysis 2021, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Sharma, B. Direct surface enhanced Raman spectroscopic detection of cortisol at physiological concentrations. Anal. Chem. 2019, 92, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Z.; Zhang, Y.; Song, Y.; Yang, H.; Wang, F. Molecularly imprinted Monolithic column-based SERS sensor for selective detection of cortisol in dog saliva. Talanta 2022, 249, 123609. [Google Scholar] [CrossRef]
- Jo, S.; Lee, W.; Park, J.; Kim, W.; Kim, W.; Lee, G.; Lee, H.-J.; Hong, J.; Park, J. Localized surface plasmon resonance aptasensor for the highly sensitive direct detection of cortisol in human saliva. Sens. Actuators B Chem. 2020, 304, 127424. [Google Scholar] [CrossRef]
- Nan, M.; Darmawan, B.A.; Go, G.; Zheng, S.; Lee, J.; Kim, S.; Lee, T.; Choi, E.; Park, J.-O.; Bang, D. Wearable Localized Surface Plasmon Resonance-Based Biosensor with Highly Sensitive and Direct Detection of Cortisol in Human Sweat. Biosensors 2023, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Pali, M.; Garvey, J.E.; Small, B.; Suni, I.I. Detection of fish hormones by electrochemical impedance spectroscopy and quartz crystal microbalance. Sens. Bio Sens. Res. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Sasaki, Y.; Zhang, Y.; Fan, H.; Ohshiro, K.; Zhou, Q.; Tang, W.; Lyu, X.; Minami, T. Accurate cortisol detection in human saliva by an extended-gate-type organic transistor functionalized with a molecularly imprinted polymer. Sens. Actuators B Chem. 2023, 382, 133458. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, Y.; Dong, W.; Zhao, Y.; Sun, L. Bio-Inspired Imprinting Materials for Biomedical Applications. Adv. Sci. 2022, 9, 2202038. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly imprinted polymers for chemical sensing: A tutorial review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Tang, W.; Yin, L.; Sempionatto, J.R.; Moon, J.M.; Teymourian, H.; Wang, J. Touch-based stressless cortisol sensing. Adv. Mater. 2021, 33, 2008465. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Row, K.H. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Parlak, O.; Keene, S.T.; Marais, A.; Curto, V.F.; Salleo, A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 2018, 4, eaar2904. [Google Scholar] [CrossRef] [PubMed]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, G.; Reynolds, B.; Smith, R.; Zhou, K.; Liu, Y. Electropolymerized Molecularly Imprinted Polymer Synthesis Guided by an Integrated Data-Driven Framework for Cortisol Detection. ACS Appl. Mater. Interfaces 2022, 14, 25972–25983. [Google Scholar] [CrossRef]
- Yang, L.; Wei, W.; Xia, J.; Tao, H.; Yang, P. Capacitive biosensor for glutathione detection based on electropolymerized molecularly imprinted polymer and kinetic investigation of the recognition process. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2005, 17, 969–977. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yang, R.; Li, J.; Qu, L. A highly sensitive and selective electrochemical sensor based on polydopamine functionalized graphene and molecularly imprinted polymer for the 2, 4-dichlorophenol recognition and detection. Talanta 2019, 195, 691–698. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Tan, Y.; Hu, J. Biosensor for the determination of sorbitol based on molecularly imprinted electrosynthesized polymers. Biosens. Bioelectron. 2004, 19, 1513–1519. [Google Scholar] [CrossRef]
- Kazemi, R.; Potts, E.I.; Dick, J.E. Quantifying interferent effects on molecularly imprinted polymer sensors for per-and polyfluoroalkyl substances (PFAS). Anal. Chem. 2020, 92, 10597–10605. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zhao, F.; Zhang, W.; Ye, Z. Molecularly imprinted polymer-based sensors for atrazine detection by electropolymerization of o-phenylenediamine. RSC Adv. 2015, 5, 56534–56540. [Google Scholar] [CrossRef]
- Sayyah, S.; El-Deeb, M.; Kamal, S.; Azooz, R. Electropolymerization of o-phenylenediamine on Pt-electrode from aqueous acidic solution: Kinetic, mechanism, electrochemical studies and characterization of the polymer obtained. J. Appl. Polym. Sci. 2009, 112, 3695–3706. [Google Scholar] [CrossRef]
- Lorenzo, R.A.; Carro, A.M.; Alvarez-Lorenzo, C.; Concheiro, A. To remove or not to remove? The challenge of extracting the template to make the cavities available in molecularly imprinted polymers (MIPs). Int. J. Mol. Sci. 2011, 12, 4327–4347. [Google Scholar] [CrossRef] [PubMed]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Q.-J.; Wang, L. Development and characterization of an amperometric sensor for triclosan detection based on electropolymerized molecularly imprinted polymer. Microchem. J. 2009, 91, 222–226. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Karadas-Bakirhan, N.; Taskin-Tok, T.; Ozkan, S.A. A selective and molecular imaging approach for anticancer drug: Pemetrexed by nanoparticle accelerated molecularly imprinting polymer. Electrochim. Acta 2020, 354, 136665. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kaya, S.I.; Corman, M.E.; Karakaya, M.; Atici, E.B.; Ozkan, S.A. A highly sensitive and selective electrochemical sensor based on computer-aided design of molecularly imprinted polymer for the determination of leflunomide. Microchem. J. 2022, 179, 107496. [Google Scholar] [CrossRef]
- Gómez-Caballero, A.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Evaluation of the selective detection of 4, 6-dinitro-o-cresol by a molecularly imprinted polymer based microsensor electrosynthesized in a semiorganic media. Sens. Actuators B Chem. 2008, 130, 713–722. [Google Scholar] [CrossRef]
- Elshafey, R.; Radi, A.-E. Molecularly imprinted copolymer/reduced graphene oxide for the electrochemical detection of herbicide propachlor. J. Appl. Electrochem. 2022, 52, 1761–1771. [Google Scholar] [CrossRef]
- Radi, A.E.; Ragaa Abd-Ellatief, M. Molecularly Imprinted Poly-o-phenylenediamine Electrochemical Sensor for Entacapone. Electroanalysis 2021, 33, 1578–1584. [Google Scholar] [CrossRef]
- Jang, D.H.; Yu, Y.S. Electropolymerization Mechanism for Poly (o-phenylenediamine)(PPD) and Its Electrocatalytic Behavior for O2 Reduction. Bull. Korean Chem. Soc. 1995, 16, 392–397. [Google Scholar]
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. MIP sensors—The electrochemical approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, E.; Yang, X. Capacitive detection of glucose using molecularly imprinted polymers. Biosens. Bioelectron. 2001, 16, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, C.; Wang, C.; Li, T.; Hu, S. The preparation of molecularly imprinted poly (o-phenylenediamine) membranes for the specific O, O-dimethyl-α-hydroxylphenyl phosphonate sensor and its characterization by AC impedance and cyclic voltammetry. J. Appl. Polym. Sci. 2006, 101, 2222–2227. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Ramanavičius, S.; Morkvėnaitė-Vilkončienė, I.; Samukaitė-Bubnienė, U.; Ratautaitė, V.; Plikusienė, I.; Viter, R.; Ramanavičius, A. Electrochemically deposited molecularly imprinted polymer-based sensors. Sensors 2022, 22, 1282. [Google Scholar] [CrossRef]
- Waffo, A.; Yesildag, C.; Caserta, G.; Katz, S.; Zebger, I.; Lensen, M.; Wollenberger, U.; Scheller, F.; Altintas, Z. Fully electrochemical MIP sensor for artemisinin. Sens. Actuators B Chem. 2018, 275, 163–173. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Park, D.; Park, J.; Park, J. Bio-Inspired Molecularly Imprinted Polymer Electrochemical Sensor for Cortisol Detection Based on O-Phenylenediamine Optimization. Biomimetics 2023, 8, 282. https://doi.org/10.3390/biomimetics8030282
Kim M, Park D, Park J, Park J. Bio-Inspired Molecularly Imprinted Polymer Electrochemical Sensor for Cortisol Detection Based on O-Phenylenediamine Optimization. Biomimetics. 2023; 8(3):282. https://doi.org/10.3390/biomimetics8030282
Chicago/Turabian StyleKim, Minwoo, Daeil Park, Joohyung Park, and Jinsung Park. 2023. "Bio-Inspired Molecularly Imprinted Polymer Electrochemical Sensor for Cortisol Detection Based on O-Phenylenediamine Optimization" Biomimetics 8, no. 3: 282. https://doi.org/10.3390/biomimetics8030282






