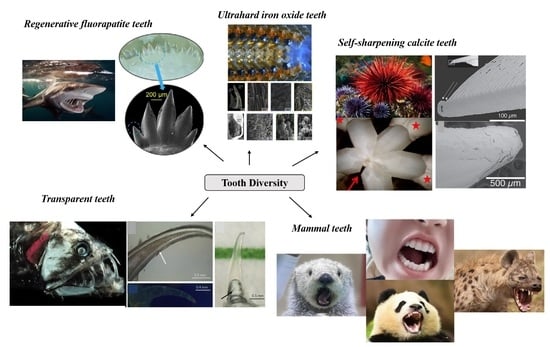
Tooth Diversity Underpins Future Biomimetic Replications
Abstract
:1. Background
2. Tooth Diversity
2.1. Mammal Teeth
2.1.1. Human Teeth
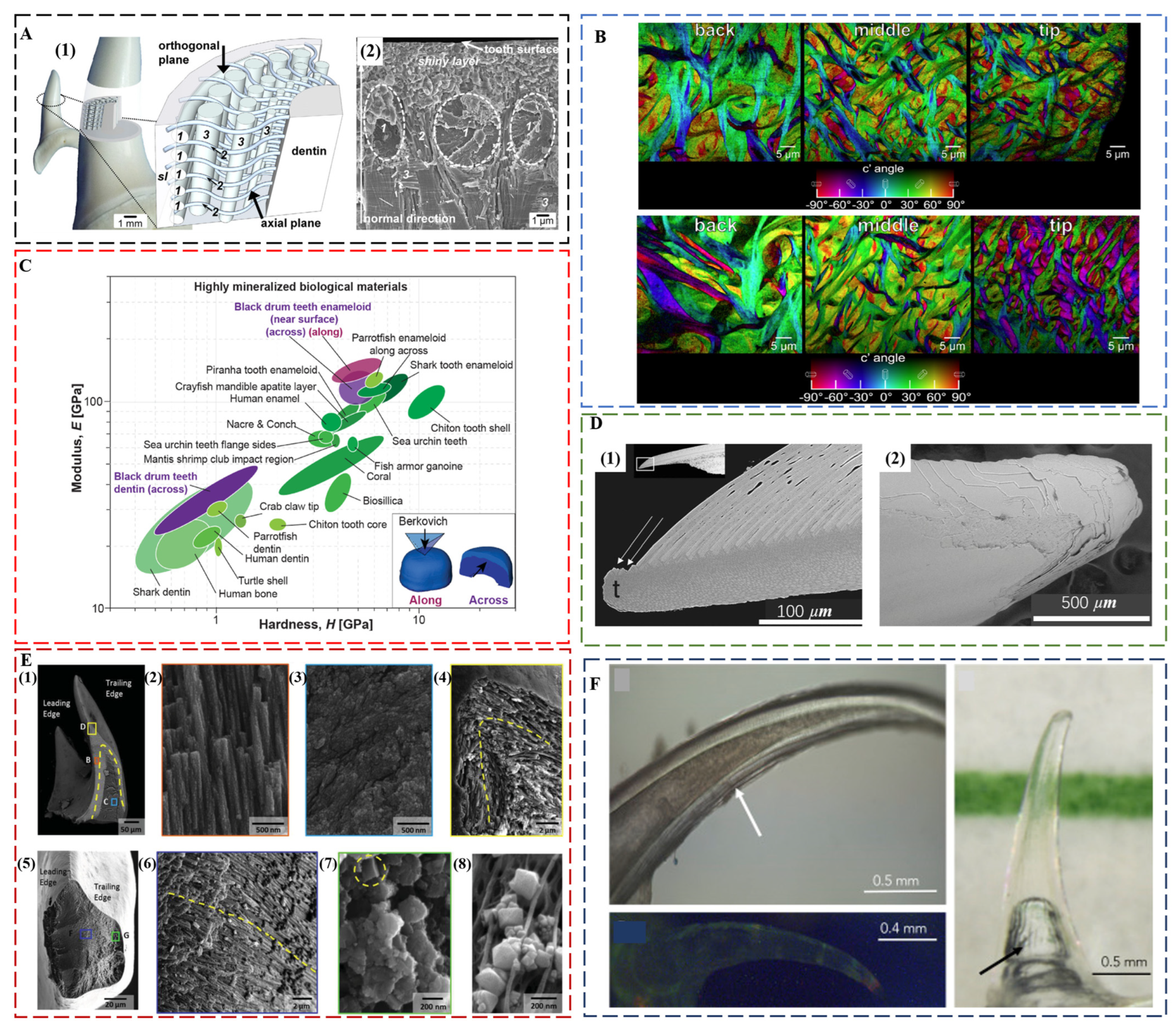
Enamel
Dentin and the Dentin–Enamel Junction (DEJ)
2.1.2. Other Mammal Teeth

2.2. Aquatic Animal Teeth
2.2.1. Fluorapatite Teeth
Shark Enameloid
Parrotfish Enameloid
Black Drum Fish Enameloid
Crayfish Mandible
2.2.2. Self-Sharpening Teeth
Calcite Teeth in Sea Urchin
Iron Oxide Teeth in Chiton and Limpet
2.2.3. Transparent Teeth
An Invisible Weapon for Dragonfish
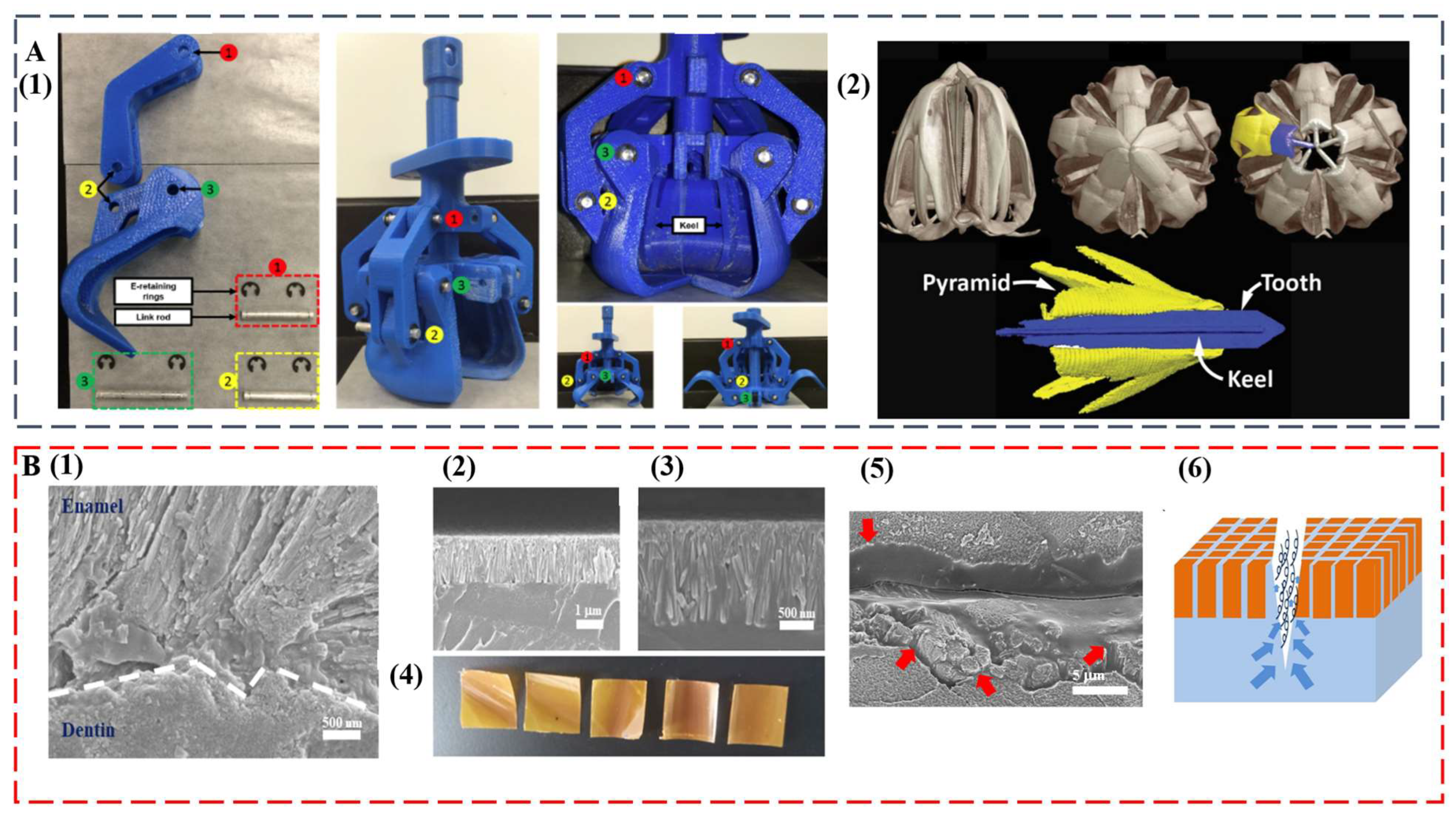
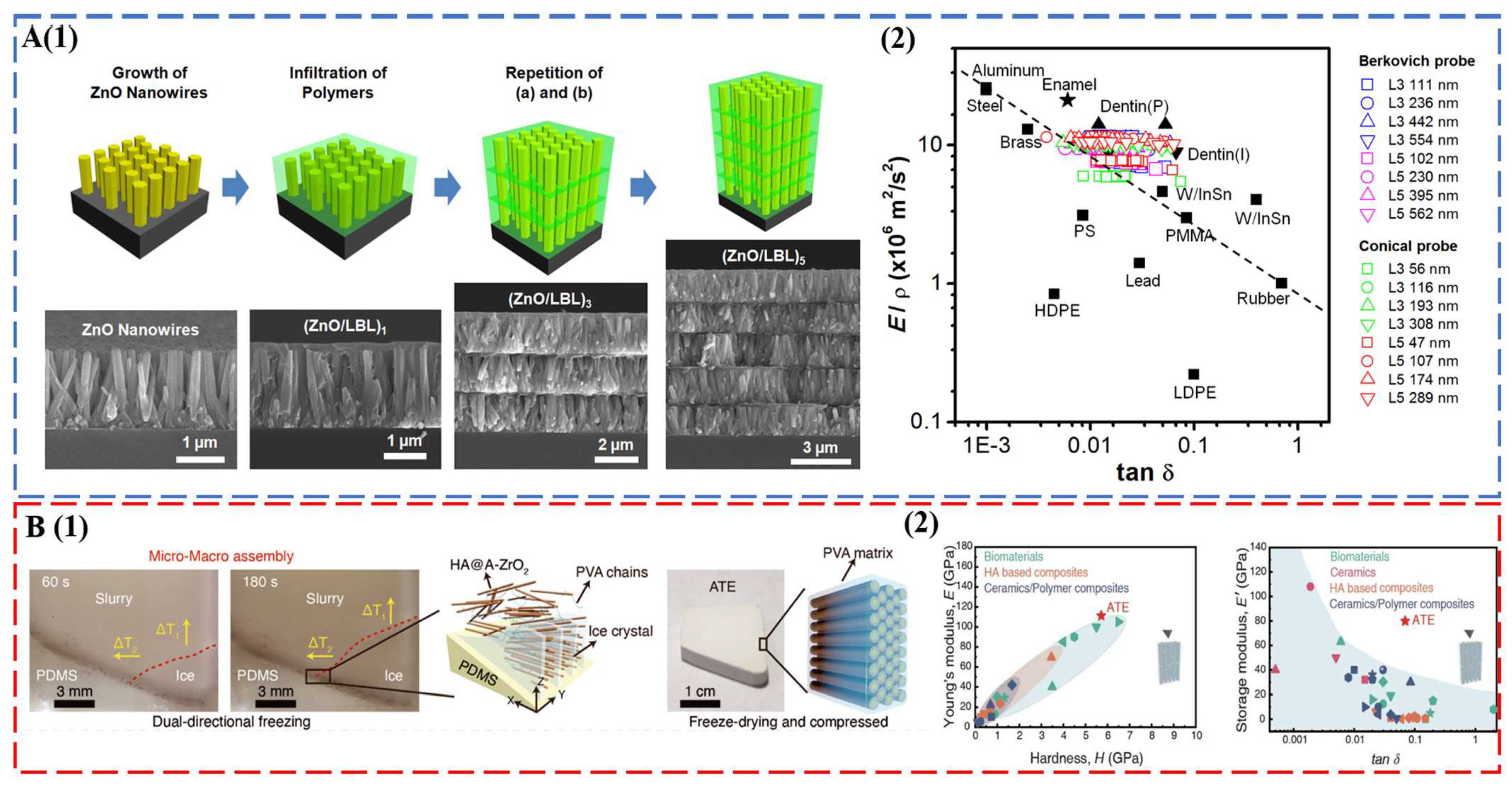
3. Enamel-Inspired Biomimetic Materials
4. Outlook and Perspective
4.1. Hierarchical and Gradient Structures
4.2. Multifunctionalities
4.3. Synthesis Challenge: Precision vs. Scalability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jheon, A.H.; Seidel, K.; Biehs, B.; Klein, O.D. From molecules to mastication: The development and evolution of teeth. Wires Dev. Biol. 2013, 2, 165–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, R.K.M.; LeBlanc, A.R.H.; Berman, D.S.; Reisz, R.R. Dental histology of Coelophysis bauri and the evolution of tooth attachment tissues in early dinosaurs. J. Morphol. 2016, 277, 916–924. [Google Scholar] [CrossRef]
- Higgins, D.; Austin, J.J. Teeth as a source of DNA for forensic identification of human remains: A Review. Sci. Justice 2013, 53, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Rohland, N.; Glocke, I.; Aximu-Petri, A.; Meyer, M. Extraction of highly degraded DNA from ancient bones, teeth and sediments for high-throughput sequencing. Nat. Protoc. 2018, 13, 13. [Google Scholar] [CrossRef]
- Kreier, F. Ancient tooth DNA reveals how ‘cold sore’ herpes virus has evolved. Nature 2022, 609, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.C.S.; Neves, L.X.; Pessoa-Lima, C.; Langer, M.C.; Domont, G.B.; Line, S.R.P.; Leme, A.F.P.; Gerlach, R.F. Ancient enamel peptides recovered from the South American Pleistocene species Notiomastodon platensis and Myocastor cf. coypus. J. Proteom. 2021, 240, 104187. [Google Scholar] [CrossRef]
- McCollum, M.; Sharpe, P.T. Evolution and development of teeth. J. Anat. 2001, 199, 153–159. [Google Scholar] [CrossRef]
- Bokor, J.; Broo, J.; Mahoney, J. Using Fossil Teeth to Study the Evolution of Horses in Response to a Changing Climate. Am. Biol. Teach. 2016, 78, 163–169. [Google Scholar] [CrossRef]
- Bechtle, S.; Ozcoban, H.; Lilleodden, E.T.; Huber, N.; Schreyer, A.; Swain, M.V.; Schneider, G.A. Hierarchical flexural strength of enamel: Transition from brittle to damage-tolerant behaviour. J. R Soc. Interface 2012, 9, 1265–1274. [Google Scholar] [CrossRef]
- He, L.H.; Swain, M.V. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J. Mech. Behav. Biomed. Mater. 2008, 1, 18–29. [Google Scholar] [CrossRef]
- He, L.H.; Swain, M.V. Nanoindentation creep behavior of human enamel. J. Biomed. Mater. Res. A 2009, 91A, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Munch, E.; Launey, M.E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Tough, Bio-Inspired Hybrid Materials. Science 2008, 322, 1516–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidoni, G.; Swain, M.; Jager, I. Enamel: From brittle to ductile like tribological response. J. Dent. 2008, 36, 786–794. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Swain, M.V. Enamel—A “metallic-like” deformable biocomposite. J. Dent. 2007, 35, 431–437. [Google Scholar] [CrossRef]
- Park, S.; Wang, D.H.; Dongsheng, Z.; Romberg, E.; Arola, D. Mechanical properties of human enamel as a function of age and location in the tooth. J. Mater. Sci.-Mater. Med. 2008, 19, 2317–2324. [Google Scholar] [CrossRef]
- Cuy, J.L.; Mann, A.B.; Livi, K.J.; Teaford, M.F.; Weihs, T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 2002, 47, 281–291. [Google Scholar] [CrossRef]
- DeRocher, K.A.; Smeets, P.J.M.; Goodge, B.H.; Zachman, M.J.; Balachandran, P.V.; Stegbauer, L.; Cohen, M.J.; Gordon, L.M.; Rondinelli, J.M.; Kourkoutis, L.F.; et al. Chemical gradients in human enamel crystallites (vol 47, pg 511, 2020). Nature 2020, 584, E3. [Google Scholar] [CrossRef]
- Yahyazadehfar, M.; Arola, D. The role of organic proteins on the crack growth resistance of human enamel. Acta Biomater. 2015, 19, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Gordon, L.M.; Cohen, M.J.; MacRenaris, K.W.; Pasteris, J.D.; Seda, T.; Joester, D. Amorphous intergranular phases control the properties of rodent tooth enamel. Science 2015, 347, 746–750. [Google Scholar] [CrossRef] [Green Version]
- Lucas, P.W. Mammal teeth: Origin, evolution, and diversity. Am. J. Phys. Anthropol. 2012, 147, 678. [Google Scholar] [CrossRef]
- Dorka, M.; Heinrich, W.D. Tetrapod teeth from a Rhaetian (Upper Triassic) bonebed near Friedland (NW-Germany). Palaeontogr. Abt. A 2006, 278, 1–13. [Google Scholar] [CrossRef]
- Fraser, G.J.; Standing, A.; Underwood, C.; Thiery, A.P. The Dental Lamina: An Essential Structure for Perpetual Tooth Regeneration in Sharks. Integr. Comp. Biol. 2020, 60, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Finger, J.W.; Thomson, P.C.; Isberg, S.R. A pilot study to understand tooth replacement in near-harvest farmed saltwater crocodiles (Crocodylus porosus): Implications for blemish induction. Aquaculture 2019, 504, 102–106. [Google Scholar] [CrossRef]
- Jernvall, J.; Thesleff, I. Tooth shape formation and tooth renewal: Evolving with the same signals. Development 2012, 139, 3487–3497. [Google Scholar] [CrossRef] [Green Version]
- Imbeni, V.; Kruzic, J.J.; Marshall, G.W.; Marshall, S.J.; Ritchie, R.O. The dentin-enamel junction and the fracture of human teeth. Nat. Mater. 2005, 4, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Pyenson, N.D.; Koch, P.L. Oh, the shark has such teeth: Did megatooth sharks play a larger role in prehistoric food webs? Sci. Adv. 2022, 8, eadd2674. [Google Scholar] [CrossRef]
- Goetz, A.J.; Griesshaber, E.; Abel, R.; Fehr, T.; Ruthensteiner, B.; Schmahl, W.W. Tailored order: The mesocrystalline nature of sea urchin teeth. Acta Biomater. 2014, 10, 3885–3898. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.Y.; Wang, S.N.; Qu, S.X.; Wang, X.X. Microstructure and self-sharpening of the magnetite cap in chiton tooth. Mat Sci. Eng. C-Mater. 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Gordon, L.M.; Roman, J.K.; Everly, R.M.; Cohen, M.J.; Wilker, J.J.; Joester, D. Selective Formation of Metastable Ferrihydrite in the Chiton Tooth. Angew Chem. Int. Ed. 2014, 53, 11506–11509. [Google Scholar] [CrossRef]
- Grunenfelder, L.K.; de Obaldia, E.E.; Wang, Q.Q.; Li, D.S.; Weden, B.; Salinas, C.; Wuhrer, R.; Zavattieri, P.; Kisailus, D. Stress and Damage Mitigation from Oriented Nanostructures within the Radular Teeth of Cryptochiton stelleri. Adv. Funct. Mater. 2014, 24, 6093–6104. [Google Scholar] [CrossRef]
- Mu, Y.; Tian, R.; Xiao, L.; Sun, D.; Zhang, Z.; Xu, S.; Yang, G. Molecular Evolution of Tooth-Related Genes Provides New Insights into Dietary Adaptations of Mammals. J. Mol. Evol. 2021, 89, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Rivals, F.; Takatsuki, S. Within-island local variations in tooth wear of sika deer (Cervus nippon centralis) in northern Japan. Mamm. Biol. 2015, 80, 333–339. [Google Scholar] [CrossRef]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Wegst, U.G.K.; Ashby, M.F. The mechanical efficiency of natural materials. Philos. Mag. 2004, 84, 2167–2181. [Google Scholar] [CrossRef]
- Launey, M.E.; Ritchie, R.O. On the Fracture Toughness of Advanced Materials. Adv. Mater. 2009, 21, 2103–2110. [Google Scholar] [CrossRef]
- Ji, B.H.; Gao, H.J. Mechanical Principles of Biological Nanocomposites. Annu. Rev. Mater. Res. 2010, 40, 77–100. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yu, T.; Yang, T.Y.; Zheng, L.X.; Liao, K. Bio-Inspired Nacre-like Composite Films Based on Graphene with Superior Mechanical, Electrical, and Biocompatible Properties. Adv. Mater. 2012, 24, 3426–3431. [Google Scholar] [CrossRef]
- Mao, L.B.; Gao, H.L.; Yao, H.B.; Liu, L.; Colfen, H.; Liu, G.; Chen, S.M.; Li, S.K.; Yan, Y.X.; Liu, Y.Y.; et al. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Ye, Z.; Natan, A.; Ma, Y.; Li, H.Y.; Chen, Y.; Wan, L.Q.; Aparicio, C.; Zhu, H.L. Bone-Inspired Mineralization with Highly Aligned Cellulose Nanofibers as Template. ACS Appl. Mater. Inter 2019, 11, 42486–42495. [Google Scholar] [CrossRef]
- Yin, Z.; Hannard, F.; Barthelat, F. Impact-resistant nacre-like transparent materials. Science 2019, 364, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Kaushik, A.K.; Arruda, E.M.; Waas, A.M.; Shim, B.S.; Xu, J.D.; Nandivada, H.; Pumplin, B.G.; Lahann, J.; Ramamoorthy, A.; et al. Ultrastrong and stiff layered polymer nanocomposites. Science 2007, 318, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Yeom, B.; Sain, T.; Lacevic, N.; Bukharina, D.; Cha, S.H.; Waas, A.M.; Arruda, E.M.; Kotov, N.A. Abiotic tooth enamel. Nature 2017, 543, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.J.; Ping, H.; Xie, J.J.; Zou, Z.Y.; Wang, K.; Xie, H.; Wang, W.M.; Lei, L.W.; Fu, Z.Y. Bioprocess-Inspired Microscale Additive Manufacturing of Multilayered TiO2/Polymer Composites with Enamel-Like Structures and High Mechanical Properties. Adv. Funct. Mater. 2020, 30, 1904880. [Google Scholar] [CrossRef]
- Zhao, H.W.; Liu, S.J.; Wei, Y.; Yue, Y.H.; Gao, M.R.; Li, Y.B.; Zeng, X.L.; Deng, X.L.; Kotov, N.A.; Guo, L.; et al. Multiscale engineered artificial tooth enamel. Science 2022, 375, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; An, B.B.; Yahyazadehfar, M.; Zhang, D.; Arola, D.D. Contact fatigue of human enamel: Experiments, mechanisms and modeling. J. Mech. Behav. Biomed. Mater. 2016, 60, 438–450. [Google Scholar] [CrossRef]
- Shen, L.; de Sousa, F.B.; Tay, N.; Lang, T.S.; Kaixin, V.L.; Han, J.; Kilpatrick-Liverman, L.; Wang, W.; Lavender, S.; Pilch, S.; et al. Deformation behavior of normal human enamel: A study by nanoindentation. J. Mech. Behav. Biomed. 2020, 108, 103799. [Google Scholar] [CrossRef]
- Bajaj, D.; Arola, D.D. On the R-curve behavior of human tooth enamel. Biomaterials 2009, 30, 4037–4046. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, D.; Park, S.; Quinn, G.D.; Arola, D. Fracture Processes and Mechanisms of Crack Growth Resistance in Human Enamel. JOM-US 2010, 62, 76–82. [Google Scholar] [CrossRef]
- Cui, F.Z.; Ge, J. New observations of the hierarchical structure of human enamel, from nanoscale to microscale. J. Tissue Eng. Regen. Med. 2007, 1, 185–191. [Google Scholar] [CrossRef]
- Maas, M.C.; Dumont, E.R. Built to last: The structure, function, and evolution of primate dental enamel. Evol. Anthropol. 1999, 8, 133–152. [Google Scholar] [CrossRef]
- Bajaj, D.; Arola, D. Role of prism decussation on fatigue crack growth and fracture of human enamel. Acta Biomater. 2009, 5, 3045–3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborn, J.W. The nature of the Hunter-Schreger bands in enamel. Arch. Oral Biol. 1965, 10, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.D.; O’Sullivan, V.R.; Dockery, P.; McGillycuddy, C.T.; Sloan, A.J. Hunter-Schreger Band patterns in human tooth enamel. J. Anat. 2010, 217, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Risnes, S. Growth tracks in dental enamel. J. Hum. Evol. 1998, 35, 331–350. [Google Scholar] [CrossRef]
- Farah, R.A.; Swain, M.V.; Drummond, B.K.; Cook, R.; Atieh, M. Mineral density of hypomineralised enamel. J. Dent. 2010, 38, 50–58. [Google Scholar] [CrossRef]
- Zhao, H.W.; Liu, S.J.; Yang, X.Y.; Guo, L. Role of Inorganic Amorphous Constituents in Highly Mineralized Biomaterials and Their Imitations. ACS Nano 2022, 16, 11. [Google Scholar] [CrossRef]
- Angker, L.; Swain, M.V. Nanoindentation: Application to dental hard tissue investigations. J. Mater. Res. 2006, 21, 1893–1905. [Google Scholar] [CrossRef]
- Yan, J.H.; Taskonak, B.; Platt, J.A.; Mecholsky, J.J. Evaluation of fracture toughness of human dentin using elastic-plastic fracture mechanics. J. Biomech. 2008, 41, 1253–1259. [Google Scholar] [CrossRef]
- Chan, Y.L.; Ngan, A.H.W.; King, N.M. Nano-scale structure and mechanical properties of the human dentine-enamel junction. J. Mech. Behav. Biomed. Mater. 2011, 4, 785–795. [Google Scholar] [CrossRef]
- Weng, Z.Y.; Liu, Z.Q.; Ritchie, R.O.; Jiao, D.; Li, D.S.; Wu, H.L.; Deng, L.H.; Zhang, Z.F. Giant panda’s tooth enamel: Structure, mechanical behavior and toughening mechanisms under indentation. J. Mech. Behav. Biomed. 2016, 64, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, X.; Xiang, W.S.; Zhong, Y.J.; Yan, F.X.; Jiang, B.L. Hierarchy structure and fracture mechanisms of the wild wolf tusk’s enamel. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 106, 110277. [Google Scholar] [CrossRef] [PubMed]
- Arango-Santander, S.; Montoya, C.; Pelaez-Vargas, A.; Ossa, E.A. Chemical, structural and mechanical characterization of bovine enamel. Arch. Oral Biol. 2020, 109, 104573. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, N.; Zhong, Y.J.; Yan, F.X.; Jiang, B.L. Wild boar’s tusk enamel: Structure and mechanical behavior. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 100, 354–362. [Google Scholar] [CrossRef]
- Xiao, H.; Lei, L.; Peng, J.P.; Yang, D.; Zeng, Q.H.; Zheng, J.; Zhou, Z.R. Research of the role of microstructure in the wear mechanism of canine and bovine enamel. J. Mech. Behav. Biomed. 2019, 92, 33–39. [Google Scholar] [CrossRef]
- O’Brien, S.; Keown, A.J.; Constantino, P.; Xie, Z.H.; Bush, M.B. Revealing the structural and mechanical characteristics of ovine teeth. J. Mech. Behav. Biomed. 2014, 30, 176–185. [Google Scholar] [CrossRef]
- Ortiz-Ruiz, A.J.; Teruel-Fernandez, J.D.; Alcolea-Rubio, L.A.; Hernandez-Fernandez, A.; Martinez-Beneyto, Y.; Gispert-Guirado, F. Structural differences in enamel and dentin in human, bovine, porcine, and ovine teeth. Ann. Anat. 2018, 218, 7–17. [Google Scholar] [CrossRef]
- Ziscovici, C.; Lucas, P.W.; Constantino, P.J.; Bromage, T.G.; van Casteren, A. Sea otter dental enamel is highly resistant to chipping due to its microstructure. Biol. Lett. 2014, 10, 20140484. [Google Scholar] [CrossRef] [Green Version]
- Popowics, T.E.; Rensberger, J.M.; Herring, S.W. Enamel microstructure and microstrain in the fracture of human and pig molar cusps. Arch. Oral Biol. 2004, 49, 595–605. [Google Scholar] [CrossRef]
- Wu, Y.H.; Liu, J.X.; Yang, Y.Q.; Tu, S.T.; Liu, Z.C.; Wang, Y.Y.; Peng, C.; Liu, G.; Jin, Y.P. Special architecture and anti-wear strategies for giant panda tooth enamel: Based on wear simulation findings. Front. Vet. Sci. 2022, 9, 985733. [Google Scholar] [CrossRef]
- Rensberger, J.M.; Wang, X. Microstructural Reinforcement in the Canine Enamel of the Hyaenid Crocuta crocuta, the FelidPuma concolorand the Late Miocene Canid Borophagus secundus. J. Mamm. Evol. 2005, 12, 379–403. [Google Scholar] [CrossRef]
- Lee, J.J.W.; Morris, D.; Constantino, P.J.; Lucas, P.W.; Smith, T.M.; Lawn, B.R. Properties of tooth enamel in great apes. Acta Biomater. 2010, 6, 4560–4565. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.Y.S.; Nagelkerken, I.; Pistevos, J.C.A.; Xie, Z.H.; Zhang, S.; Connell, S.D. Shark teeth can resist ocean acidification. Glob. Chang. Biol. 2022, 28, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Enax, J.; Prymak, O.; Raabe, D.; Epple, M. Structure, composition, and mechanical properties of shark teeth. J. Struct. Biol. 2012, 178, 290–299. [Google Scholar] [CrossRef]
- Chen, P.Y.; Schirer, J.; Simpson, A.; Nay, R.; Lin, Y.S.; Yang, W.; Lopez, M.I.; Li, J.A.; Olevsky, E.A.; Meyers, M.A. Predation versus protection: Fish teeth and scales evaluated by nanoindentation. J. Mater. Res. 2012, 27, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.F.; Loh, H.C.; Jia, Z.; Stifler, C.A.; Masic, A.; Gilbert, P.U.P.A.; Shahar, R.; Li, L. Black Drum Fish Teeth: Built for Crushing Mollusk Shells. Acta Biomater. 2022, 137, 147–161. [Google Scholar] [CrossRef]
- He, C.; Zhou, W.; Wang, H.T.; Shi, S.Q.; Yao, H.M. Mechanics of Pharyngeal Teeth of Black Carp (Mylopharyngodon piceus) Crushing Mollusk Shells. Adv. Eng. Mater. 2013, 15, 684–690. [Google Scholar] [CrossRef]
- Enax, J.; Janus, A.M.; Raabe, D.; Epple, M.; Fabritius, H.O. Ultrastructural organization and micromechanical properties of shark tooth enameloid. Acta Biomater. 2014, 10, 3959–3968. [Google Scholar] [CrossRef]
- Enault, S.; Guinot, G.; Koot, M.B.; Cuny, G. Chondrichthyan tooth enameloid: Past, present, and future. Zool. J. Linn. Soc.-Lond. 2015, 174, 549–570. [Google Scholar] [CrossRef]
- Amini, S.; Razi, H.; Seidel, R.; Werner, D.; White, W.T.; Weaver, J.C.; Dean, M.N.; Fratzl, P. Shape-preserving erosion controlled by the graded microarchitecture of shark tooth enameloid. Nat. Commun. 2020, 11, 5971. [Google Scholar] [CrossRef]
- Marcus, M.A.; Amini, S.; Stifler, C.A.; Sun, C.-Y.; Tamura, N.; Bechtel, H.A.; Parkinson, D.Y.; Barnard, H.S.; Zhang, X.X.X.; Chua, J.Q.I.; et al. Parrotfish Teeth: Stiff Biominerals Whose Microstructure Makes Them Tough and Abrasion-Resistant To Bite Stony Corals. ACS Nano 2017, 11, 11856–11865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentov, S.; Zaslansky, P.; Al-Sawalmih, A.; Masic, A.; Fratzl, P.; Sagi, A.; Berman, A.; Aichmayer, B. Enamel-like apatite crown covering amorphous mineral in a crayfish mandible. Nat. Commun. 2012, 3, 839. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Cohen, S.R.; Addadi, L.; Weiner, S. Sea urchin tooth design: An “All-Calcite” polycrystalline reinforced fiber composite for grinding rocks. Adv. Mater. 2008, 20, 1555–1559. [Google Scholar] [CrossRef]
- Killian, C.E.; Metzler, R.A.; Gong, Y.T.; Churchill, T.H.; Olson, I.C.; Trubetskoy, V.; Christensen, M.B.; Fournelle, J.H.; De Carlo, F.; Cohen, S.; et al. Self-Sharpening Mechanism of the Sea Urchin Tooth. Adv. Funct. Mater. 2011, 21, 682–690. [Google Scholar] [CrossRef]
- Ma, Y.R.; Aichmayer, B.; Paris, O.; Fratzl, P.; Meibom, A.; Metzler, R.A.; Politi, Y.; Addadi, L.; Gilbert, P.U.P.A.; Weiner, S. The grinding tip of the sea urchin tooth exhibits exquisite control over calcite crystal orientation and Mg distribution. Proc. Natl. Acad. Sci. USA 2009, 106, 6048–6053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, H.D.; Zaheri, A.; Nguyen, H.; Restrepo, D.; Daly, M.; Frank, M.; McKittrick, J. In situ Wear Study Reveals Role of Microstructure on Self-Sharpening Mechanism in Sea Urchin Teeth. Matter-US 2019, 1, 1246–1261. [Google Scholar] [CrossRef]
- Weaver, J.C. Analysis of an ultra hard magnetic biomineral in chiton radular teeth (vol 13, pg 42, 2010). Mater. Today 2010, 13, 13. [Google Scholar] [CrossRef]
- Faivre, D.; Godec, T.U. From Bacteria to Mollusks: The Principles Underlying the Biomineralization of Iron Oxide Materials. Angew. Chem. Int. Ed. 2015, 54, 4728–4747. [Google Scholar] [CrossRef]
- Barber, A.H.; Lu, D.; Pugno, N.M. Extreme strength observed in limpet teeth. J. R Soc. Interface 2015, 12, 20141326. [Google Scholar] [CrossRef] [Green Version]
- Velasco-Hogan, A.; Deheyn, D.D.; Koch, M.; Nothdurft, B.; Arzt, E.; Meyers, M.A. On the Nature of the Transparent Teeth of the Deep-Sea Dragonfish, Aristostomias scintillans. Matter-US 2019, 1, 235–249. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, Y.F.; Yu, T.T.; Peng, L.Y.; Sun, Q.N.; Han, B. Engineered Fabrication of Enamel-Mimetic Materials. Engineering 2022, 14, 113–123. [Google Scholar] [CrossRef]
- Zhao, H.W.; Liu, S.J.; Lu, J.F.; Yang, X.Y.; Yang, Z.; Li, F.S.; Guo, L. Natural tooth enamel and its analogs. Cell Rep. Phys. Sci. 2022, 3, 100945. [Google Scholar] [CrossRef]
- Wong, H.M.; Zhang, Y.Y.; Li, Q.L. An enamel-inspired bioactive material with multiscale structure and antibacterial adhesion property. Bioact Mater. 2022, 7, 491–503. [Google Scholar] [CrossRef] [PubMed]
- de Obaldia, E.E.; Jeong, C.; Grunenfelder, L.K.; Kisailus, D.; Zavattieri, P. Analysis of the mechanical response of biomimetic materials with highly oriented microstructures through 3D printing, mechanical testing and modeling. J. Mech. Behav. Biomed. Mater. 2015, 48, 70–85. [Google Scholar] [CrossRef]
- Le Ferrand, H.; Bouville, F.; Niebel, T.P.; Studart, A.R. Magnetically assisted slip casting of bioinspired heterogeneous composites. Nat. Mater. 2015, 14, 1172–1179. [Google Scholar] [CrossRef] [Green Version]
- Stifler, C.A.; Jakes, J.E.; North, J.D.; Green, D.R.; Weaver, J.C.; Gilbert, P.U.P.A. Crystal misorientation correlates with hardness in tooth enamels. Acta Biomater. 2021, 120, 124–134. [Google Scholar] [CrossRef]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P.U.P.A. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.B.; Naleway, S.E.; Wirth, T.S.; Jung, J.Y.; Cheung, C.L.; Loera, F.B.; Medina, S.; Sato, K.N.; Taylor, J.R.A.; McKittrick, J. A Protocol for Bioinspired Design: A Ground Sampler Based on Sea Urchin Jaws. JoVE-J. Vis. Exp. 2016, e53554. [Google Scholar] [CrossRef] [Green Version]
- Lew, A.J.; Beniash, E.; Gilbert, P.U.P.A.; Buehler, M.J. Role of the Mineral in the Self-Healing of Cracks in Human Enamel. ACS Nano 2022, 16, 8. [Google Scholar] [CrossRef]
- Chai, H.; Lee, J.J.W.; Constantino, P.J.; Lucas, P.W.; Lawn, B.R. Remarkable resilience of teeth. Proc. Natl. Acad. Sci. USA 2009, 106, 7289–7293. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, S.J.; Xiao, Z.H.; Zhao, H.W.; Luo, J.; Deng, X.L.; Guo, L. Enamel Repair with Amorphous Ceramics. Adv. Mater. 2020, 32, 1907067. [Google Scholar] [CrossRef]
- Shao, C.Y.; Jin, B.A.; Mu, Z.; Lu, H.; Zhao, Y.Q.; Wu, Z.F.; Yan, L.M.; Zhang, Z.S.; Zhou, Y.C.; Pan, H.H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Deng, J.J.; Deng, X.L.; Fang, C.Q.; Zhang, X.; Yang, P. Controlling Enamel Remineralization by Amyloid-Like Amelogenin Mimics. Adv. Mater. 2020, 32, 2002080. [Google Scholar] [CrossRef]
- Yan, Y.; Niu, K.; Zhang, W.; Sun, W.; Jiang, Y.; Jiang, Z.; Zhao, L.; Yang, M.; Li, B.; Hou, Y.; et al. A Biomimetic Tooth Replicate That Is Hard, Damage-Tolerant, and Self-Healable. CCS Chem. 2023, 5, 95–105. [Google Scholar] [CrossRef]
- Yang, T.; Chen, H.S.; Jia, Z.A.; Deng, Z.F.; Chen, L.N.; Peterman, E.M.; Weaver, J.C.; Li, L. A damage-tolerant, dual-scale, single-crystalline microlattice in the knobby starfish, Protoreaster nodosus. Science 2022, 375, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Keten, S.; Xu, Z.P.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nepal, D.; Kang, S.; Adstedt, K.M.; Kanhaiya, K.; Bockstaller, M.R.; Brinson, L.C.; Buehler, M.J.; Coveney, P.V.; Dayal, K.; El-Awady, J.A.; et al. Hierarchically structured bioinspired nanocomposites. Nat. Mater. 2022, 22, 18–35. [Google Scholar] [CrossRef]
- Velasco-Hogan, A.; Huang, W.; Serrano, C.; Kisailus, D.; Meyers, M.A. Tooth structure, mechanical properties, and diet specialization of Piranha and Pacu (Serrasalmidae): A comparative study. Acta Biomater. 2021, 134, 531–545. [Google Scholar] [CrossRef]
- Mirmohammadi, S.A.; Pasini, D.; Barthelat, F. Modeling, design and tailoring of a tough, strong and stiff multilayered bone graft material. J. Mech. Behav. Biomed. Mater. 2022, 134, 105369. [Google Scholar] [CrossRef]
- Yourdkhani, M.; Pasini, D.; Barthelat, F. Multiscale Mechanics and Optimization of Gastropod Shells. J. Bionic Eng. 2011, 8, 357–368. [Google Scholar] [CrossRef]
- Rivera, J.; Murata, S.; Hosseini, M.S.; Trikanad, A.A.; James, R.; Pickle, A.; Yaraghi, N.; Matsumoto, N.; Yang, W.; Parkinson, D.Y.; et al. Structural Design Variations in Beetle Elytra. Adv. Funct. Mater. 2021, 31, 3189–3197. [Google Scholar] [CrossRef]
- Gu, G.X.; Chen, C.T.; Richmond, D.J.; Buehler, M.J. Bioinspired hierarchical composite design using machine learning: Simulation, additive manufacturing, and experiment. Mater. Horiz. 2018, 5, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Bastek, J.H.; Kumar, S.; Telgen, B.; Glaesener, R.N.; Kochmann, D.M. Inverting the structure-property map of truss metamaterials by deep learning. Proc. Natl. Acad. Sci. USA 2022, 119, e2111505119. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.D.; Zhang, D.; Ma, Z.B.; Cao, W.X.; Hou, Y.; Zhu, J.Q.; Gan, Y.; Yang, M. An Epidermis-like Hierarchical Smart Coating with a Hardness of Tooth Enamel. ACS Nano 2018, 12, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.D.; Hou, Y.; Yang, M. Scalable Synthesis of Multifunctional Epidermis-Like Smart Coatings. Adv. Funct. Mater. 2019, 29, 1903984. [Google Scholar] [CrossRef]
- Elder, B.; Neupane, R.; Tokita, E.; Ghosh, U.; Hales, S.; Kong, Y.L. Nanomaterial Patterning in 3D Printing. Adv. Mater. 2020, 32, 1907142. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Han, S.; Yang, M. Tooth Diversity Underpins Future Biomimetic Replications. Biomimetics 2023, 8, 42. https://doi.org/10.3390/biomimetics8010042
Wang D, Han S, Yang M. Tooth Diversity Underpins Future Biomimetic Replications. Biomimetics. 2023; 8(1):42. https://doi.org/10.3390/biomimetics8010042
Chicago/Turabian StyleWang, Di, Shuangxia Han, and Ming Yang. 2023. "Tooth Diversity Underpins Future Biomimetic Replications" Biomimetics 8, no. 1: 42. https://doi.org/10.3390/biomimetics8010042