Biomimetic Self-Assembled Chiral Inorganic Nanomaterials: A New Strategy for Solving Medical Problems
Abstract
:1. Introduction
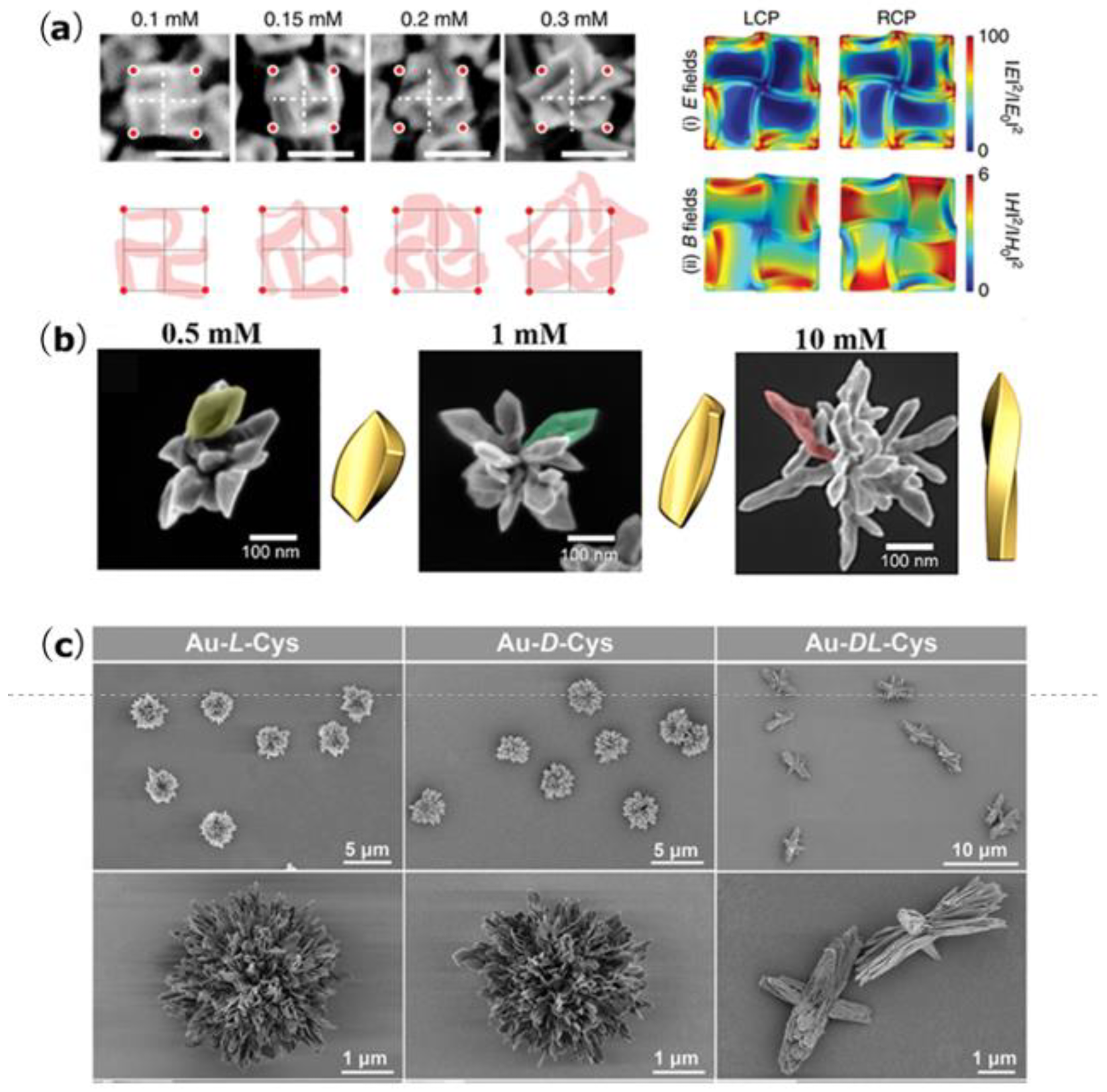
2. Synthesis of Biomimetic Self-Assembled Chiral Structures
3. Medical Applications of Biomimetic Chiral Self-Assembled Particles
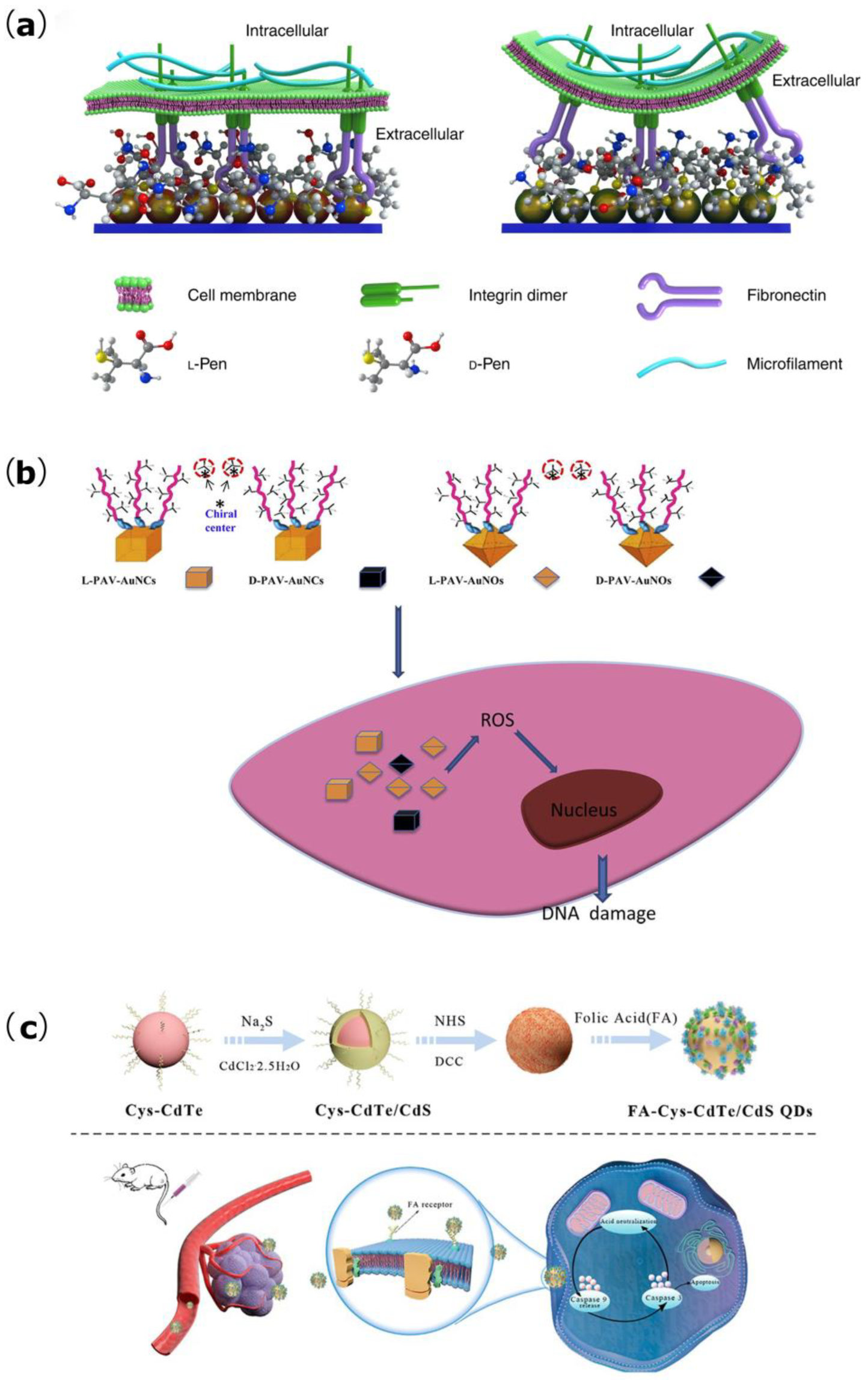
3.1. Effects on Cell Adhesion, Proliferation, and Differentiation
3.2. Molecular Probes
3.3. Tumor Treatment
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kondepudi, D. Chapter 1—Chiral Asymmetry in Nature. In Chiral Analysis, 2nd ed.; Polavarapu, P.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Shen, Z.; Liu, M. Chiral Nanoarchitectonics: Towards the Design, Self-Assembly, and Function of Nanoscale Chiral Twists and Helices. Adv. Mater. 2015, 28, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Cölfen, H. Chirality communications between inorganic and organic compounds. SmartMat 2021, 2, 17–32. [Google Scholar] [CrossRef]
- Ma, L.; Falkowski, J.M.; Abney, C.; Lin, W. A series of isoreticular chiral metal–organic frameworks as a tunable platform for asymmetric catalysis. Nat. Chem. 2010, 2, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yashima, E. Helical Polyacetylenes Induced via Noncovalent Chiral Interactions and Their Applications as Chiral Materials. Top. Curr. Chem. 2017, 375, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, H.; Tsukube, H. Coordination chemistry strategies for dynamic helicates: Time-programmable chirality switching with labile and inert metal helicates. Chem. Soc. Rev. 2012, 41, 6977–6991. [Google Scholar] [CrossRef]
- Ha, C.; Ryu, J.; Park, C.B.J.B. Metal Ions Differentially Influence the Aggregation and Deposition of Alzheimer’s β-Amyloid on a Solid Template. Biochemistry 2007, 46, 6118–6125. [Google Scholar] [CrossRef]
- Wang, F.; Feng, C.-L. Metal-Ion-Mediated Supramolecular Chirality of l -Phenylalanine Based Hydrogels. Angew. Chem. Int. Ed. 2018, 57, 5655–5659. [Google Scholar] [CrossRef]
- Kokan, Z.; Perić, B.; Vazdar, M.; Marinić, Z.; Vikić-Topić, D.; Meštrović, E.; Kirin, S.I. Metal-induced supramolecular chirality inversion of small self-assembled molecules in solution. Chem. Commun. 2017, 53, 1945–1948. [Google Scholar] [CrossRef]
- Liu, C.; Xie, L.; Zhang, R. Ca2+ Mediates the Self-Assembly of the Foot Proteins of Pinctada fucata from the Nanoscale to the Microscale. Biomacromolecules 2016, 17, 3347–3355. [Google Scholar] [CrossRef]
- Bertula, K.; Nonappa; Myllymäki, T.T.; Yang, H.; Zhu, X.; Ikkala, O. Hierarchical self-assembly from nanometric micelles to colloidal spherical superstructures. Polymer 2017, 126, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Mendes, A.C.L.; Baran, E.T.; Reis, R.L.; Azevedo, H.S. Self-assembly in nature: Using the principles of nature to create complex nanobiomaterials. WIREs Nanomed. Nanobiotechnology 2013, 5, 582–612. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P.; O’Brien, E.T. Microtubule Dynamic Instability and GTP Hydrolysis. Annu. Rev. Biophys. Biomol. Struct. 1992, 21, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. The cytoskeleton, cellular motility and the reductionist agenda. Nature 2003, 422, 741–745. [Google Scholar] [CrossRef]
- Ben-Jacob, E.; Cohen, I.; Gutnick, D.L. Cooperative Organization of Bacterial Colonies: From Genotype to Morphotype. Annu. Rev. Microbiol. 1998, 52, 779–806. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Li, P.; Gao, D.; Lyu, B.; Ma, J.; Zhang, J.; Lyu, L. High-efficiency antibacterial and anti-mildew properties under self-assembly: An environmentally friendly nanocomposite. Adv. Powder Technol. 2021, 32, 2433–2440. [Google Scholar] [CrossRef]
- Lee, H.-E.; Kim, R.M.; Ahn, H.-Y.; Lee, Y.Y.; Byun, G.H.; Im, S.W.; Mun, J.; Rho, J.; Nam, K.T. Cysteine-encoded chirality evolution in plasmonic rhombic dodecahedral gold nanoparticles. Nat. Commun. 2020, 11, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dijken, D.J.; Štacko, P.; Stuart, M.C.A.; Browne, W.R.; Feringa, B.L. Chirality controlled responsive self-assembled nanotubes in water. Chem. Sci. 2016, 8, 1783–1789. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-E.; Lee, J.; Ju, M.; Ahn, H.-Y.; Lee, Y.Y.; Jang, H.-S.; Nam, K.T. Identifying peptide sequences that can control the assembly of gold nanostructures. Mol. Syst. Des. Eng. 2018, 3, 581–590. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, X.; Han, L.; Asahina, S.; Xu, D.; Cao, Y.; Yao, Y.; Che, S. Optically Active Chiral CuO “Nanoflowers”. J. Am. Chem. Soc. 2014, 136, 7193–7196. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Xu, J.-X.; Chen, Y.-H.; Lu, I.-C.; Han, J.-L.; Lin, P.-H. Self-assembled lanthanide-based helixes: Synthetic control of the helical handedness by chirality of the ligand. Dalton Trans. 2021, 51, 69–73. [Google Scholar] [CrossRef]
- Zhang, Q.; Hernandez, T.; Smith, K.W.; Jebeli, S.A.H.; Dai, A.X.; Warning, L.; Baiyasi, R.; McCarthy, L.A.; Guo, H.; Chen, D.-H.; et al. Unraveling the origin of chirality from plasmonic nanoparticle-protein complexes. Science 2019, 365, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Qu, Z.-B.; Kumar, P.; Vecchio, D.; Wang, Y.; Ma, Y.; Bahng, J.H.; Bernardino, K.; Gomes, W.R.; Colombari, F.M.; et al. Emergence of complexity in hierarchically organized chiral particles. Science 2020, 368, 642–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Hao, T.; Li, X.; Qu, A.; Xu, L.; Hao, C.; Xu, C.; Kuang, H. Direct observation of selective autophagy induction in cells and tissues by self-assembled chiral nanodevice. Nat. Commun. 2018, 9, 4494. [Google Scholar] [CrossRef] [Green Version]
- Dou, X.; Wu, B.; Liu, J.; Zhao, C.; Qin, M.; Wang, Z.; Schönherr, H.; Feng, C.-L. Effect of Chirality on Cell Spreading and Differentiation: From Chiral Molecules to Chiral Self-Assembly. ACS Appl. Mater. Interfaces 2019, 11, 38568–38577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Q.; Wang, Y.; Hao, D.; Qi, W.; Su, R.; He, Z. Topology-Induced Chiral Amplification and Inversion in Self-Assembling Dipeptide Films. Adv. Mater. Interfaces 2022, 9, 2102089. [Google Scholar] [CrossRef]
- Yan, J.; Yao, Y.; Yan, S.; Gao, R.; Lu, W.; He, W. Chiral Protein Supraparticles for Tumor Suppression and Synergistic Immunotherapy: An Enabling Strategy for Bioactive Supramolecular Chirality Construction. Nano Lett. 2020, 20, 5844–5852. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Dou, X.; Tang, Z.; Zhang, D.; Ni, N.; Wang, J.; Gao, H.; Ju, Y.; Dai, X.; Zhao, C.; et al. Bio-inspired chiral self-assemblies promoted neuronal differentiation of retinal progenitor cells through activation of metabolic pathway. Bioact. Mater. 2020, 6, 990–997. [Google Scholar] [CrossRef]
- Levin, A.; Hakala, T.A.; Schnaider, L.; Bernardes, G.J.L.; Gazit, E.; Knowles, T.P.J. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 2020, 4, 615–634. [Google Scholar] [CrossRef]
- Du, P.; Xu, S.; Xu, Z.; Wang, Z. Bioinspired Self-Assembling Materials for Modulating Enzyme Functions. Adv. Funct. Mater. 2021, 31, 2104819. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, K.; Tian, J.; Liu, C.; Tian, J.; Jiang, F.; Yuan, D.; Zhang, J.; Chen, Q.; Hong, M. Induction of Chirality in a Metal–Organic Framework Built from Achiral Precursors. Angew. Chem. Int. Ed. 2020, 60, 3087–3094. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, E.; Tan, J.; Yang, W.; Kim, B.; Moon, J. A new class of chiral semiconductors: Chiral organic molecule incorporating organic inorganic hybrid perovskites. Mater. Horizons 2017, 4, 851–856. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, X.; Cheng, P.; Jia, X.; Xu, J.; Bu, X.-H. Induction of Chiral Hybrid Metal Halides from Achiral Building Blocks. J. Am. Chem. Soc. 2022, 144, 16471–16479. [Google Scholar] [CrossRef] [PubMed]
- Ben-Moshe, A.; da Silva, A.; Müller, A.; Abu-Odeh, A.; Harrison, P.; Waelder, J.; Niroui, F.; Ophus, C.; Minor, A.M.; Asta, M.; et al. The chain of chirality transfer in tellurium nanocrystals. Science 2021, 372, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Kuang, H.; Wang, L.; Xu, L.; Chang, W.-S.; Zhang, H.; Sun, M.; Zhu, Y.; Zhao, Y.; Liu, L.; et al. Chiral plasmonics of self-assembled nanorod dimers. Sci. Rep. 2013, 3, srep01934. [Google Scholar] [CrossRef] [Green Version]
- Duan, T.; Ai, J.; Duan, Y.; Han, L.; Che, S. Self-Assembly of Chiral Nematic-Like Films with Chiral Nanorods Directed by Chiral Molecules. Chem. Mater. 2021, 33, 6227–6232. [Google Scholar] [CrossRef]
- Zheng, G.; Bao, Z.; Pérez-Juste, J.; Du, R.; Liu, W.; Dai, J.; Zhang, W.; Lee, L.Y.S.; Wong, K.-Y. Tuning the Morphology and Chiroptical Properties of Discrete Gold Nanorods with Amino Acids. Angew. Chem. Int. Ed. 2018, 57, 16452–16457. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Li, Y.; Cheng, Z.; Li, T.; Zhang, H.; Lu, Z.; Yang, B. Self-Assembly of Nanoclusters into Mono-, Few-, and Multilayered Sheets via Dipole-Induced Asymmetric van der Waals Attraction. ACS Nano 2015, 9, 6315–6323. [Google Scholar] [CrossRef]
- Yanagimoto, Y.; Negishi, Y.; Fujihara, H.; Tsukuda, T. Chiroptical Activity of BINAP-Stabilized Undecagold Clusters. J. Phys. Chem. B 2006, 110, 11611–11614. [Google Scholar] [CrossRef]
- Lan, X.; Lu, X.; Shen, C.; Ke, Y.; Ni, W.; Wang, Q. Au Nanorod Helical Superstructures with Designed Chirality. J. Am. Chem. Soc. 2014, 137, 457–462. [Google Scholar] [CrossRef]
- Lee, H.-E.; Ahn, H.-Y.; Mun, J.; Lee, Y.Y.; Kim, M.; Cho, N.H.; Chang, K.; Kim, W.S.; Rho, J.; Nam, K.T. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 2018, 556, 360–365. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, X.; Zhang, H.; Bai, X.; Ai, R.; Shao, L.; Wang, J. Gold Nanorods: The Most Versatile Plasmonic Nanoparticles. Chem. Rev. 2021, 121, 13342–13453. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Ding, Q.; Yuan, R.; Yuan, Y. AuNPs/CdS QDs/CeO2 ternary nanocomposite coupled with scrollable three-dimensional DNA walker mediated cycling amplification for sensitive photoelectrochemical miRNA assay. Anal. Chim. Acta 2022, 1228, 340344. [Google Scholar] [CrossRef]
- Sharma, J.; Chhabra, R.; Cheng, A.; Brownell, J.; Liu, Y.; Yan, H. Control of Self-Assembly of DNA Tubules Through Integration of Gold Nanoparticles. Science 2009, 323, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzyk, A.; Schreiber, R.; Fan, Z.Y.; Pardatscher, G.; Roller, E.M.; Hogele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-Based Self-Assembly of Chiral Plasmonic Nanostructures with Tailored Optical Response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Chekini, M.; Qu, Z.-B.; Wang, Y.; Yeltik, A.; Liu, Y.; Kotlyar, A.; Zhang, T.; Li, B.; Demir, H.V.; et al. Chiral Ceramic Nanoparticles and Peptide Catalysis. J. Am. Chem. Soc. 2017, 139, 13701–13712. [Google Scholar] [CrossRef]
- Yan, W.; Xu, L.; Xu, C.; Ma, W.; Kuang, H.; Wang, L.; Kotov, N.A. Self-Assembly of Chiral Nanoparticle Pyramids with Strong R/S Optical Activity. J. Am. Chem. Soc. 2012, 134, 15114–15121. [Google Scholar] [CrossRef]
- Lan, X.; Zhou, X.; McCarthy, L.A.; Govorov, A.O.; Liu, Y.; Link, S. DNA-Enabled Chiral Gold Nanoparticle–Chromophore Hybrid Structure with Resonant Plasmon–Exciton Coupling Gives Unusual and Strong Circular Dichroism. J. Am. Chem. Soc. 2019, 141, 19336–19341. [Google Scholar] [CrossRef]
- Srivastava, S.; Santos, A.; Critchley, K.; Kim, K.-S.; Podsiadlo, P.; Sun, K.; Lee, J.; Xu, C.; Lilly, G.D.; Glotzer, S.C.; et al. Light-Controlled Self-Assembly of Semiconductor Nanoparticles into Twisted Ribbons. Science 2010, 327, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, X.; Wang, W.; Sun, M.; Choi, W.J.; Kim, J.-Y.; Hao, C.; Li, S.; Qu, A.; Lu, M.; et al. Enantiomer-dependent immunological response to chiral nanoparticles. Nature 2022, 601, 366–373. [Google Scholar] [CrossRef]
- Purcell-Milton, F.; McKenna, R.; Brennan, L.J.; Cullen, C.P.; Guillemeney, L.; Tepliakov, N.V.; Baimuratov, A.S.; Rukhlenko, I.D.; Perova, T.S.; Duesberg, G.S.; et al. Induction of Chirality in Two-Dimensional Nanomaterials: Chiral 2D MoS2 Nanostructures. ACS Nano 2018, 12, 954–964. [Google Scholar] [CrossRef]
- Yeom, J.; Santos, U.S.; Chekini, M.; Cha, M.; de Moura, A.F.; Kotov, N.A. Chiromagnetic nanoparticles and gels. Science 2018, 359, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Li, Q.; Gao, Z.; Wang, H.; Shi, B.; Wu, Y.; Shangguan, L.; Hong, X.; Wang, F.; Huang, F. Pillararene Host–Guest Complexation Induced Chirality Amplification: A New Way to Detect Cryptochiral Compounds. Angew. Chem. Int. Ed. 2020, 59, 10868–10872. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.S.M.; Machalska, M.S.E.; Oleszkiewicz, M.S.T.; Grzebelus, H.E.; Baranski, H.R.; Szcześniak, P.; Mlynarski, H.J.; Zajac, G.; Kaczor, H.A.; Baranska, H.M. Chiral Amplification in Nature: Studying Cell-Extracted Chiral Carotenoid Microcrystals via the Resonance Raman Optical Activity of Model Systems. Angew. Chem. Int. Ed. 2019, 58, 8383–8388. [Google Scholar] [CrossRef]
- Grason, G.M. Chiral and achiral mechanisms of self-limiting assembly of twisted bundles. Soft Matter 2019, 16, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, S.; Miao, K.; Miao, X.; Deng, W. Same building block, but diverse surface-confined self-assemblies: Solvent and concentration effects-induced structural diversity towards chirality and achirality. Phys. Chem. Chem. Phys. 2018, 20, 17367–17379. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Lin, J.; Zhu, X.; Gong, S.; Wang, X.-S.; Wang, L. Superhelices with Designed Helical Structures and Temperature-Stimulated Chirality Transitions. Macromolecules 2015, 49, 15–22. [Google Scholar] [CrossRef]
- Kumar, P.; Kotov, N. Early Growth Stages of Hierarchically Organized Chiral Structures. Microsc. Microanal. 2020, 26, 550–551. [Google Scholar] [CrossRef]
- Feng, W.; Kim, J.-Y.; Wang, X.; Calcaterra, H.A.; Qu, Z.; Meshi, L.; Kotov, N.A. Assembly of mesoscale helices with near-unity enantiomeric excess and light-matter interactions for chiral semiconductors. Sci. Adv. 2017, 3, e1601159. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Feng, W.; Kim, J.-Y.; Lu, J.; Kumar, P.; Mu, Z.; Wu, X.; Mao, X.; Kotov, N.A. Self-Assembly of Chiral Nanoparticles into Semiconductor Helices with Tunable near-Infrared Optical Activity. Chem. Mater. 2019, 32, 476–488. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Ramesar, N.S.; Heinz, H.; Xu, L.; Xu, C.; Kotov, N.A. Single- and multi-component chiral supraparticles as modular enantioselective catalysts. Nat. Commun. 2019, 10, 4826. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Pacella, M.S.; Vali, H.; Gray, J.J.; McKee, M.D. Chiral switching in biomineral suprastructures induced by homochiral l -amino acid. Sci. Adv. 2018, 4, eaas9819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, S.; de Frutos, M.; Davidson, P.; Abécassis, B. Ligand-induced twisting of nanoplatelets and their self-assembly into chiral ribbons. Sci. Adv. 2017, 3, e1701483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Chan, H.; Baskin, A.; Gelman, E.; Repnin, N.; Král, P.; Klajn, R. Self-assembly of magnetite nanocubes into helical superstructures. Science 2014, 345, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Bahng, J.H.; Yeom, B.; Wang, Y.; Tung, S.O.; Hoff, J.; Kotov, N. Anomalous dispersions of ‘hedgehog’ particles. Nature 2015, 517, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Vecchio, D.; Emre, A.; Rahmani, S.; Cheng, C.; Zhu, J.; Misra, A.C.; Lahann, J.; Kotov, N.A. Graph Theoretical Design of Biomimetic Aramid Nanofiber Nanocomposites as Insulation Coatings for Implantable Bioelectronics. bioRxiv 2020, 12, 424604. [Google Scholar]
- Winogradoff, D.; Li, P.; Joshi, H.; Quednau, L.; Maffeo, C.; Aksimentiev, A. Chiral Systems Made from DNA. Adv. Sci. 2021, 8, 2003113. [Google Scholar] [CrossRef]
- Zhang, M.; Qing, G.; Sun, T. Chiral biointerface materials. Chem. Soc. Rev. 2011, 41, 1972–1984. [Google Scholar] [CrossRef]
- Timsit, Y. DNA Self-Assembly: From Chirality to Evolution. Int. J. Mol. Sci. 2013, 14, 8252–8270. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; He, M.-Q.; Yu, Y.-L.; Wang, J.-H. Biomolecule-mediated chiral nanostructures: A review of chiral mechanism and application. Adv. Colloid Interface Sci. 2021, 289, 102376. [Google Scholar] [CrossRef]
- Skeete, Z.; Cheng, H.; Crew, E.; Lin, L.; Zhao, W.; Joseph, P.; Shan, S.; Cronk, H.; Luo, J.; Li, Y.; et al. Design of Functional Nanoparticles and Assemblies for Theranostic Applications. ACS Appl. Mater. Interfaces 2014, 6, 21752–21768. [Google Scholar] [CrossRef]
- Thang, D.C.; Wang, Z.; Lu, X.; Xing, B. Precise cell behaviors manipulation through light-responsive nano-regulators: Recent advance and perspective. Theranostics 2019, 9, 3308–3340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, L.; Sun, M.; Ma, W.; Wu, X.; Xu, C.; Kuang, H. Tuning the interactions between chiral plasmonic films and living cells. Nat. Commun. 2017, 8, 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Gong, J.; Wang, Y.; He, J.; You, D.; Zhou, Y.; Li, Q.; Liu, Y.; Cheng, K.; Qian, J.; et al. Chiral geometry regulates stem cell fate and activity. Biomaterials 2019, 222, 119456. [Google Scholar] [CrossRef]
- Deng, J.; Yao, M.; Gao, C. Cytotoxicity of gold nanoparticles with different structures and surface-anchored chiral polymers. Acta Biomater. 2017, 53, 610–618. [Google Scholar] [CrossRef]
- Mazur, M.; Kudrynska, A.; Pawlak, A.; Hernandez-Suarez, B.; Obmińska-Mrukowicz, B.; Gładkowski, W. Biotechnological Approach for the Production of Enantiomeric Hydroxylactones Derived from Benzaldehyde and Evaluation of Their Cytotoxic Activity. Catalysts 2020, 10, 1313. [Google Scholar] [CrossRef]
- Abramkin, S.A.; Jungwirth, U.; Valiahdi, S.M.; Dworak, C.; Habala, L.; Meelich, K.; Berger, W.; Jakupec, M.A.; Hartinger, C.G.; Nazarov, A.A.; et al. {(1R,2R,4R)-4-Methyl-1,2-cyclohexanediamine}oxalatoplatinum(II): A Novel Enantiomerically Pure Oxaliplatin Derivative Showing Improved Anticancer Activity in Vivo. J. Med. Chem. 2010, 53, 7356–7364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stensbøl, T.B.; Borre, L.; Johansen, T.N.; Egebjerg, J.; Madsen, U.; Ebert, B.; Krogsgaard-Larsen, P. Resolution, absolute stereochemistry and molecular pharmacology of the enantiomers of ATPA. Eur. J. Pharmacol. 1999, 380, 153–162. [Google Scholar] [CrossRef]
- Rivara, S.; Diamantini, G.; Di Giacomo, B.; Lamba, D.; Gatti, G.; Lucini, V.; Pannacci, M.; Mor, M.; Spadoni, G.; Tarzia, G. Reassessing the melatonin pharmacophore—Enantiomeric resolution, pharmacological activity, structure analysis, and molecular modeling of a constrained chiral melatonin analogue. Bioorganic Med. Chem. 2006, 14, 3383–3391. [Google Scholar] [CrossRef]
- Hao, C.; Xu, L.; Kuang, H.; Xu, C. Artificial Chiral Probes and Bioapplications. Adv. Mater. 2019, 32, e1802075. [Google Scholar] [CrossRef]
- Ghislieri, D.; Green, A.P.; Pontini, M.; Willies, S.C.; Rowles, I.; Frank, A.; Grogan, G.; Turner, N.J. Engineering an Enantioselective Amine Oxidase for the Synthesis of Pharmaceutical Building Blocks and Alkaloid Natural Products. J. Am. Chem. Soc. 2013, 135, 10863–10869. [Google Scholar] [CrossRef]
- Murai, T.; Matsuoka, D.; Morishita, K. 1,1‘-Binaphthyl-2,2‘-diyl Phosphoroselenoyl Chloride as a Chiral Molecular Tool for the Preparation of Enantiomerically Pure Alcohols and Amines. J. Am. Chem. Soc. 2006, 128, 4584–4585. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R. Chiral Metal Complexes as Discriminating Molecular Catalysts. Science 1990, 248, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Asmari, M.; Wang, X.; Casado, N.; Piponski, M.; Kovalenko, S.; Logoyda, L.; Hanafi, R.S.; El Deeb, S. Chiral Monolithic Silica-Based HPLC Columns for Enantiomeric Separation and Determination: Functionalization of Chiral Selector and Recognition of Selector-Selectand Interaction. Molecules 2021, 26, 5241. [Google Scholar] [CrossRef]
- Ali, I.; Zaid, M.E.A.; Belboukhari, N.; Sekkoum, K.; Al-Qahtani, W.H.; Karami, A.M.; Locatelli, M. Chiral HPLC separation and simulation studies of two chiral centered bis-imino flavans (Schiff base). Microchem. J. 2022, 178, 107429. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, Y.; Ma, Q.; Zhang, J.; Wang, Z. Experimental and computational studies of enantioseparation of three profen enantiomers with a focus on quantification of the enantiomeric impurities present in the corresponding enantiopure S-profen drugs. J. Chromatogr. A 2022, 1673, 463095. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Wang, J.; Chen, J.; Guan, M.; Qiu, H. Solid membranes for chiral separation: A review. Chem. Eng. J. 2020, 410, 128247. [Google Scholar] [CrossRef]
- Pu, L. Chemoselective and enantioselective fluorescent identification of specific amino acid enantiomers. Chem. Commun. 2022, 58, 8038–8048. [Google Scholar] [CrossRef] [PubMed]
- Huo, B.; Lu, K.; Tian, J.; Zhao, F.; Wang, Y.; Yu, S.; Yu, X.; Pu, L. From MonoBINOL to BisBINOL: Expanded Enantioselective Fluorescent Recognition of Amino Acids. J. Org. Chem. 2021, 86, 6780–6786. [Google Scholar] [CrossRef]
- Jejurkar, V.P.; Yashwantrao, G.; Kumar, P.; Neekhra, S.; Maliekal, P.J.; Badani, P.; Srivastava, R.; Saha, S. Design and Development of Axially Chiral Bis(naphthofuran) Luminogens as Fluorescent Probes for Cell Imaging. Chem. A Eur. J. 2020, 27, 5470–5482. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Oh, J.-W.; Kim, Y.-R.; Kim, J.S.; Kim, H. Chiral gold nanoparticle-based electrochemical sensor for enantioselective recognition of 3,4-dihydroxyphenylalanine. Chem. Commun. 2010, 46, 5665–5667. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Sun, M.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Hybrid Nanoparticle Pyramids for Intracellular Dual MicroRNAs Biosensing and Bioimaging. Adv. Mater. 2017, 29, 1606086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Du, Y.; Tian, J.; Shi, D.; Wang, Y.; Hu, L.; Yu, S.; Yu, X.; Pu, L. Enantioselective Fluorescent Recognition of Amino Acids in Aqueous Solution by Using a Chiral Aldehyde Probe. Eur. J. Org. Chem. 2018, 2018, 1891–1895. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.; Prasad, S.; Kumar, B.; Sharma, S.K.; Vats, P. High-altitude hypoxia induced reactive oxygen species generation, signaling, and mitigation approaches. Int. J. Biometeorol. 2020, 65, 601–615. [Google Scholar] [CrossRef]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, R.; Adibhatla, R.; Hatcher, J.; Franklin, J. Loss of cardiolipin and mitochondria during programmed neuronal death: Evidence of a role for lipid peroxidation and autophagy. Neuroscience 2002, 115, 587–602. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Ichimura, Y.; Kirisako, T.; Takao, T.; Satomi, Y.; Shimonishi, Y.; Ishihara, N.; Mizushima, N.; Tanida, I.; Kominami, E.; Ohsumi, M.; et al. A ubiquitin-like system mediates protein lipidation. Nature 2000, 408, 488–492. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, X.; Kang, J.; Su, J.; Li, Y.; Zhong, J.; Sun, L. Oxidative Stress Induces Parallel Autophagy and Mitochondria Dysfunction in Human Glioma U251 Cells. Toxicol. Sci. 2009, 110, 376–388. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, P.; Wang, X.; Wang, L.; Zhu, Y.; Song, Y.; Gao, W. Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, Y.-F.; Liu, X.; Feng, G.; Xiong, D.; Mu, G.-F.; Chen, F.-P. ROS Promote Ox-LDL-Induced Platelet Activation by Up-Regulating Autophagy Through the Inhibition of the PI3K/AKT/mTOR Pathway. Cell. Physiol. Biochem. 2018, 50, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, S.; Zhao, J.; Sun, M.; Wang, W.; Lu, M.; Qu, A.; Hao, C.; Chen, C.; Xu, C.; et al. Ultrasmall Magneto-chiral Cobalt Hydroxide Nanoparticles Enable Dynamic Detection of Reactive Oxygen Species In Vivo. J. Am. Chem. Soc. 2022, 144, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Y. Near-infrared optically active Cu2−xS nanocrystals: Sacrificial template-ligand exchange integration fabrication and chirality dependent autophagy effects. J. Mater. Chem. B 2020, 8, 7921–7930. [Google Scholar] [CrossRef]
- Hou, X.-X.; Ren, Y.-P.; Luo, Z.-H.; Jiang, B.-L.; Lu, T.-T.; Huang, F.-P.; Qin, X.-Y. Two novel chiral tetranucleate copper-based complexes: Syntheses, crystal structures, inhibition of angiogenesis and the growth of human breast cancer in vitro and in vivo. Dalton Trans. 2021, 50, 14684–14694. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Fei, X.; Li, J.; Liu, H.; Liu, W.; Yang, Y.; Li, B.; Liu, M.; Yang, G.; et al. Chiral FA Conjugated CdTe/CdS Quantum Dots for Selective Cancer Ablation. ACS Nano 2022, 16, 12991–13001. [Google Scholar] [CrossRef]
- Miao, J.; Cai, Y.; Shao, Y.; Yang, G.; Huang, H.; Shang, Z.; Cheng, J.; Li, Y.; Xu, X. Multiple cell death pathways triggered by temperature-mediated synergistic effect derived from chiral phototheranostic ablation nanoagents. Appl. Mater. Today 2021, 23, 101001. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Wang, F.; Yang, M.; Xie, L.; Zeng, X. Biosafety, Nontoxic Nanoparticles for VL–NIR Photothermal Therapy Against Oral Squamous Cell Carcinoma. ACS Omega 2021, 6, 11240–11247. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.; Hao, C.; Hu, S.; Chen, C.; Cao, Y.; Xu, Z.; Guo, J.; Xu, L.; Sun, M.; et al. The Development of Chiral Nanoparticles to Target NK Cells and CD8+ T Cells for Cancer Immunotherapy. Adv. Mater. 2022, 34, 2109354. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.; de Moura, A.F.; Wu, X.; Kuang, H.; Xu, C.; Kotov, N.A. Chiral Inorganic Nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Qin, W. Organic Chiral Spin-Optics: The Interaction between Spin and Photon in Organic Chiral Materials. Adv. Opt. Mater. 2021, 9, 2101201. [Google Scholar] [CrossRef]
- Kotov, N.A.; Liz-Marzán, L.M.; Weiss, P.S. Chiral Nanostructures: New Twists. ACS Nano 2021, 15, 12457–12460. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, R.; Gao, X.; Cao, Z.; Wang, J.; Ma, Y. Biomimetic Self-Assembled Chiral Inorganic Nanomaterials: A New Strategy for Solving Medical Problems. Biomimetics 2022, 7, 165. https://doi.org/10.3390/biomimetics7040165
Wei R, Gao X, Cao Z, Wang J, Ma Y. Biomimetic Self-Assembled Chiral Inorganic Nanomaterials: A New Strategy for Solving Medical Problems. Biomimetics. 2022; 7(4):165. https://doi.org/10.3390/biomimetics7040165
Chicago/Turabian StyleWei, Rong, Xueying Gao, Ziwei Cao, Jing Wang, and Yu Ma. 2022. "Biomimetic Self-Assembled Chiral Inorganic Nanomaterials: A New Strategy for Solving Medical Problems" Biomimetics 7, no. 4: 165. https://doi.org/10.3390/biomimetics7040165




