Organismal Design and Biomimetics: A Problem of Scale
Abstract
:1. Introduction
2. Scaling Effects in Organismal Design and Artefacts
2.1. Mechanics
2.1.1. Statics
2.1.2. Dynamics
Walking, Jumping, and Running
Swimming
Flying
Tropic, Nastic, and Other Movements
2.2. Optics
2.2.1. Pigmentary Colours
2.2.2. Structural Colours
2.2.3. Bioluminescence
2.3. Electricity
2.4. Acoustics
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, J.F.; Bogatyreva, O.A.; Bogatyrev, N.R.; Bowyer, A.; Pahl, A.K. Biomimetics: Its practice and theory. J. R. Soc. Interface 2006, 3, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Gruber, P. Biomimetics in Architecture; Springer: Vienna, Austria, 2011. [Google Scholar]
- Vogel, S. Comparative Biomechanics: Life’s Physical World; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Speck, O.; Speck, D.; Horn, R.; Gantner, J.; Sedlbauer, K.P. Biomimetic bio-inspired biomorph sustainable? An attempt to classify and clarify biology-derived technical developments. Bioinspir. Biomim. 2017, 12, 011004. [Google Scholar] [CrossRef]
- Vogel, S. Cats’ Paws and Catapults: Mechanical Worlds of Nature and People; WW Norton & Company: New York, NY, USA, 2000. [Google Scholar]
- Perricone, V. Constructional design of organisms. Diid Ind. Des. Des. 2030 Saperi. 2020, 70/20, 96–103. Available online: https://www.researchgate.net/publication/352816849_Perricone_V_2020_Constructional_design_of_organisms_diid_disegno_industriale_industrial_design_Design_2030_Saperi_7020_96-103_ISBN_9788832080506 (accessed on 24 September 2021).
- Ayre, M. Biomimetics applied to space exploration. WIT Trans. Ecol. Environ. 2004, 73. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Biomimetics—Using nature to inspire human innovation. Bioinspir. Biomim. 2006, 1, P1. [Google Scholar] [CrossRef] [PubMed]
- Gebeshuber, I.C.; Drack, M. An attempt to reveal synergies between biology and mechanical engineering. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2008, 222, 1281–1287. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics: Lessons from nature—An overview. Philos. Trans. A Math Phys. Eng. Sci. 2009, 367, 1445–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebeshuber, I.C.; Gruber, P.; Drack, M. A gaze into the crystal ball: Biomimetics in the year 2059. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2009, 223, 2899–2918. [Google Scholar] [CrossRef]
- Knippers, J.; Speck, T. Design and construction principles in nature and architecture. Bioinspir. Biomim. 2012, 7, 015002. [Google Scholar] [CrossRef]
- Seilacher, A.; Gishlick, A.D. Morphodynamics; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- West, G.B.; Brown, J.H. Life’s universal scaling laws. Phys. Today 2004, 57, 36–43. [Google Scholar] [CrossRef]
- Huber, H.; Hohn, M.J.; Rachel, R.; Fuchs, T.; Wimmer, V.C.; Stetter, K.O. A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont. Nature 2002, 417, 63–67. [Google Scholar] [CrossRef]
- Sillett, S.C.; Van Pelt, R.; Carroll, A.L.; Campbell-Spickler, J.; Antoine, M.E. Structure and dynamics of forests dominated by Sequoiadendron giganteum. For. Ecol. Manag. 2019, 448, 218–239. [Google Scholar] [CrossRef]
- Sears, R.; Perrin, W.F. Blue whale: Balaenoptera musculus. In Encyclopedia of Marine Mammals; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Thomson, J.A. On growth and form. Nature 1917, 100, 21–22. [Google Scholar] [CrossRef]
- Perez, A.; Linsey, J.; Tsenn, J.; Glier, M. Identifying product scaling principles: A step towards enhancing biomimetic design. ASME Int. Mech. Eng. Congr. Expo. 2011, 54884, 789–798. [Google Scholar]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Benyus, J.M. Biomimicry: Innovation Inspired by Nature; Quill-William Morrow: New York, NY, USA, 1997. [Google Scholar]
- Pauli, G.A. The Blue Economy: 10 Years, 100 Innovations, 100 Million Jobs; Paradigm Publications: Boulder, CO, USA, 2010. [Google Scholar]
- Vincent, J.; Bogatyreva, O.; Bogatyrev, N.; Pahl, A.K.; Bowyer, A. A Theoretical Basis for Biomimetics; MRS Online Proceedings Library (OPL); Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Shingleton, A. Allometry: The study of biological scaling. Nat. Educ. Knowl. 2010, 3, 2. [Google Scholar]
- Huxley, J.S. Problems of Relative Growth; Dial Press: New York, NY, USA, 1932. [Google Scholar]
- Moore, K.L. The Developing Human; W. B. Saunders: Philadelphia, PA, USA, 1983. [Google Scholar]
- Spence, A.J. Scaling in biology. Curr. Biol. 2009, 19, R57–R61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonner, J.T. From Bacteria to Blue Wales, Why Size Matters; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Hickman, C.P., Jr.; Roberts, L.S.; Larson, A. Integrated Principles of Zoology, 11th ed.; McGraw-Hill: Boston, MA, USA, 2001. [Google Scholar]
- Brusca, R.C.; Brusca, G.J. Invertebrates; Sinauer Associates: Sunderland, MA, USA, 2003. [Google Scholar]
- Smith, F.A.; Payne, J.L.; Heim, N.A.; Balk, M.A.; Finnegan, S.; Kowalewski, M.; Lyons, S.K.; McClain, C.; McShea, D.W.; Novack-Gottshall, P.; et al. Body size evolution across the Geozoic. Annu. Rev. Earth Planet. Sci. 2016, 44, 523–553. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: New York, NY, USA, 1983. [Google Scholar]
- Foster, J.B. Evolution of mammals on islands. Nature 1964, 202, 234–235. [Google Scholar] [CrossRef]
- Langhaar, H.L. Dimensional Analysis and Theory of Models; John Wiley & Sons, Inc.: New York, NY, USA; Chapman & Hall, Limited: London, UK, 1951. [Google Scholar]
- Lauer, C.; Schmier, S.; Speck, T.; Nickel, K.G. Strength-size relationships in two porous biological materials. Acta Biomater. 2018, 77, 322–332. [Google Scholar] [CrossRef]
- Carpinteri, A.; Cornetti, P.; Puzzi, S. Scaling laws and multiscale approach in the mechanics of heterogeneous and disordered materials. Appl. Mech. Rev. 2006, 59, 283. [Google Scholar] [CrossRef] [Green Version]
- Peterson, H. Scaling laws applied to wind turbine design. Wind Eng. 1984, 8, 99–108. [Google Scholar]
- Vincent, J. TRIZ as a primary tool for biomimetics. In Research and Practice on the Theory of Inventive Problem Solving (TRIZ); Chechurin, L., Ed.; Springer: Cham, Switzerland, 2016; pp. 225–235. [Google Scholar]
- Yang, Y.; Song, X.; Li, X.; Chen, Z.; Zhou, C.; Zhou, Q.; Chen, Y. Recent progress in biomimetic additive manufacturing technology: From materials to functional structures. Adv. Mater. 2018, 30, 1706539. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, Z.; Cai, C.; Shen, H.; Liang, F.; Wang, D.; Wang, C.; Zhu, T.; Guo, J.; Wang, Y.; et al. Bioinspired materials: From low to high dimensional structure. Adv. Mater. 2014, 26, 6994–7017. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, A.; Broeckhoven, C. Looking deep into nature: A review of micro-computed tomography in biomimicry. Acta Biomater. 2019, 85, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Degl’Innocenti, A.; Mazzolai, B. Three-dimensional reconstruction of root shape in the moth orchid Phalaenopsis sp.: A biomimicry methodology for robotic applications. BMC Res. Notes 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayfield, E.J. Finite element analysis and understanding the biomechanics and evolution of living and fossil organisms. Annu. Rev. Earth Planet. Sci. 2007, 35, 541–576. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Quake, S.R.; Scherer, A. From micro-to nanofabrication with soft materials. Science 2000, 290, 1536–1540. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Tricinci, O.; Terencio, T.; Pugno, N.M.; Greco, F.; Mazzolai, B.; Mattoli, V. Air trapping mechanism in artificial Salvinia-like micro-hairs fabricated via direct laser lithography. Micromachines 2017, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Koehl, M.A.R. When does morphology matter? Annu. Rev. Ecol. Syst. 1996, 27, 501–542. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, S.A.; Biggs, W.D.; Currey, J.D. Mechanical Design in Organisms; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Hildebrand, M. Analysis of Vertebrate Structure, 3rd ed.; Zanichelli: Bologna, Italy, 1992. [Google Scholar]
- Kingsolver, J.G.; Koehl, M.A.R. Aerodynamics, thermoregulation, and the evolution of insect wings: Differential scaling and evolutionary change. Evolution 1985, 39, 488–504. [Google Scholar] [CrossRef]
- Imani, M.; Donn, M.; Balador, Z. Bio-inspired materials contribution of biology to energy efficiency of buildings. In Handbook of Ecomaterials; Springer International Publishing: New York, NY, USA, 2018; pp. 2213–2236. [Google Scholar]
- Fratzl, P. Biomimetic materials research: What can we really learn from nature’s structural materials? J. R. Soc. Interface 2007, 4, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Mcadams, D.A.; Grunlan, J.C. Nano/micro-manufacturing of bioinspired materials: A review of methods to mimic natural structures. Adv. Mater. 2016, 28, 6292–6321. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, J.; Wang, J.; Zhu, Y.; Jiang, L. Bioinspired hierarchical alumina–graphene oxide–poly (vinyl alcohol) artificial nacre with optimized strength and toughness. ACS Appl. Mater. Interfaces 2015, 7, 9281–9286. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J. Biomechanics of cellular solids. J. Biomech. 2005, 38, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Saleh, E.; Woolliams, P.; Clarke, B.; Gregory, A.; Greedy, S.; Smartt, C.; Wildman, R.; Ashcroft, I.; Hague, R.; Dickens, P.; et al. 3D inkjet-printed UV-curable inks for multi-functional electromagnetic applications. Addit. Manuf. 2017, 13, 143–148. [Google Scholar] [CrossRef]
- Song, J.; Reichert, S.; Kallai, I.; Gazit, D.; Wund, M.; Boyce, M.C.; Ortiz, C. Quantitative microstructural studies of the armor of the marine threespine stickleback (Gasterosteus aculeatus). J. Struct. Biol. 2010, 171, 318–331. [Google Scholar] [CrossRef]
- Bach, K.; Burkhardt, B. Diatoms I, Shells in Nature and Technics; Cramer: Braunschweig, Germany, 1984. [Google Scholar]
- Hamm, C. Evolution of Lightweight Structures: Analyses and Technical Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Hamm, C.E.; Smetacek, V. Armor: Why, When and How? In Evolution of Primary Producers in the Sea; Falkowski, P., Knoll, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
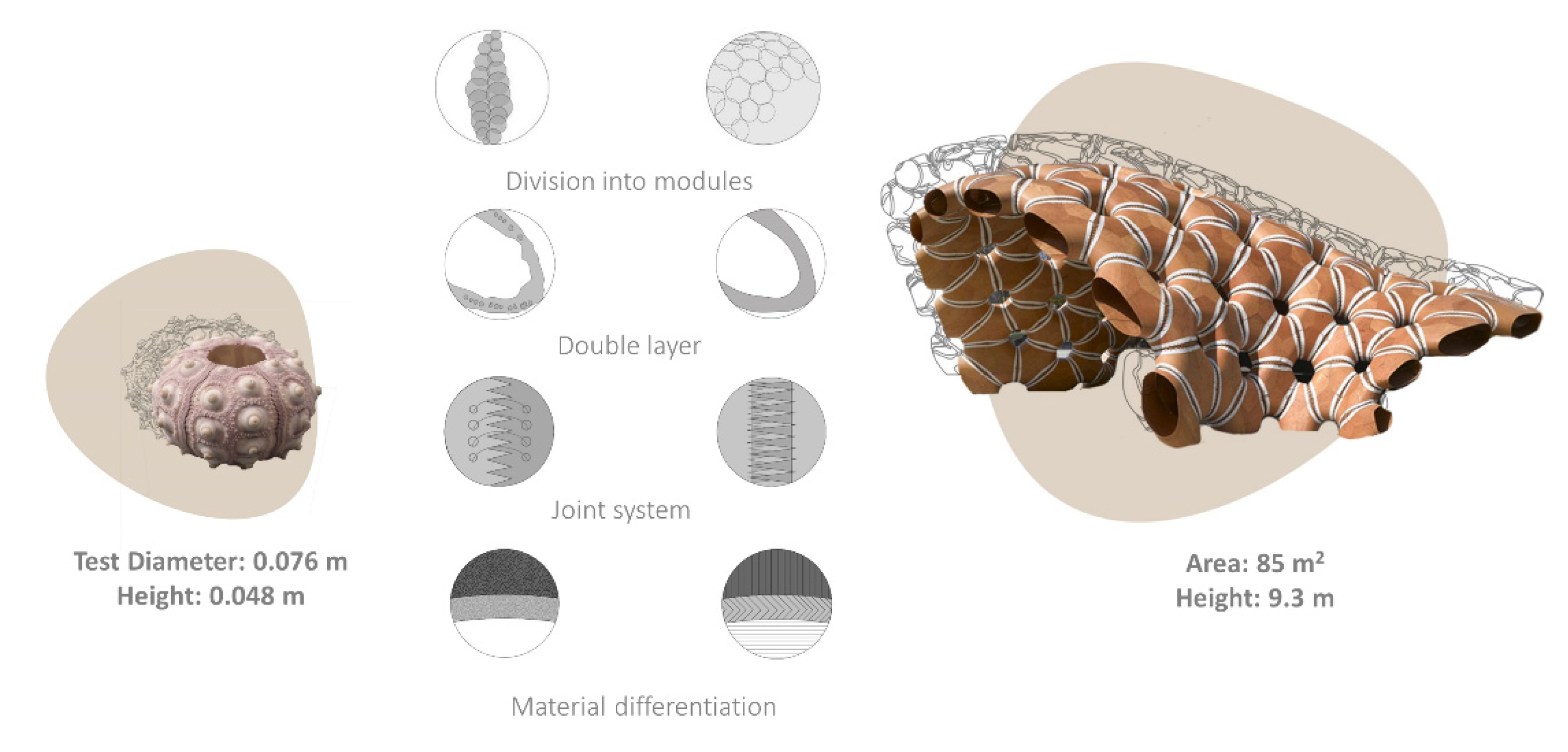
- Marmo, F.; Perricone, V.; Langella, C.; Pontillo, G.; Rosati, L. Bioinspired design of shell structures: A lesson from echinoids. In Proceedings of IASS Annual Symposia; International Association for Shell and Spatial Structures (IASS): Barcelona, Spain, 2019. [Google Scholar]
- Frampton, K.; Futagawa, Y. Modern Architecture 1851–1945; Random House Incorporated: New York, NY, USA, 1983. [Google Scholar]
- Perricone, V.; Grun, T.; Marmo, F.; Langella, C.; Carnevali, M.D.C. Constructional design of echinoid endoskeleton: Main structural components and their potential for biomimetic applications. Bioinspir. Biomim. 2020, 16, 011001. [Google Scholar] [CrossRef]
- Grun, T.B.; Dehkordi, L.K.F.; Schwinn, T.; Sonntag, D.; von Scheven, M.; Bischoff, M.; Knippers, J.; Menges, A.; Nebelsick, J.H. The skeleton of the sand dollar as a biological role model for segmented shells in building construction: A research review. In Biomimetic Research for Architecture and Building Construction; Springer: Cham, Switzerland, 2016; pp. 217–242. [Google Scholar]
- Magna, R.L.; Gabler, M.; Reichert, S.; Schwinn, T.; Waimer, F.; Menges, A.; Knippers, J. From nature to fabrication: Biomimetic design principles for the production of complex spatial structures. Int. J. Space Struct. 2013, 28, 27–39. [Google Scholar] [CrossRef]
- ICD/ITKE Research Pavilion 2015-16. Available online: https://www.itke.uni-stuttgart.de/research/icd-itke-research-pavilions/icd-itke-research-pavilion-2015-16 (accessed on 24 September 2021).
- Charpentier, V.; Adriaenssens, S. Effect of gravity on the scale of compliant shells. Biomimetics 2020, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, M.; Ali, M.N.; Sami, J.; Ansari, U. Review of mechanics and applications of auxetic structures. Adv. Mater. Sci. Eng. 2014, 2014, 753496. [Google Scholar] [CrossRef] [Green Version]
- Frohlich, L.M.; La Barbera, M.; Stevens, W.P. Poisson’s ratio of a crossed fibre sheath: The skin of aquatic salamanders. J. Zool. 1994, 232, 231–252. [Google Scholar] [CrossRef]
- Lees, C.; Vincent, J.F.; Hillerton, J.E. Poisson’s ratio in skin. Biomed. Mater. Eng. 1991, 1, 19–23. [Google Scholar] [CrossRef]
- Veronda, D.R.; Westmann, R.A. Mechanical characterization of skin—Finite deformations. J. Biomech. 1970, 3, 111–124. [Google Scholar] [CrossRef]
- Evans, K.E.; Alderson, A. Auxetic materials: Functional materials and structures from lateral thinking! Adv. Mater. 2000, 12, 617–628. [Google Scholar] [CrossRef]
- Lakes, R. A broader view of membranes. Nature 2001, 414, 503–504. [Google Scholar] [CrossRef]
- Joseph, A.; Mahesh, V.; Harursampath, D. On the application of additive manufacturing methods for auxetic structures: A review. Adv. Manuf. 2021, 9, 342–368. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H. A review on auxetic structures and polymeric materials. Sci. Res. Essays 2010, 5, 1052–1063. [Google Scholar]
- Santulli, C.; Langella, C. Study and development of concepts of auxetic structures in bio-inspired design. Int. J. Sustain. Des. 2016, 3, 20–37. [Google Scholar] [CrossRef]
- Panico, M.; Langella, C.; Santulli, C. Development of a biomedical neckbrace through tailored auxetic shapes. Emerg. Sci. J. 2017, 1, 105–117. [Google Scholar] [CrossRef]
- Mackenzie, D. A flapping of wings. Science 2012, 335, 1430–1433. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, T. A review of design and fabrication of the bionic flapping wing micro air vehicles. Micromachines 2019, 10, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudek, G.; Giguere, P.; Prahacs, C.; Saunderson, S.; Sattar, J.; Torres-Mendez, L.A.; Jenkin, M.; German, A.; Hogue, A.; Ripsman, A.; et al. Aqua: An amphibious autonomous robot. Computer 2007, 40, 46–53. [Google Scholar] [CrossRef]
- Ren, K.; Yu, J. Research status of bionic amphibious robots: A review. Ocean Eng. 2021, 227, 108862. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Ruan, J.; Rong, X. Research of mammal bionic quadruped robots: A review. In Proceedings of the 2011 IEEE 5th International Conference on Robotics, Automation and Mechatronics (RAM), Qingdao, China, 17–19 September 2011; pp. 166–171. [Google Scholar]
- Neppalli, S.; Jones, B.; McMahan, W.; Chitrakaran, V.; Walker, I.; Pritts, M.; Csencsits, M.; Rahn, C.; Grissom, M. Octarm—A soft robotic manipulator. In Proceedings of the 2007 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Diego, CA, USA, 29 October–2 November 2007; p. 2569. [Google Scholar]
- Borst, H.G.; Walterbusch, G.; Schaps, D. Extensive aortic replacement using “elephant trunk” prosthesis. J. Thorac. Cardiovasc. Surg. 1983, 31, 37–40. [Google Scholar] [CrossRef]
- Diller, E.; Sitti, M. Micro-Scale Mobile Robotics; Now Publishers: Delft, The Netherlands, 2013. [Google Scholar]
- Cuthill, I.C. Camouflage. J. Zool. 2019, 308, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Pikul, J.H.; Li, S.; Bai, H.; Hanlon, R.T.; Cohen, I.; Shepherd, R.F. Stretchable surfaces with programmable 3D texture morphing for synthetic camouflaging skins. Science 2017, 358, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, H.; Wang, J.; Ding, L.; Liu, T.; Gao, H. Scale effect mechanism research of insect-imitating hexapod robot. J. Mech. Sci. Technol. 2019, 33, 2873–2882. [Google Scholar] [CrossRef]
- Kaspari, M.; Weiser, M.D. The size-grain hypothesis and interspecific scaling in ants. Func. Ecol. 1999, 13, 530–538. [Google Scholar] [CrossRef]
- Kovac, M. Bio-Inspired Jumping Locomotion for Miniature Robotics. Ph.D. Thesis, Swiss Federal Institute of Technology, Zürich, Switzerland, 2010. [Google Scholar]
- Chen, D.; Yin, J.; Zhao, K.; Zheng, W.; Wang, T. Bionic mechanism and kinematics analysis of hopping robot inspired by locust jumping. J. Bionic Eng. 2011, 8, 429–439. [Google Scholar] [CrossRef]
- Niiyama, R.; Nagakubo, A.; Kuniyoshi, Y. Mowgli: A bipedal jumping and landing robot with an artificial musculoskeletal system. In Proceedings of the 2007 IEEE International Conference on Robotics and Automation, Rome, Italy, 10–14 April 2007; pp. 2546–2551. [Google Scholar]
- Graichen, K.; Hentzelt, S.; Hildebrandt, A.; Kärcher, N.; Gaißert, N.; Knubben, E. Control design for a bionic kangaroo. Control Eng. Pract. 2015, 42, 106–117. [Google Scholar] [CrossRef]
- Li, C.; Iscen, A.; Sai, H.; Sato, K.; Sather, N.A.; Chin, S.M.; Álvarez, Z.; Palmer, L.C.; Schatz, G.C.; Stupp, S.I. Supramolecular–covalent hybrid polymers for light-activated mechanical actuation. Nat. Mater. 2020, 19, 900–909. [Google Scholar] [CrossRef]
- Nirody, J.A.; Sun, Y.R.; Lo, C.J. The biophysicist’s guide to the bacterial flagellar motor. Adv. Phys. X 2017, 2, 324–343. [Google Scholar] [CrossRef]
- Aleyev, Y.G. Creation of propulsive force (locomotion). In Nekton; Springer: Dordrecht, The Netherlands, 1977. [Google Scholar]
- Fish, F.E. Limits of nature and advances of technology: What does biomimetics have to offer to aquatic robots? App. Bionics Biomech. 2006, 3, 49–60. [Google Scholar] [CrossRef]
- Dean, B.; Bhushan, B. Shark-skin surfaces for fluid-drag reduction in turbulent flow: A review. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 4775–4806. [Google Scholar] [PubMed] [Green Version]
- Bechert, D.W.; Bruse, M.; Hage, W.V.; Van der Hoeven, J.T.; Hoppe, G. Experiments on drag-reducing surfaces and their optimization with an adjustable geometry. J. Fluid Mech. 1997, 338, 59–87. [Google Scholar] [CrossRef]
- Wen, L.; Weaver, J.C.; Thornycroft, P.J.; Lauder, G.V. Hydrodynamic function of biomimetic shark skin: Effect of denticle pattern and spacing. Bioinspir. Biomim. 2015, 10, 066010. [Google Scholar] [CrossRef] [Green Version]
- Oeffner, J.; Lauder, G.V. The hydrodynamic function of shark skin and two biomimetic applications. J. Exp. Biol. 2012, 215, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Lee, C.; Lee, D.; Won, Y.J.; Lee, G.W.; Shin, M.G.; Chun, B.; Kim, T.-S.; Park, H.-D.; Jung, H.W.; et al. Sharkskin-mimetic desalination membranes with ultralow biofouling. J. Mater. Chem. A 2018, 6, 23034–23045. [Google Scholar] [CrossRef]
- Repelling Germs with ‘Sharkskin’. Available online: https://www.sciencenewsforstudents.org/article/repelling-germs-sharkskin (accessed on 24 September 2021).
- Fish, F.E. Advantages of natural propulsive systems. Mar. Technol. Soc. J. 2013, 47, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Saadat, M.; Fish, F.E.; Domel, A.G.; Di Santo, V.; Lauder, G.V.; Haj-Hariri, H. On the rules for aquatic locomotion. Phys. Rev. Fluids 2017, 2, 083102. [Google Scholar] [CrossRef]
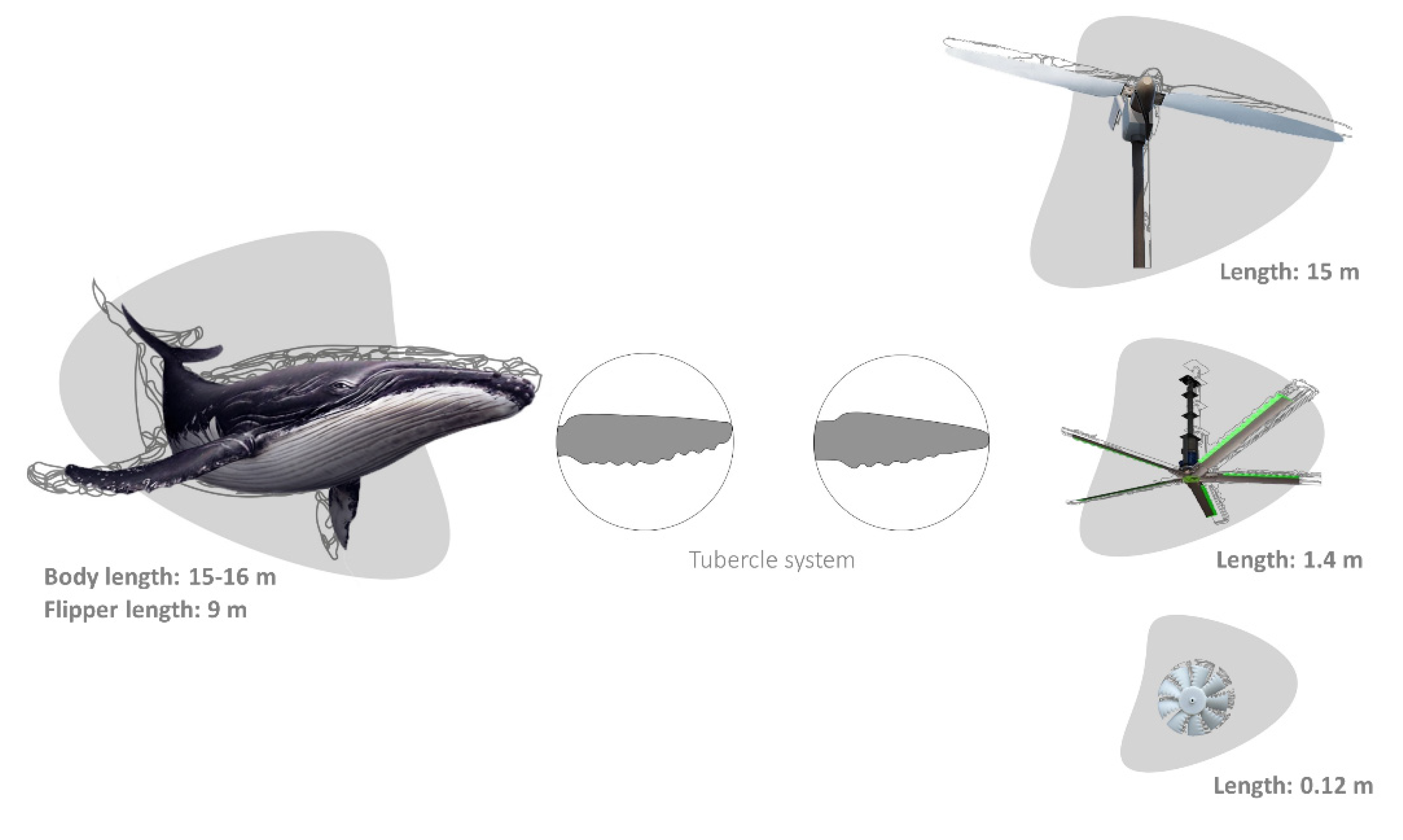
- Fish, F.E.; Weber, P.W.; Murray, M.M.; Howle, L.E. The tubercles on humpback whales’ flippers: Application of bio-inspired technology. Integr. Comp. Biol. 2011, 51, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aftab, S.M.A.; Razak, N.A.; Rafie, A.M.; Ahmad, K.A. Mimicking the humpback whale: An aerodynamic perspective. Prog. Aerosp. Sci. 2016, 84, 48–69. [Google Scholar] [CrossRef]
- Fish, F.E.; Battle, J.M. Hydrodynamic design of the humpback whale flipper. J. Morphol. 1995, 225, 51–60. [Google Scholar] [CrossRef]
- Fish, F.; Lauder, G.V. Passive and active flow control by swimming fishes and mammals. Annu. Rev. Fluid Mech. 2006, 38, 193–224. [Google Scholar] [CrossRef] [Green Version]
- Whalepower Corporation. Available online: https://whalepowercorp.wordpress.com (accessed on 24 September 2021).
- Dudley, R. Mechanisms and implications of animal flight maneuverability. Integr. Comp. Biol. 2002, 42, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ravi, S.; Kolomenskiy, D.; Tanaka, H. Biomechanics and biomimetics in insect-inspired flight systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150390. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, J.E.; Maestro, D.; Bottaro, A. Biomimetic spiroid winglets for lift and drag control. Comptes Rendus Mec. 2012, 340, 67–80. [Google Scholar] [CrossRef]
- Roderick, W.R.; Cutkosky, M.R.; Lentink, D. Touchdown to take-off: At the interface of flight and surface locomotion. Interface Focus 2017, 7, 20160094. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.W. Plant Tropisms: And Other Growth Movements; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Esmon, C.A.; Pedmale, U.V.; Liscum, E. Plant tropisms: Providing the power of movement to a sessile organism. Int. J. Dev. Biol. 2004, 49, 665–674. [Google Scholar] [CrossRef]
- McGinley, M.A. Within and among plant variation in seed mass and pappus size in Tragopogon dubious. Can. J. Bot. 1989, 67, 1298–1304. [Google Scholar] [CrossRef]
- Sisodia, R.; Bhatla, S.C. Plant movements. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2018; pp. 907–935. [Google Scholar]
- Zheliazkova, M.; Savova, B.; Naboni, R. Plant-inspired kinetic systems for architecture. In Proceedings of the 22nd Conference of the Iberoamerican Society of Digital Graphics—ISSN: 2318-6968, São Carlos, Brazil, 7–9 November 2018; pp. 338–345. [Google Scholar]
- Forterre, Y.; Skotheim, J.M.; Dumais, J.; Mahadevan, L. How the Venus flytrap snaps. Nature 2005, 433, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.; Vincent, J.F.; Rocca, A.M. How pinecones open. Nature 1997, 390, 668. [Google Scholar] [CrossRef]
- Gruber, P.; Gosztonyi, S. Skin in architecture: Towards bioinspired facades. WIT Trans. Ecol. Environ. 2010, 138, 503–513. [Google Scholar]
- Durai Prabhakaran, R.T.; Spear, M.J.; Curling, S.; Wootton-Beard, P.; Jones, P.; Donnison, I.; Ormondroyd, G.A. Plants and architecture: The role of biology and biomimetics in materials development for buildings. Intell. Build. Int. 2019, 11, 178–211. [Google Scholar] [CrossRef]
- Heinrich, M.K.; von Mammen, S.; Hofstadler, D.N.; Wahby, M.; Zahadat, P.; Skrzypczak, T.; Soorati, M.D.; Krela, R.; Kwiatkowski, W.; Schmickl, T.; et al. Constructing living buildings: A review of relevant technologies for a novel application of biohybrid robotics. J. R. Soc. Interface 2019, 16, 20190238. [Google Scholar] [CrossRef] [Green Version]
- Lienhard, J.; Schleicher, S.; Poppinga, S.; Masselter, T.; Milwich, M.; Speck, T.; Knippers, J. Flectofin: A nature based hinge-less flapping mechanism. Bioinspir. Biomim. 2011, 6, 045001. [Google Scholar] [CrossRef]
- Krieg, O.D. HygroSkin–meteorosensitive pavilion. In Advancing Wood Architecture; Routledge: Oxfordshire, UK, 2016; pp. 125–140. [Google Scholar]
- Mazzolai, B.; Beccai, L.; Mattoli, V. Plants as model in biomimetics and biorobotics: New perspectives. Front. Bioeng. Biotechnol. 2014, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, B.; Mondini, A.; Corradi, P.; Laschi, C.; Mattoli, V.; Sinibaldi, E.; Dario, P. A miniaturized mechatronic system inspired by plant roots for soil exploration. IEEE ASME Trans. Mechatron. 2011, 16, 201–212. [Google Scholar] [CrossRef]
- Tonazzini, A.; Popova, L.; Mattioli, F.; Mazzolai, B. Analysis and characterization of a robotic probe inspired by the plant root apex. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012. [Google Scholar]
- Mishra, A.K.; Tramacere, F.; Guarino, R.; Pugno, N.M.; Mazzolai, B. A study on plant root apex morphology as a model for soft robots moving in soil. PLoS ONE 2018, 13, e0197411. [Google Scholar]
- Lu, T.Y.; Zhang, X.L.; Liu, Z.Y.; Zhang, Z.N. A tumbleweed-mimicing wind-driven rover for planetary exploration. In Proceedings of the 2010 International Conference on Intelligent Computation Technology and Automation, Changsha, China, 11–12 May 2010; Volume 1, pp. 42–45. [Google Scholar]
- Ulrich, E.R.; Pines, D.J.; Humbert, J.S. From falling to flying: The path to powered flight of a robotic samara nano air vehicle. Bioinspir. Biomim. 2010, 5, 045009. [Google Scholar] [CrossRef]
- Gruber, P. The signs of life in architecture. Bioinspir. Biomim. 2008, 3, 023001. [Google Scholar] [CrossRef] [PubMed]
- Willmer, C.M. The evolution, structure and functioning of stomata. Bot. J. Scotl. 1993, 46, 433–445. [Google Scholar] [CrossRef]
- Holmes, D.A. Waterproof breathable fabrics. In Handbook of Technical Textiles; CRC Press: Boca Raton, FL, USA, 2000; pp. 282–315. [Google Scholar]
- Kapsali, V.; Vincent, J. From a pinecone to design of an active textile. Biomimetics 2020, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Fiorello, I.; Tricinci, O.; Naselli, G.A.; Mondini, A.; Filippeschi, C.; Tramacere, F.; Mishra, A.K.; Mazzolai, B. Climbing plant-inspired micropatterned devices for reversible attachment. Adv. Funct. Mater. 2020, 30, 2003380. [Google Scholar] [CrossRef]
- Johnsen, S.; Mobley, C. The Optics of Life: A Biologist’s Guide to Light in Nature; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
- Ball, P. Patterns in Nature: Why the Natural World Looks the Way It Does; The University of Chicago Press: Chicago, IL, USA, 2016. [Google Scholar]
- Sun, J.; Bhushan, B.; Tong, J. Structural coloration in nature. RSC Adv. 2013, 3, 14862–14889. [Google Scholar] [CrossRef]
- Horváth, G.; Horváth, G.; Varju, D. Polarized Light in Animal Vision: Polarization Patterns in Nature; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Gagnon, Y.L.; Templin, R.M.; How, M.J.; Marshall, N.J. Circularly polarized light as a communication signal in mantis shrimps. Curr. Biol. 2015, 25, 3074–3078. [Google Scholar] [CrossRef] [Green Version]
- Kahlke, T.; Umbers, K.D. Bioluminescence. Curr. Biol. 2016, 26, R307–R318. [Google Scholar] [CrossRef] [Green Version]
- De Tommasi, E.; Esposito, E.; Romano, S.; Crescitelli, A.; Di Meo, V.; Mocella, V.; Zito, G.; Rendina, I. Frontiers of light manipulation in natural, metallic, and dielectric nanostructures. Riv. Nuovo Cim. 2021, 44, 1–68. [Google Scholar] [CrossRef]
- Darwin, C. On the Origin of Species. John Murray; Reprinted 1985; Penguin Classics: London, UK, 1859. [Google Scholar]
- Poulton, E.B. The Colours of Animals; Kegan Paul, Trench, Trübner & Co.: London, UK, 1890. [Google Scholar]
- Komárek, S. Mimicry, Aposematism and Related Phenomena in Animals and Plants. Bibliography 1800–1990; Vesmir: Prague, Czech Republic, 1998. [Google Scholar]
- Utke, I.; Moshkalev, S.; Russell, P. Nanofabrication Using Focused Ion and Electron Beams: Principles and Applications; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- De Angelis, F.; Malerba, M.; Patrini, M.; Miele, E.; Das, G.; Toma, A.; Zaccaria, R.P.; Di Fabrizio, E. 3D hollow nanostructures as building blocks for multifunctional plasmonics. Nano Lett. 2013, 13, 3553–3558. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Katiyi, A.; Ang, A.S.; Hazan, A. On-chip nanophotonics and future challenges. Nanophotonics 2020, 9, 3733–3753. [Google Scholar] [CrossRef]
- Martin, H.D. The function of natural colorants: The biochromes. Chimia 1995, 49, 45–68. [Google Scholar]
- Qiao, Y.; Meng, Z.; Wang, P.; Yan, D. Research progress of bionic adaptive camouflage materials. Front. Mater. Sci. 2021, 8, 79. [Google Scholar]
- Stevens, M.; Merilaita, S. Defining disruptive coloration and distinguishing its functions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, M. Predator perception and the interrelation between different forms of protective coloration. Proc. R. Soc. B 2007, 274, 1457–1464. [Google Scholar] [CrossRef]
- Stevens, M.; Yule, D.H.; Ruxton, G.D. Dazzle coloration and prey movement. Proc. R. Soc. B 2008, 275, 2639–2643. [Google Scholar] [CrossRef] [Green Version]
- How, M.J.; Zanker, J.M. Motion camouflage induced by zebra stripes. Zoology 2014, 117, 163–170. [Google Scholar] [CrossRef]
- Williams, D. Naval Camouflage 1914–1945: A Complete Visual Reference; Naval Institute Press: Annapolis, MD, USA, 2001. [Google Scholar]
- Dazzle Camouflage. Available online: https://en.wikipedia.org/wiki/Dazzle_camouflage (accessed on 24 September 2021).
- Rawlings, A.E.; Bramble, J.P.; Staniland, S.S. Innovation through imitation: Biomimetic, bioinspired and biokleptic research. Soft Matter 2012, 8, 6675–6679. [Google Scholar] [CrossRef]
- Greanya, V. Bioinspired Photonics: Optical Structures and Systems Inspired by Nature; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Juhl, S.B.; Chan, E.P.; Ha, Y.H.; Maldovan, M.; Brunton, J.; Ward, V.; Vaia, R.A. Assembly of Wiseana iridovirus: Viruses for colloidal photonic crystals. Adv. Funct. Mater. 2006, 16, 1086–1094. [Google Scholar] [CrossRef]
- Kinoshita, S.; Yoshioka, S.; Miyazaki, J. Physics of structural colors. Rep. Prog. Phys. 2008, 71, 076401. [Google Scholar] [CrossRef] [Green Version]
- Kolle, M. Photonic Structures Inspired by Nature; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Schenk, F.; Wilts, B.D.; Stavenga, D.G. The Japanese jewel beetle: A painter’s challenge. Bioinspir. Biomim. 2013, 8, 045002. [Google Scholar] [CrossRef]
- Galusha, J.W.; Richey, L.R.; Gardner, J.S.; Cha, J.N.; Bartl, M.H. Discovery of a diamond-based photonic crystal structure in beetle scales. Phys. Rev. E 2008, 77, 050904. [Google Scholar] [CrossRef]
- Vukusic, P.; Sambles, J.R.; Lawrence, C.R.; Wootton, R.J. Quantified interference and diffraction in single Morpho butterfly scales. Proc. R. Soc. Lond. B Biol. Sci. 1999, 266, 1403–1411. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, M.A.; Yoshioka, S.; Liu, C.; Stavenga, D.G. Coloration mechanisms and phylogeny of Morpho butterflies. J. Exp. Biol. 2016, 219, 3936–3944. [Google Scholar] [CrossRef] [Green Version]
- Johansen, V.E.; Onelli, O.D.; Steiner, L.M.; Vignolini, S. Photonics in nature: From order to disorder. In Functional Surfaces in Biology III; Springer: Cham, Switzerland, 2017; pp. 53–89. [Google Scholar]
- Kinoshita, S.; Yoshioka, S.; Fujii, Y.; Okamoto, N. Photophysics of structural color in the Morpho butterflies. Forma 2002, 17, 103–121. [Google Scholar]
- Rossbach, V.; Patanathabutr, P.; Wichitwechkarn, J. Copying and manipulating nature: Innovation for textile materials. Fibers Polym. 2003, 4, 8–14. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y. Nanofabrication and coloration study of artificial Morpho butterfly wings with aligned lamellae layers. Sci. Rep. 2015, 5, 16637. [Google Scholar] [CrossRef] [Green Version]
- Dumanli, A.G.; Savin, T. Recent advances in the biomimicry of structural colours. Chem. Soc. Rev. 2016, 45, 6698–6724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Tommasi, E.; Congestri, R.; Dardano, P.; De Luca, A.C.; Managò, S.; Rea, I.; De Stefano, M. UV-shielding and wavelength conversion by centric diatom nanopatterned frustules. Sci. Rep. 2018, 8, 16285. [Google Scholar] [CrossRef]
- Lavanga, L.; Dardano, P.; De Stefano, L.; Rendina, I.; De Tommasi, E.; De Stefano, M.; Langella, C.; de Luca, A.C.; Dholakia, K.; Mazilu, M. Sub-diffractive light confinement: A biological-based approach. In Proceedings of the 2014 Fotonica AEIT Italian Conference on Photonics Technologies, Naples, Italy, 12–14 May 2014; pp. 1–3. [Google Scholar]
- Wilson, T.; Hastings, J.W. Bioluminescence. Annu. Rev. Cell Dev. Biol. 1998, 14, 197–230. [Google Scholar] [CrossRef]
- Prescher, J.A.; Contag, C.H. Guided by the light: Visualizing biomolecular processes in living animals with bioluminescence. Curr. Opin. Chem. Biol. 2010, 14, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Reeve, B.; Sanderson, T.; Ellis, T.; Freemont, P. How Synthetic Biology Will Reconsider Natural Bioluminescence and Its Applications; Bioluminescence: Fundamentals and Applications in Biotechnology-Volume 2; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–30. [Google Scholar]
- Singh, A.V.; Rahman, A.; Kumar, N.S.; Aditi, A.S.; Galluzzi, M.; Bovio, S.; Barozzi, S.; Montani, E.; Parazzoli, D. Bio-inspired approaches to design smart fabrics. Mater. Des. 2012, 36, 829–839. [Google Scholar] [CrossRef]
- Zupanc, G.K.; Bullock, T.H. From electrogenesis to electroreception: An overview. In Electroreception; Springer: New York, NY, USA, 2005; pp. 5–46. [Google Scholar]
- Debabov, V.G. Electricity from microorganisms. Microbiology 2008, 77, 123–131. [Google Scholar] [CrossRef]
- Meet the Electric Life Forms That Live on Pure Energy. Available online: https://www.newscientist.com/article/dn25894-meet-the-electric-life-forms-that-live-on-pure-energy/ (accessed on 24 September 2021).
- Conley, B. Microbial extracellular electron transfer is a far-out metabolism. Am. Soc. Microbiol. 2019. Available online: https://asm.org/Articles/2019/November/Microbial-Extracellular-Electron-Transfer-is-a-Far (accessed on 24 September 2021).
- Nelson, M.E.; Maciver, M.A. Prey capture in the weakly electric fish Apteronotus albifrons: Sensory acquisition strategies and electrosensory consequences. J. Exp. Biol. 1999, 202, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Vaknin, Y.; Gan-Mor, S.; Bechar, A.; Ronen, B.; Eisikowitch, D. The role of electrostatic forces in pollination. In Pollen and Pollination; Springer: Vienna, Austria, 2000; pp. 133–142. [Google Scholar]
- Greggers, U.; Koch, G.; Schmidt, V.; Dürr, A.; Floriou-Servou, A.; Piepenbrock, D.; Göpfert, M.C.; Menzel, R. Reception and learning of electric fields in bees. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130528. [Google Scholar] [CrossRef] [Green Version]
- Lissmann, H.W.; Machin, K.E. The mechanism of object location in Gymnarchus niloticus and similar fish. J. Exp. Biol. 1958, 35, 451–486. [Google Scholar] [CrossRef]
- Baffet, G.; Boyer, F.; Gossiaux, P.B. Biomimetic localization using the electrolocation sense of the electric fish. In Proceedings of the 2008 IEEE International Conference on Robotics and Biomimetics, Bangkok, Thailand, 22–25 February 2009; pp. 659–664. [Google Scholar]
- Neveln, I.D.; Bai, Y.; Snyder, J.B.; Solberg, J.R.; Curet, O.M.; Lynch, K.M.; MacIver, M.A. Biomimetic and bio-inspired robotics in electric fish research. J. Exp. Biol. 2013, 216, 2501–2514. [Google Scholar] [CrossRef] [Green Version]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Dubonos, S.V.; Grigorieva, I.V.; Novoselov, K.S.; Zhukov, A.A.; Shapoval, S.Y. Microfabricated adhesive mimicking gecko foot-hair. Nat. Mater. 2003, 2, 461–463. [Google Scholar] [CrossRef]
- Ge, L.; Sethi, S.; Ci, L.; Ajayan, P.M.; Dhinojwala, A. Carbon nanotube-based synthetic gecko tapes. Proc. Natl. Acad. Sci. USA 2007, 104, 10792–10795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parness, A.; Soto, D.; Esparza, N.; Gravish, N.; Wilkinson, M.; Autumn, K.; Cutkosky, M. A microfabricated wedge-shaped adhesive array displaying gecko-like dynamic adhesion, directionality and long lifetime. J. R. Soc. Interface 2009, 6, 1223–1232. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, M.D.; Croll, A.B.; King, D.R.; Paret, B.M.; Irschick, D.J.; Crosby, A.J. Looking beyond fibrillar features to scale gecko-like adhesion. Adv. Mater. 2012, 24, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Labonte, D.; Clemente, C.J.; Dittrich, A.; Kuo, C.Y.; Crosby, A.J.; Irschick, D.J.; Federle, W. Extreme positive allometry of animal adhesive pads and the size limits of adhesion-based climbing. Proc. Natl. Acad. Sci. USA 2016, 113, 1297–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilman, C.A.; Imburgia, M.J.; Bartlett, M.D.; King, D.R.; Crosby, A.J.; Irschick, D.J. Geckos as springs: Mechanics explain across-species scaling of adhesion. PLoS ONE 2015, 10, e0134604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, B.N.J. On the mechanism of adhesion in biological systems. J. Chem. Phys. 2003, 118, 7614–7621. [Google Scholar] [CrossRef] [Green Version]
- Arzt, E.; Gorb, S.; Spolenak, R. From micro to nano contacts in biological attachment devices. Proc. Natl. Acad. Sci. USA 2003, 100, 10603–10606. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Yao, H. Shape insensitive optimal adhesion of nanoscale fibrillar structures. Proc. Natl. Acad. Sci. USA 2004, 101, 7851–7856. [Google Scholar] [CrossRef] [Green Version]
- Peattie, A.M.; Full, J. Phylogenetic analysis of the scaling of wet and dry biological fibrillar adhesives. Proc. Natl. Acad. Sci. USA 2007, 104, 18595–18600. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, N. Animal bioacoustics. In Springer Handbook of Acoustics; Springer: New York, NY, USA, 2007; p. 785. [Google Scholar]
- Erbe, C.; Dent, M.L. What is animal bioacoustics? J. Acoust. Soc. Am. 2016, 139, 2004. [Google Scholar] [CrossRef]
- Lilley, G. A Study of the silent flight of the owl. In Proceedings of the 4th AIAA/CEAS Aeroacoustics Conference, Toulouse, France, 2–4 June 1998; pp. 1–6. [Google Scholar]
- Chen, K.; Liu, Q.; Liao, G.; Yang, Y.; Ren, L.; Yang, H.; Chen, X. The sound suppression characteristics of wing feather of owl (Bubo bubo). J. Bionic Eng. 2012, 9, 192–199. [Google Scholar] [CrossRef]
- Wagner, H.; Weger, M.; Klaas, M.; Schröder, W. Features of owl wings that promote silent flight. Interface Focus 2017, 7, 20160078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersh, A.S.; Hayden, R.E. Aerodynamic Sound Radiation from Lifting Surfaces with and without Leading-Edge Serrations; NASA Contract Report No. 114370; NASA: Washington, DC, USA, 1971. [Google Scholar]
- Sodermann, P.T. Aerodynamic Effects of Leading-Edge Serrations on a Two Dimensional Airfoil; NASA TM. X-2643; NASA: Washington, DC, USA, 1973. [Google Scholar]
- Juknevicius, A.; Chong, T.P. On the leading edge noise and aerodynamics of thin aerofoil subjected to the straight and curved serrations. J. Sound Vib. 2018, 425, 324–343. [Google Scholar] [CrossRef]
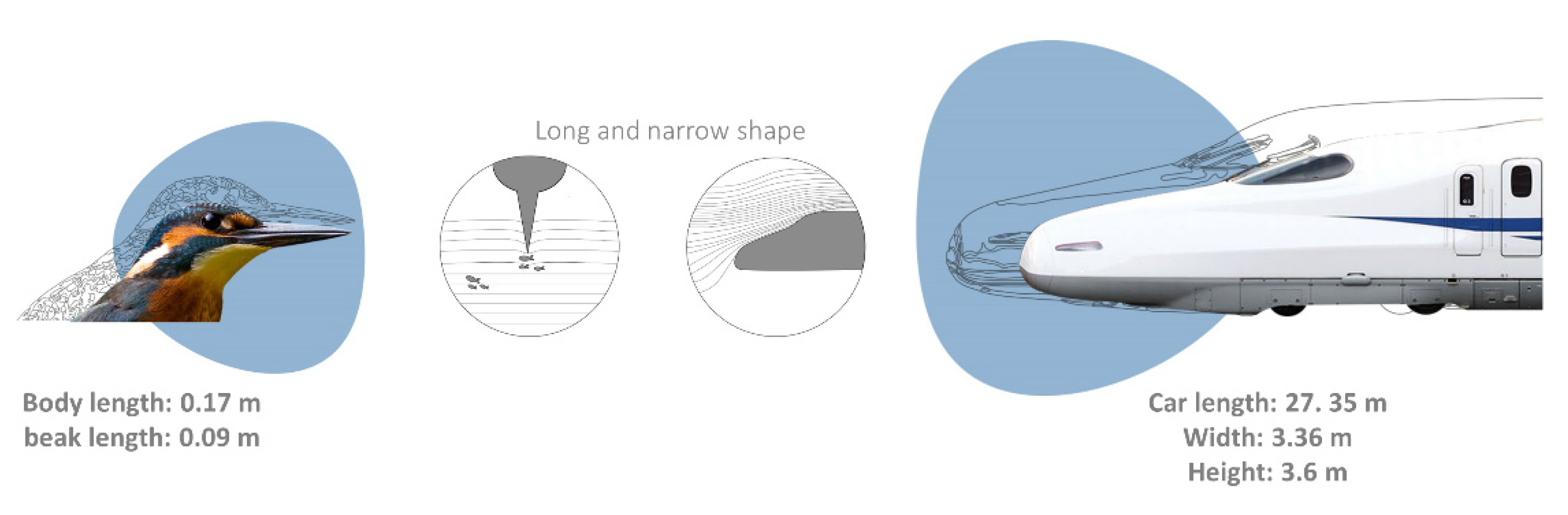
- Vincent, L.; Xiao, T.; Yohann, D.; Jung, S.; Kanso, E. Dynamics of water entry. J. Fluid Mech. 2018, 846, 508. [Google Scholar] [CrossRef]
- Crandell, K.E.; Howe, R.O.; Falkingham, P.L. Repeated evolution of drag reduction at the air–water interface in diving kingfishers. J. R. Soc. Interface 2019, 16, 20190125. [Google Scholar] [CrossRef] [Green Version]
- McKeag, T. Auspicious forms. Zygote Q. 2012, 2, 14–35. [Google Scholar]
- Shinkansen. Available online: https://pngimage.net/shinkansen-png-4 (accessed on 24 September 2021).
- Griffin, D.R. Listening in the Dark: The Acoustic Orientation of Bats and Men; Yale University Press: New Haven, CT, USA, 1958. [Google Scholar]
- Au, W.W.; Hastings, M.C. Principles of Marine Bioacoustics; Springer: New York, NY, USA, 2008. [Google Scholar]
- Erbe, C. Underwater Acoustics: Noise and the Effects on Marine Mammals, 3rd ed.; JASCO Applied Sciences: Brisbane, QLD, Australia, 2010. [Google Scholar]
- Qiao, G.; Bilal, M.; Liu, S.; Babar, Z.; Ma, T. Biologically inspired covert underwater acoustic communication—A review. Phys. Commun. 2018, 30, 107–114. [Google Scholar] [CrossRef]
- Bannasch, R.; Kebkal, K.; Yakovlev, S.; Kebkal, A. Fast and reliable underwater communication: Successful applications of biologically inspired techniques. In Proceedings of the 25th International Conference on Offshore Mechanics and Arctic Engineering, Hamburg, Germany, 4–9 June 2006; Volume 47462, pp. 741–747. [Google Scholar]
- Richard, B.; Wainwright, P. Scaling the feeding mechanism of largemouth bass (Micropterus salmoides): Kinematics of prey capture. J. Exp. Biol. 1995, 198, 419–433. [Google Scholar] [CrossRef]
- Ilton, M.; Cox, S.M.; Egelmeers, T.; Sutton, G.P.; Patek, S.N.; Crosby, A.J. The effect of size-scale on the kinematics of elastic energy release. Soft Matter 2019, 15, 9579–9586. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.C., VIII. A dynamical theory of the electromagnetic field. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1865, 155, 459–512. [Google Scholar]
- Denny, M.; McFadzean, A. Engineering Animals; Harvard University Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Bai, Y.; Snyder, J.; Silverman, Y.; Peshkin, M.; MacIver, M.A. Sensing capacitance of underwater objects in bio-inspired electrosense. In Proceedings of the 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems, Vilamoura-Algarve, Portugal, 7–12 October 2012. [Google Scholar]
- McKenna, M.F.; Cranford, T.W.; Berta, A.; Pyenson, N.D. Morphology of the odontocete melon and its implications for acoustic function. Mar. Mammal Sci. 2012, 28, 690–713. [Google Scholar] [CrossRef]
- Deb, K. Multi-Objective Optimization Using Evolutionary Algorithms; Wiley: Chichester, UK, 2001. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perricone, V.; Santulli, C.; Rendina, F.; Langella, C. Organismal Design and Biomimetics: A Problem of Scale. Biomimetics 2021, 6, 56. https://doi.org/10.3390/biomimetics6040056
Perricone V, Santulli C, Rendina F, Langella C. Organismal Design and Biomimetics: A Problem of Scale. Biomimetics. 2021; 6(4):56. https://doi.org/10.3390/biomimetics6040056
Chicago/Turabian StylePerricone, Valentina, Carlo Santulli, Francesco Rendina, and Carla Langella. 2021. "Organismal Design and Biomimetics: A Problem of Scale" Biomimetics 6, no. 4: 56. https://doi.org/10.3390/biomimetics6040056








