Biogenic Metal Oxides
Abstract
:1. Introduction
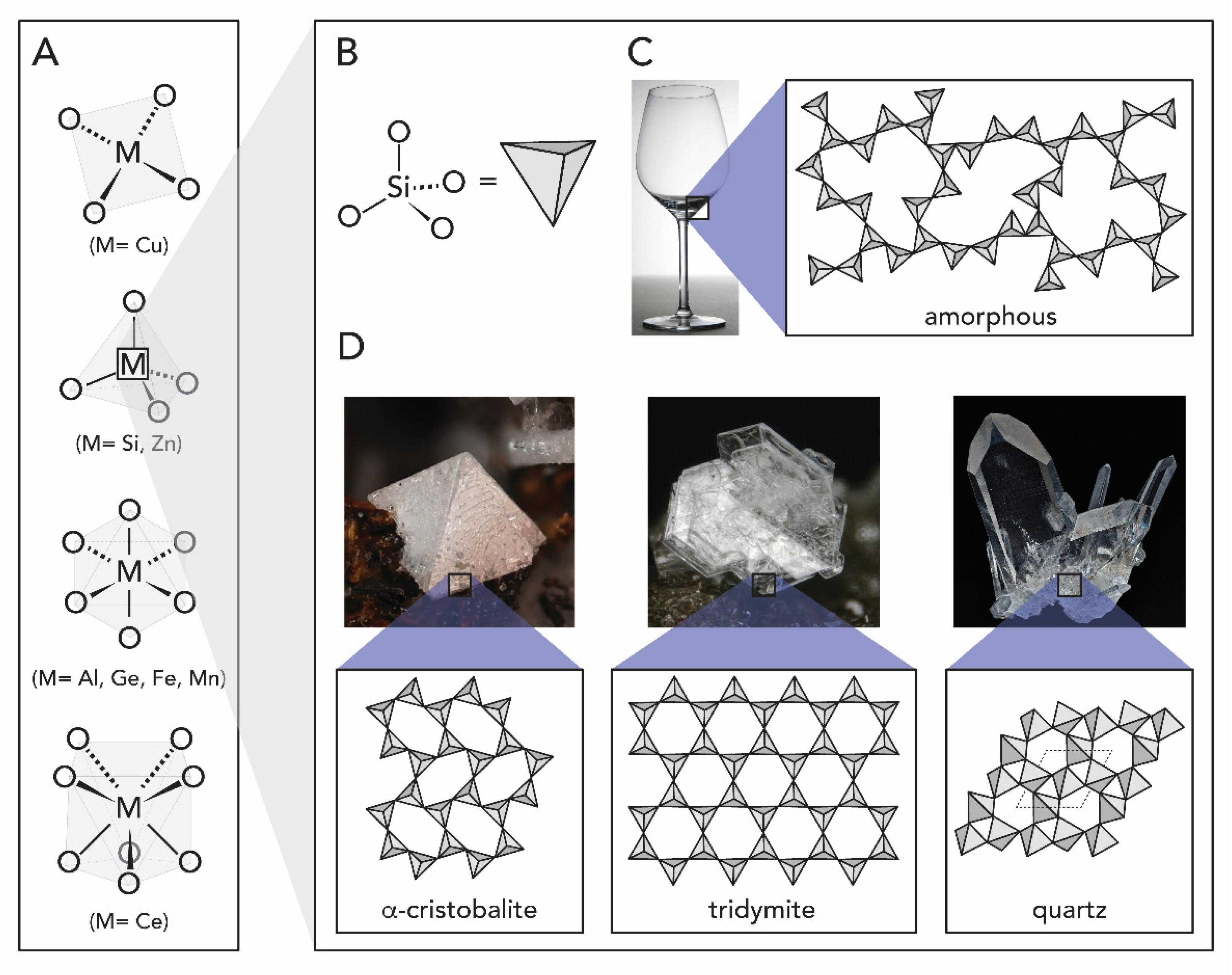
1.1. Metal Oxides
1.2. Origins of the Precursors of Biogenic Metal Oxides
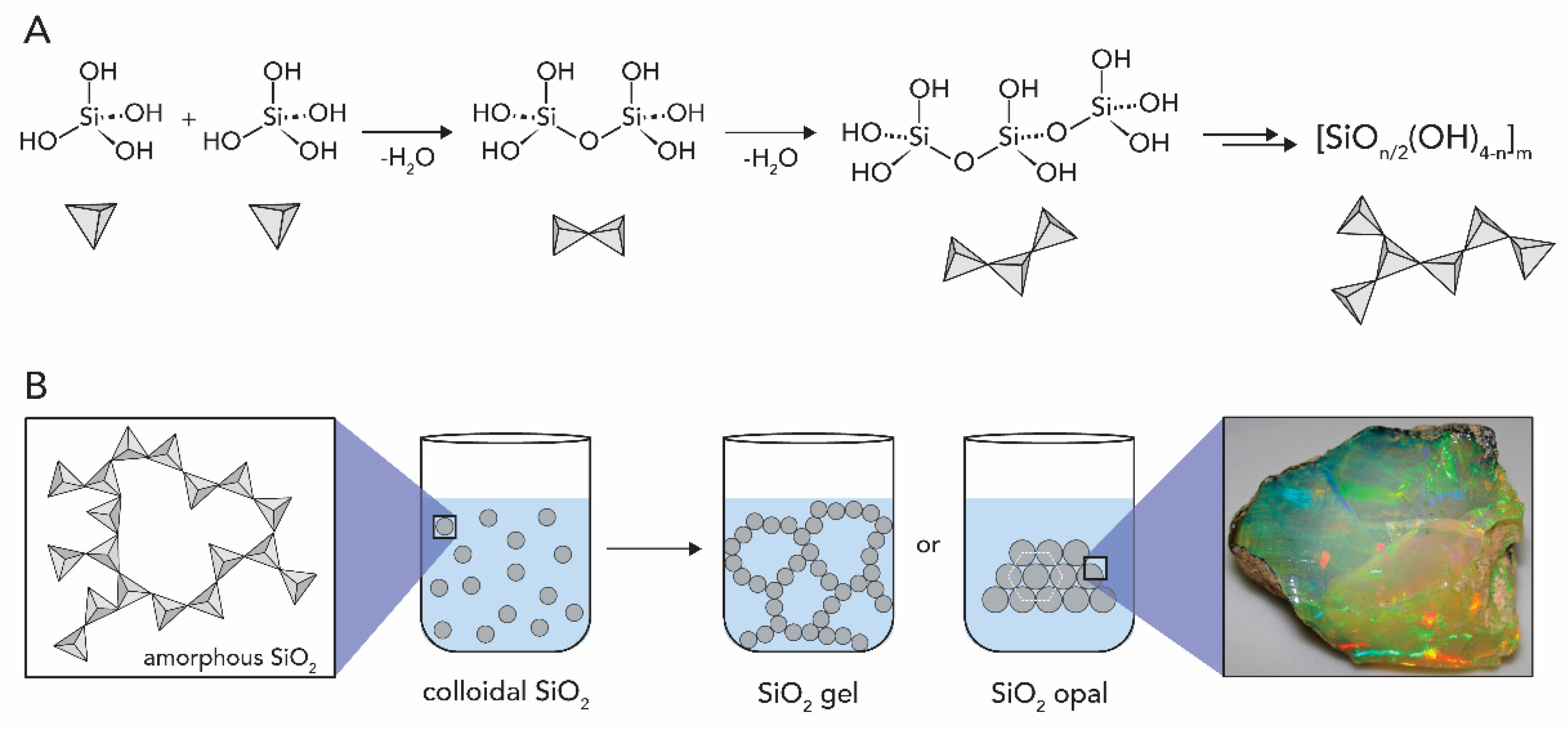
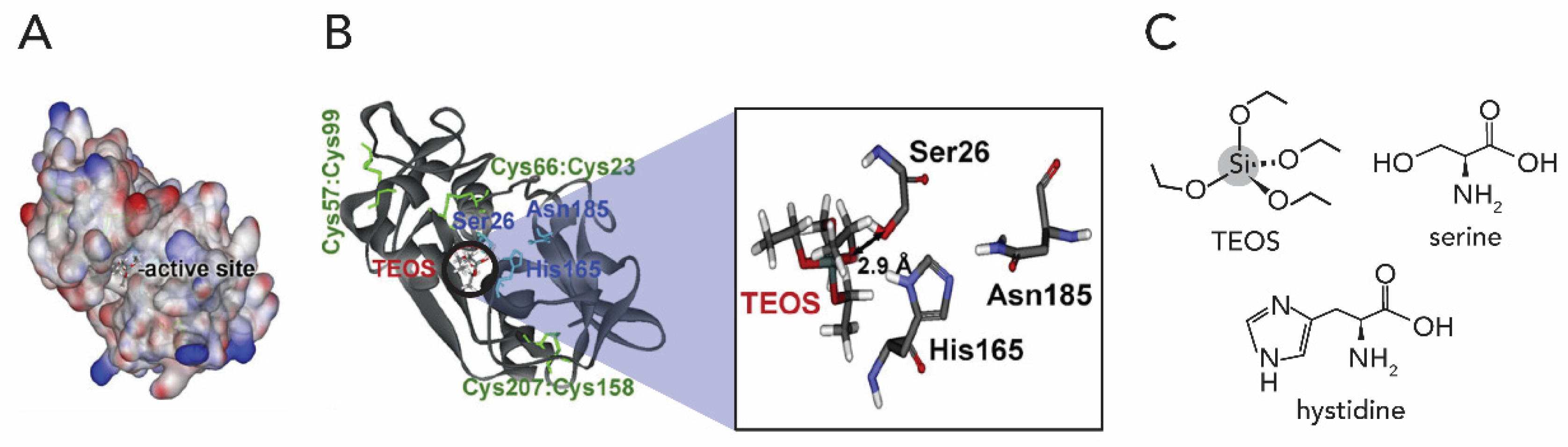
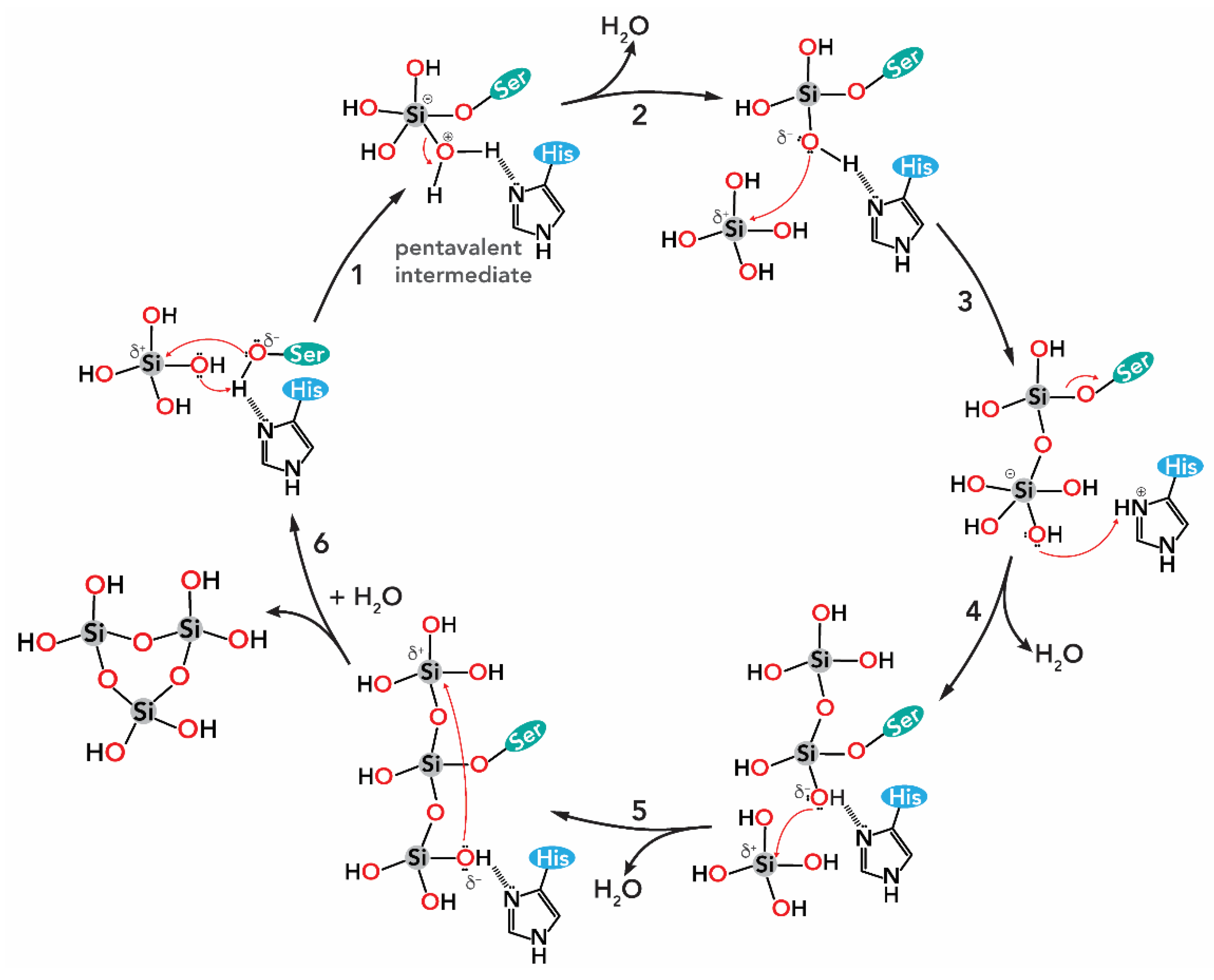
1.2.1. Silicon Oxide
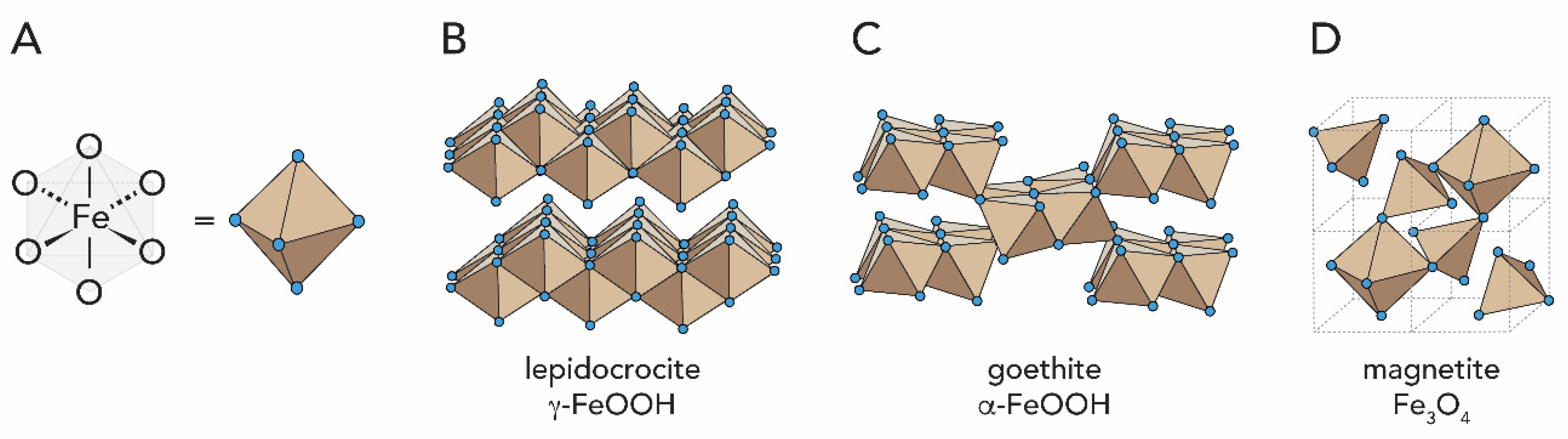
1.2.2. Iron Oxides
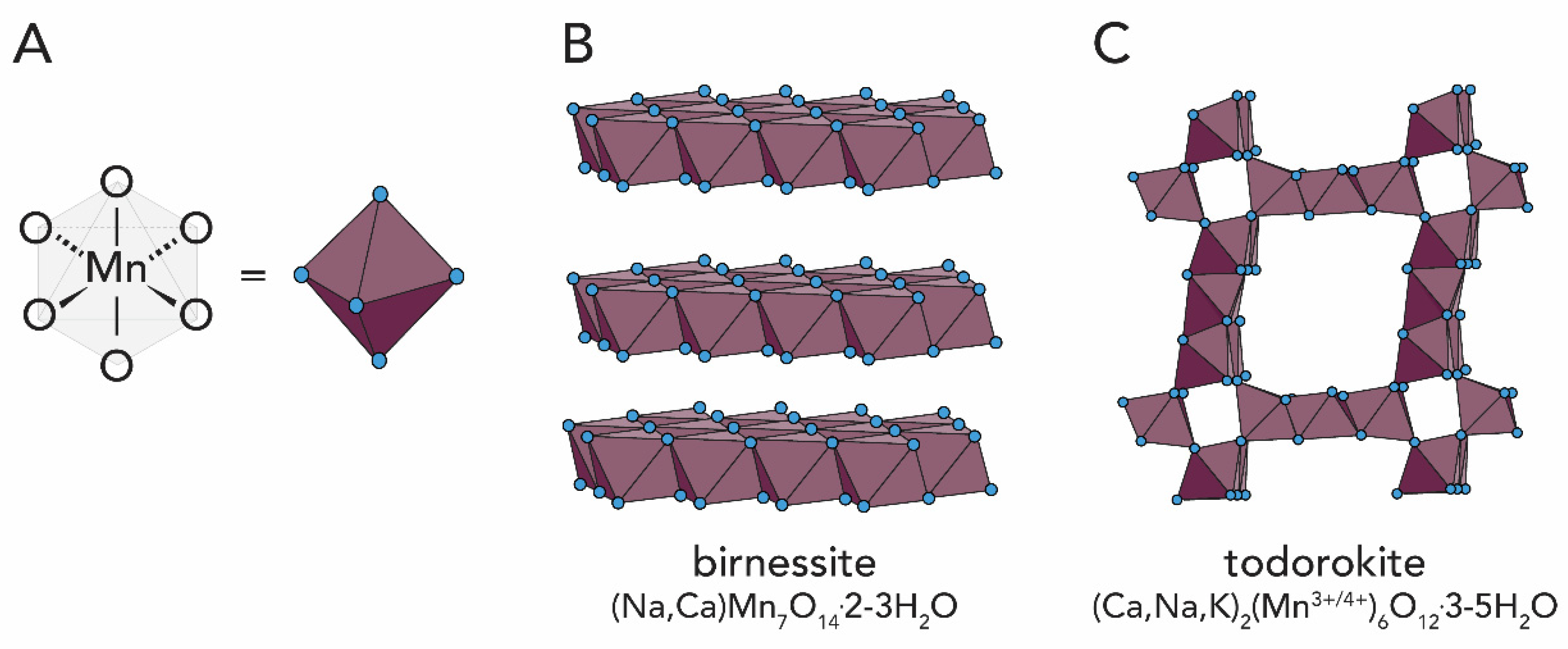
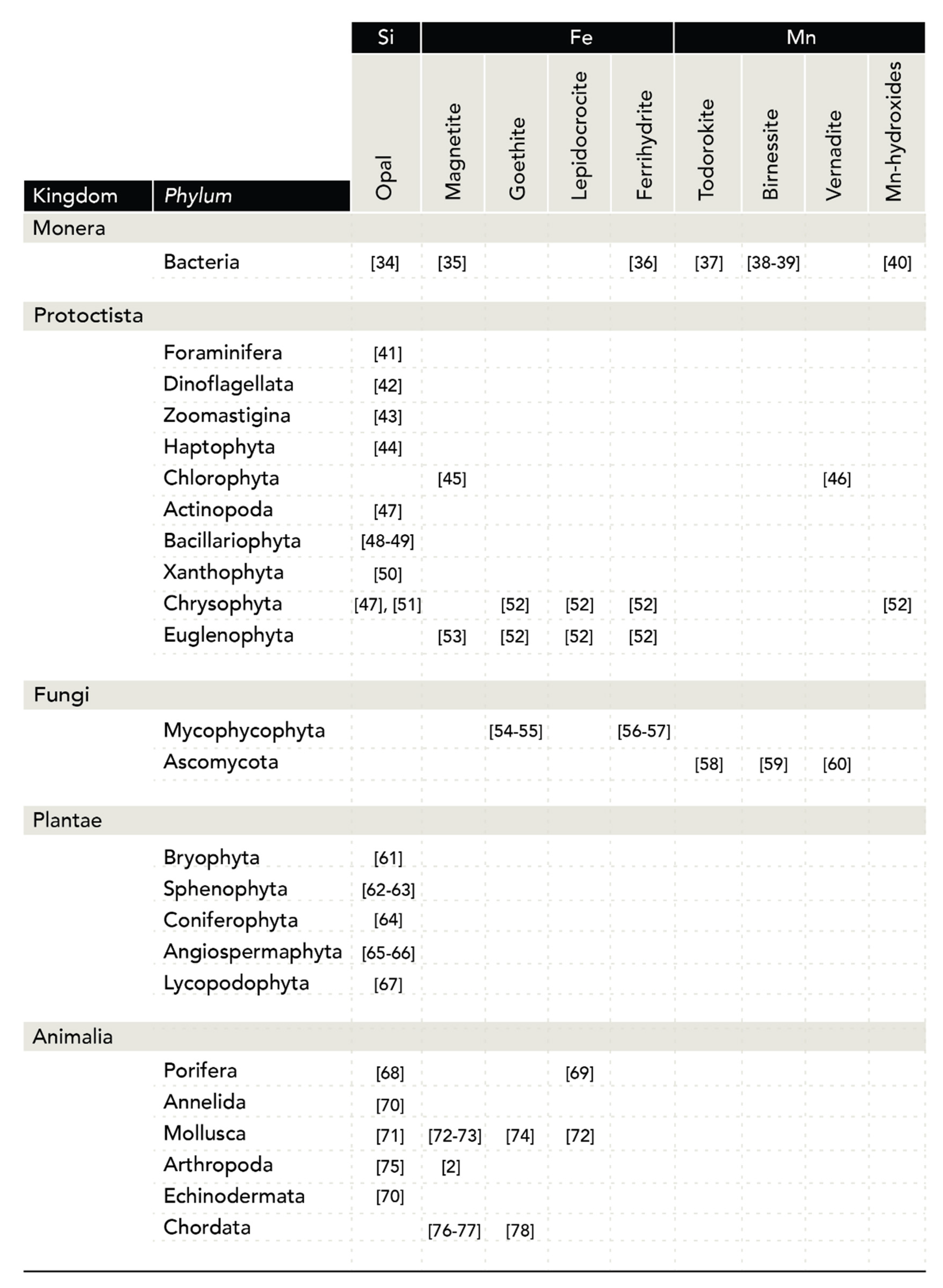
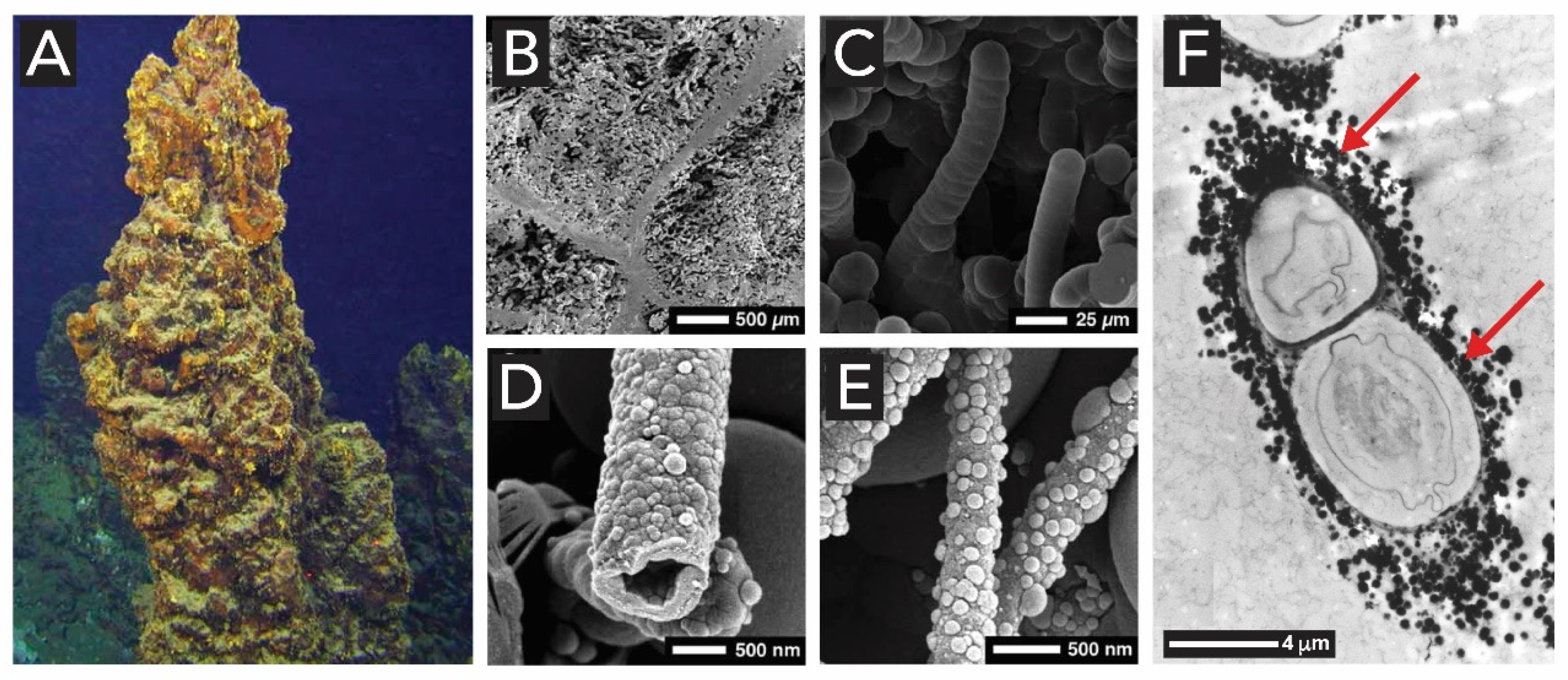
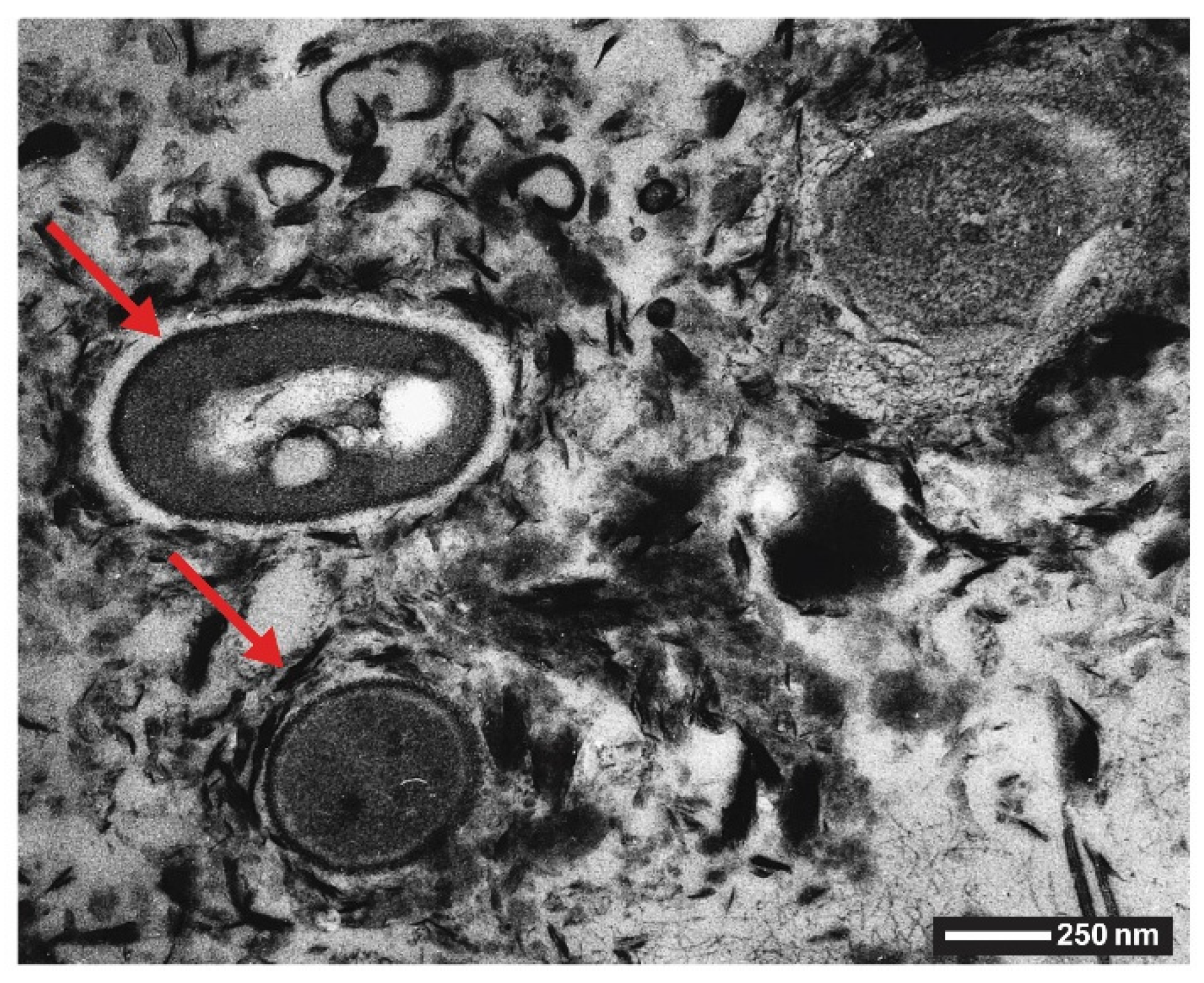
1.2.3. Manganese Oxides
2. The Biogenesis of Metal Oxides
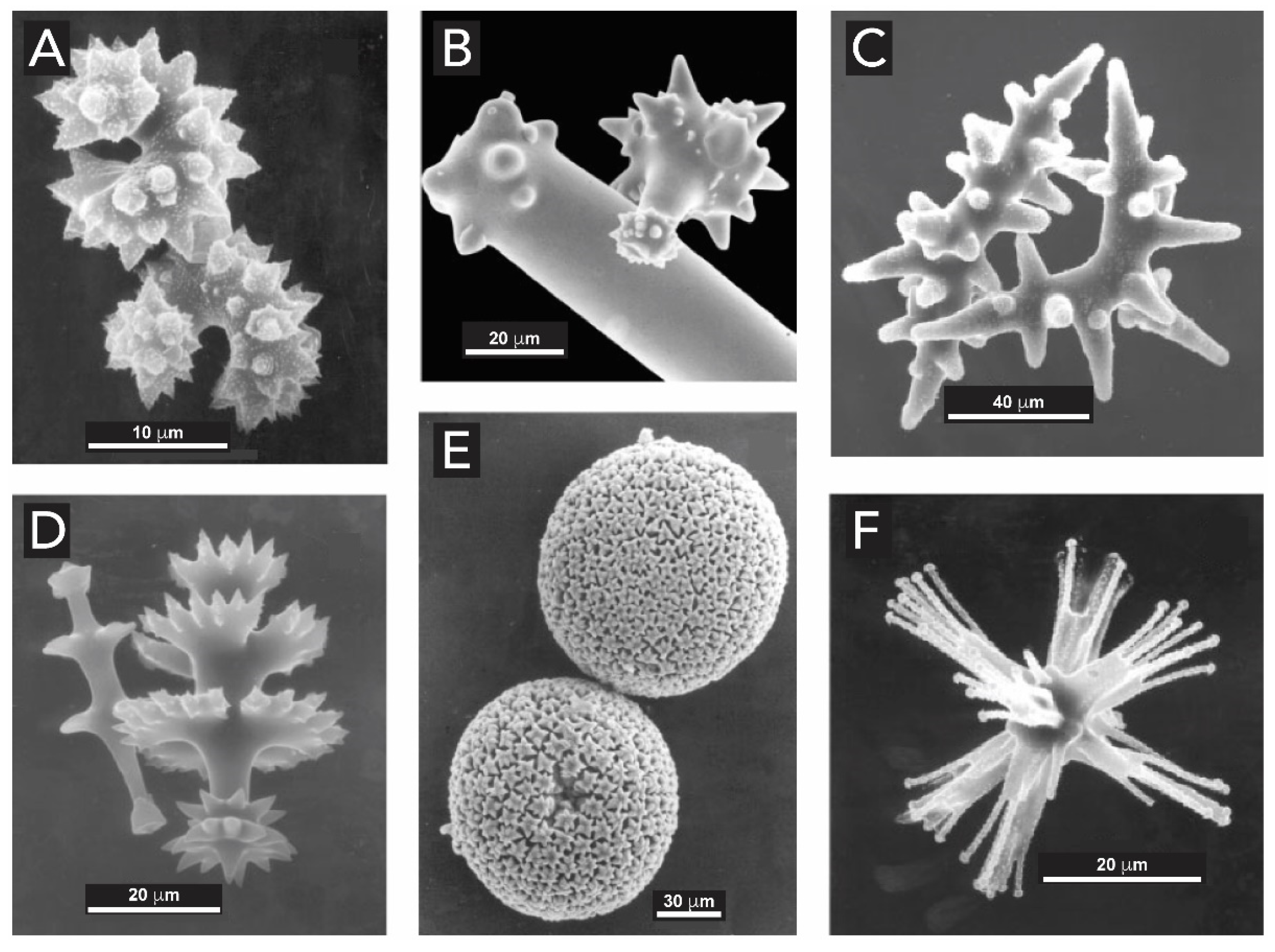
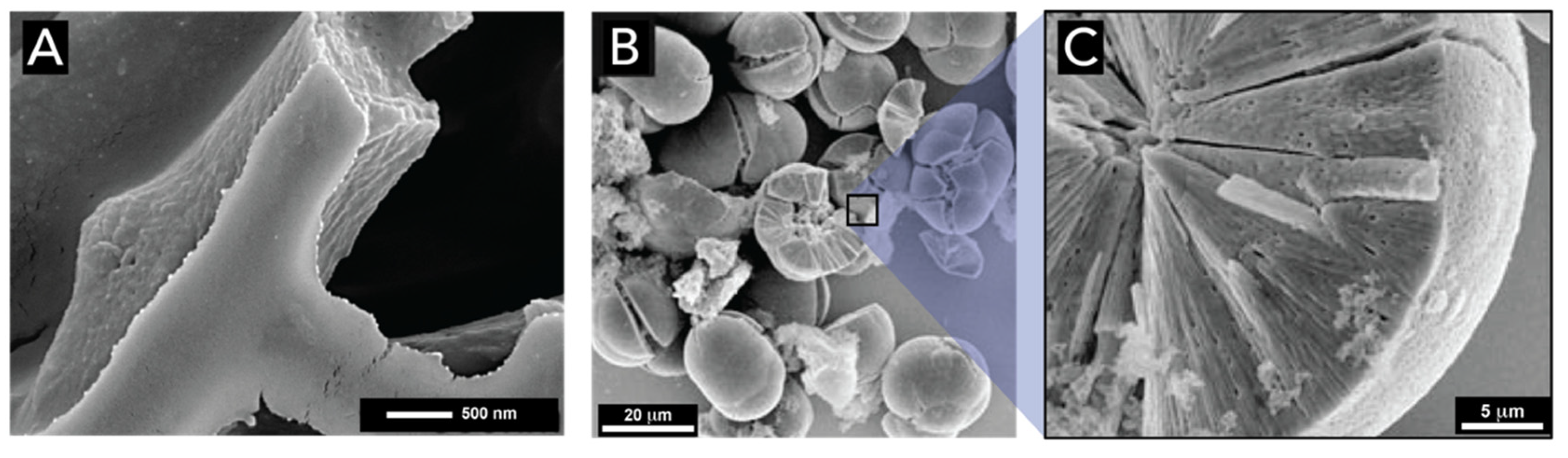
2.1. Silicon Dioxide
2.1.1. Bacteria (Kingdom Monera)
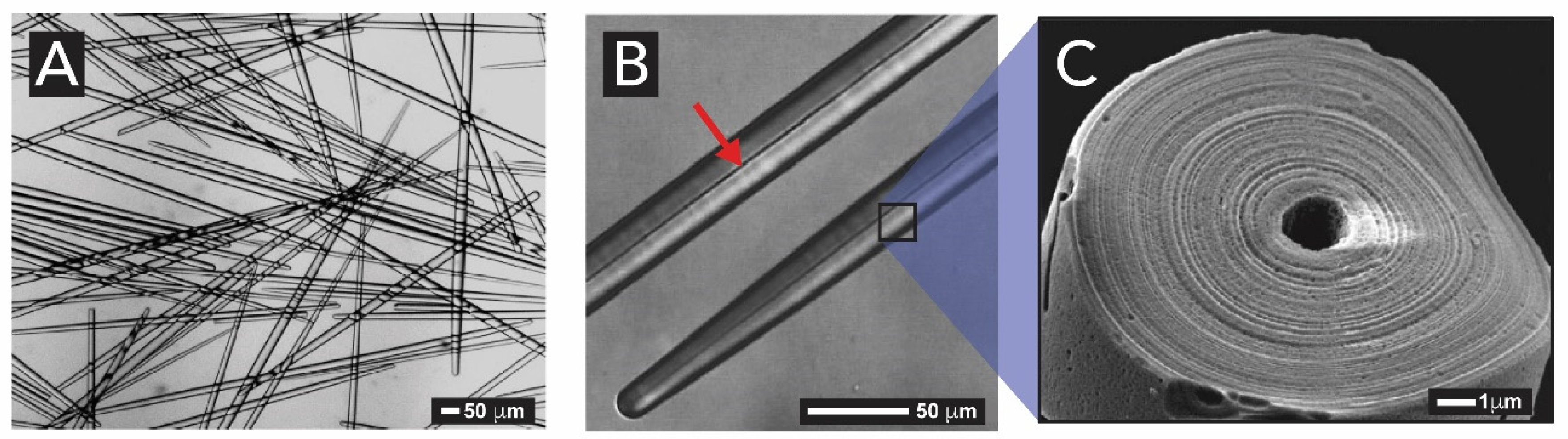
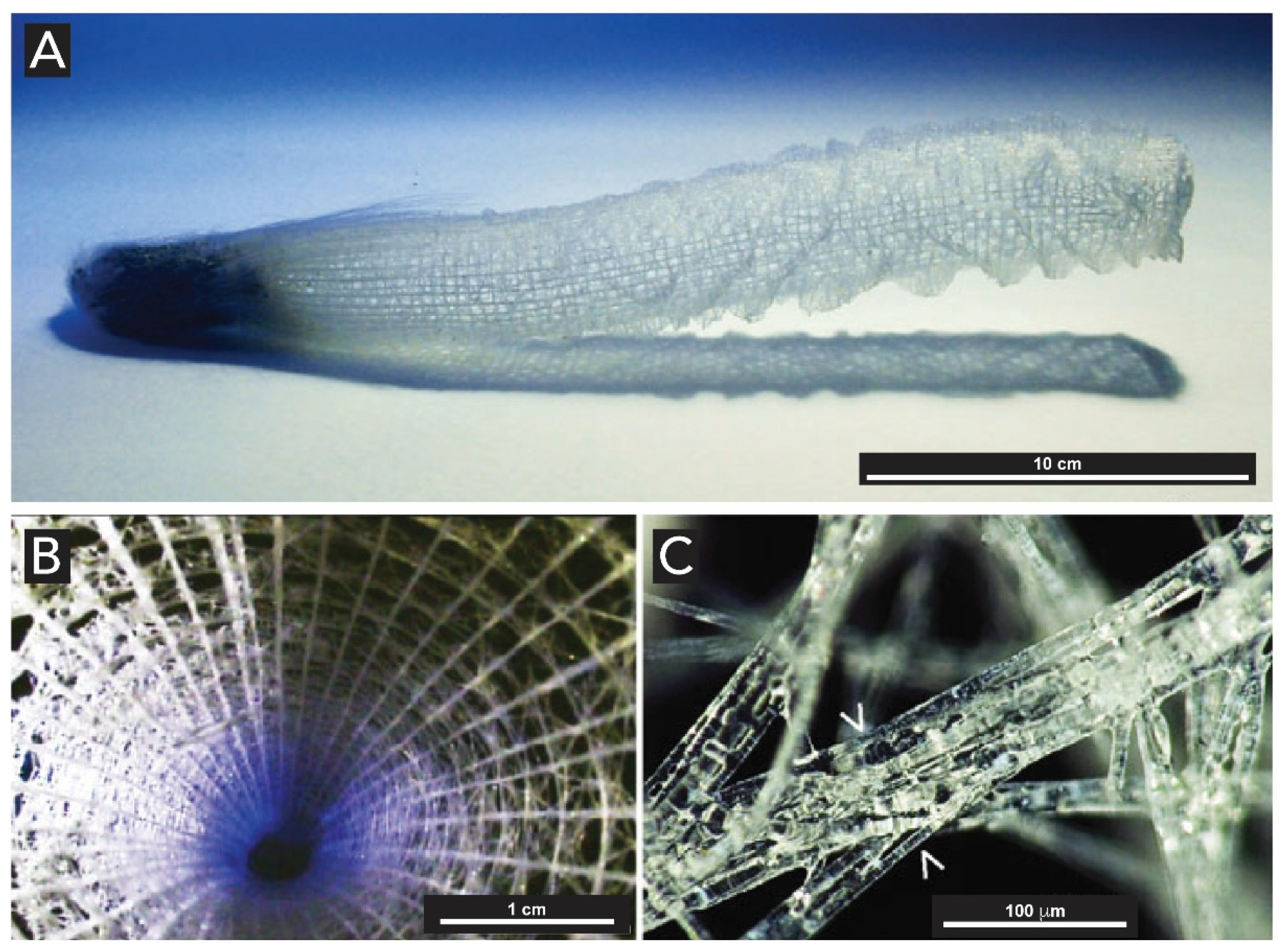
2.1.2. Sponges (Kingdom Animalia, Phylum Porifera)
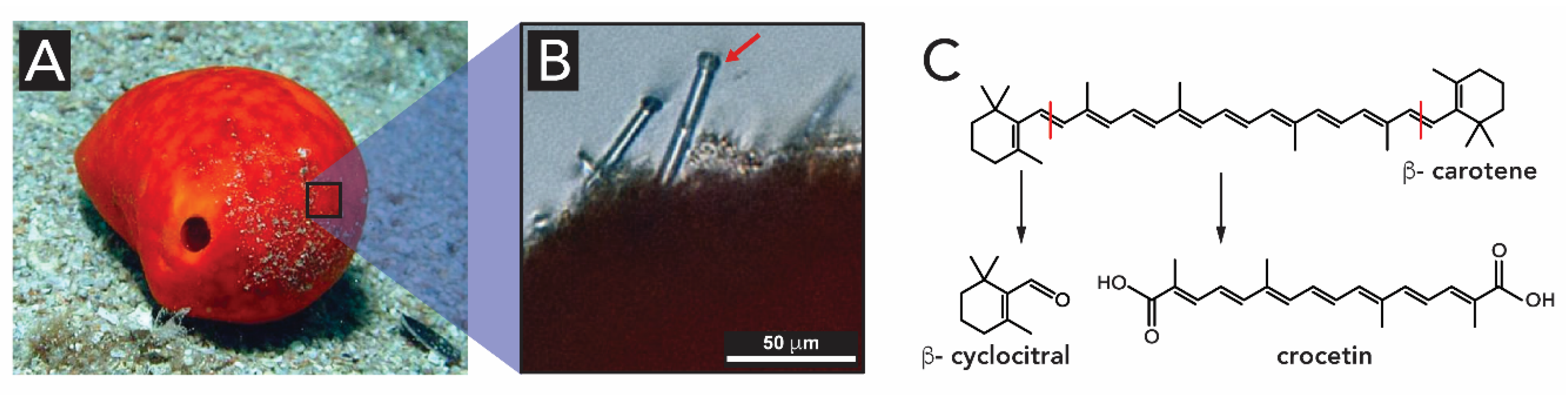
2.1.3. Light-Harvesting in Sponges
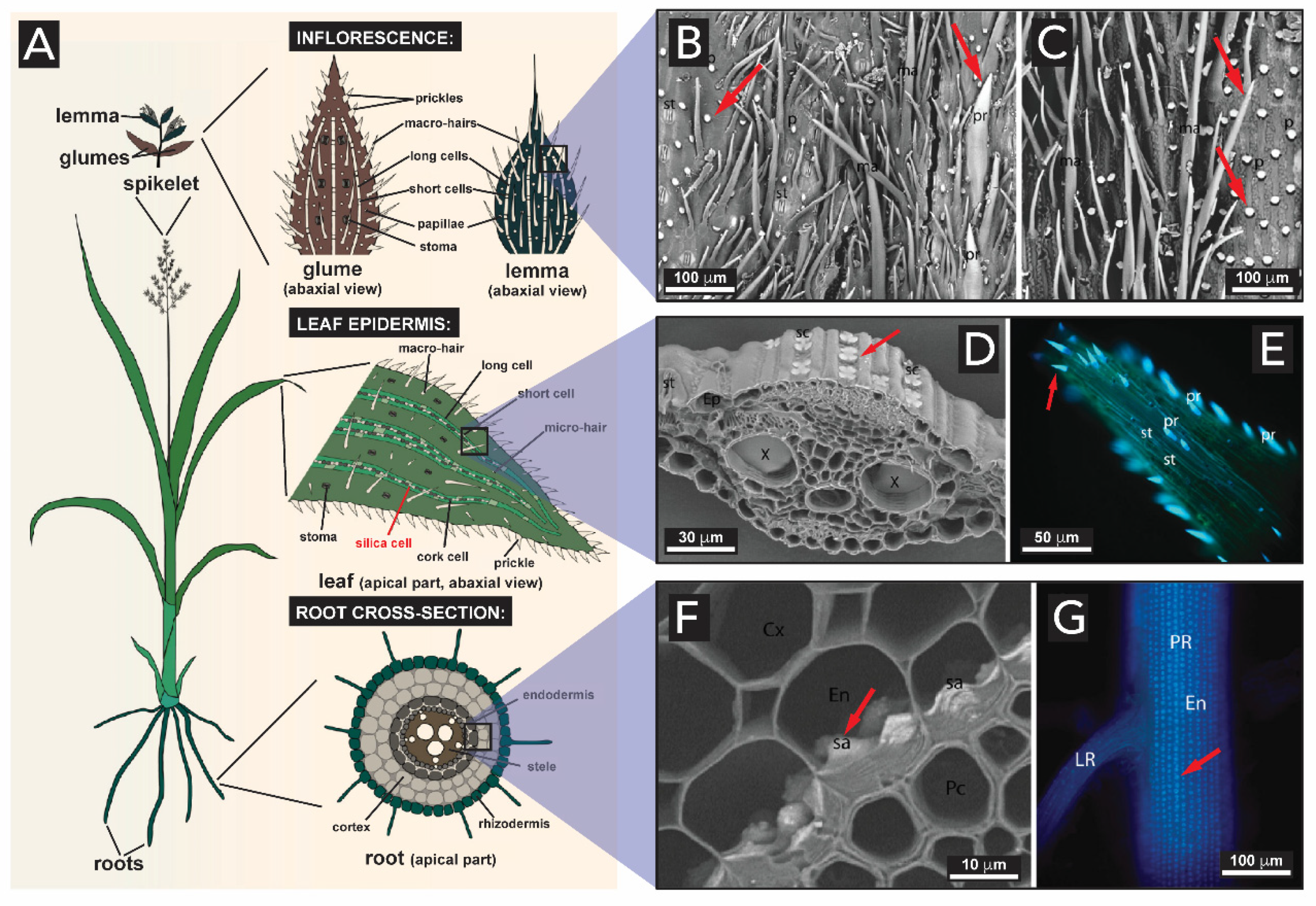
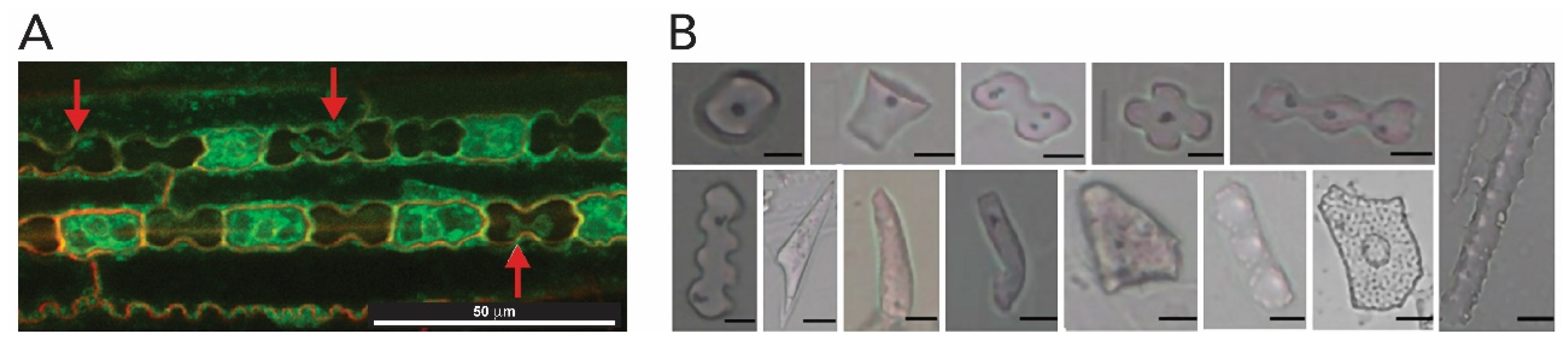
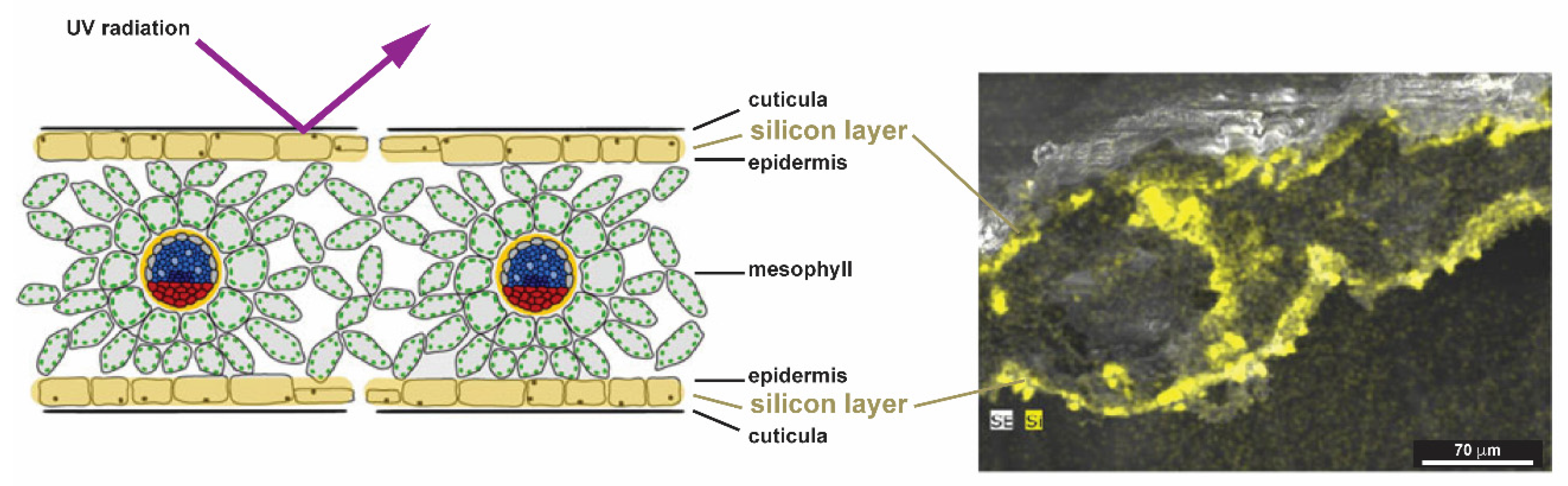
2.1.4. Plants (Kingdom Plantae)
2.2. Aluminum Oxide and Germanium Oxide
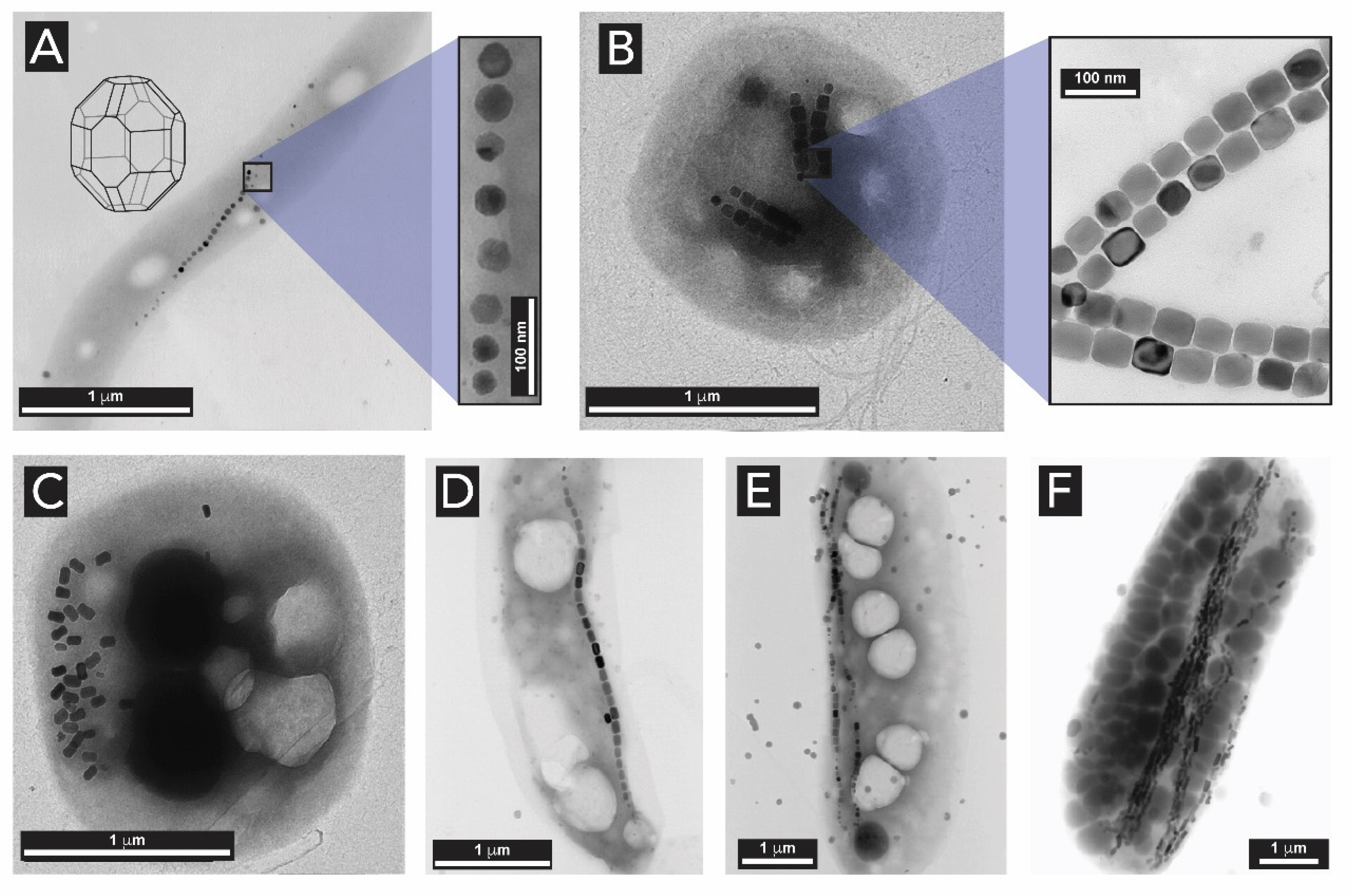
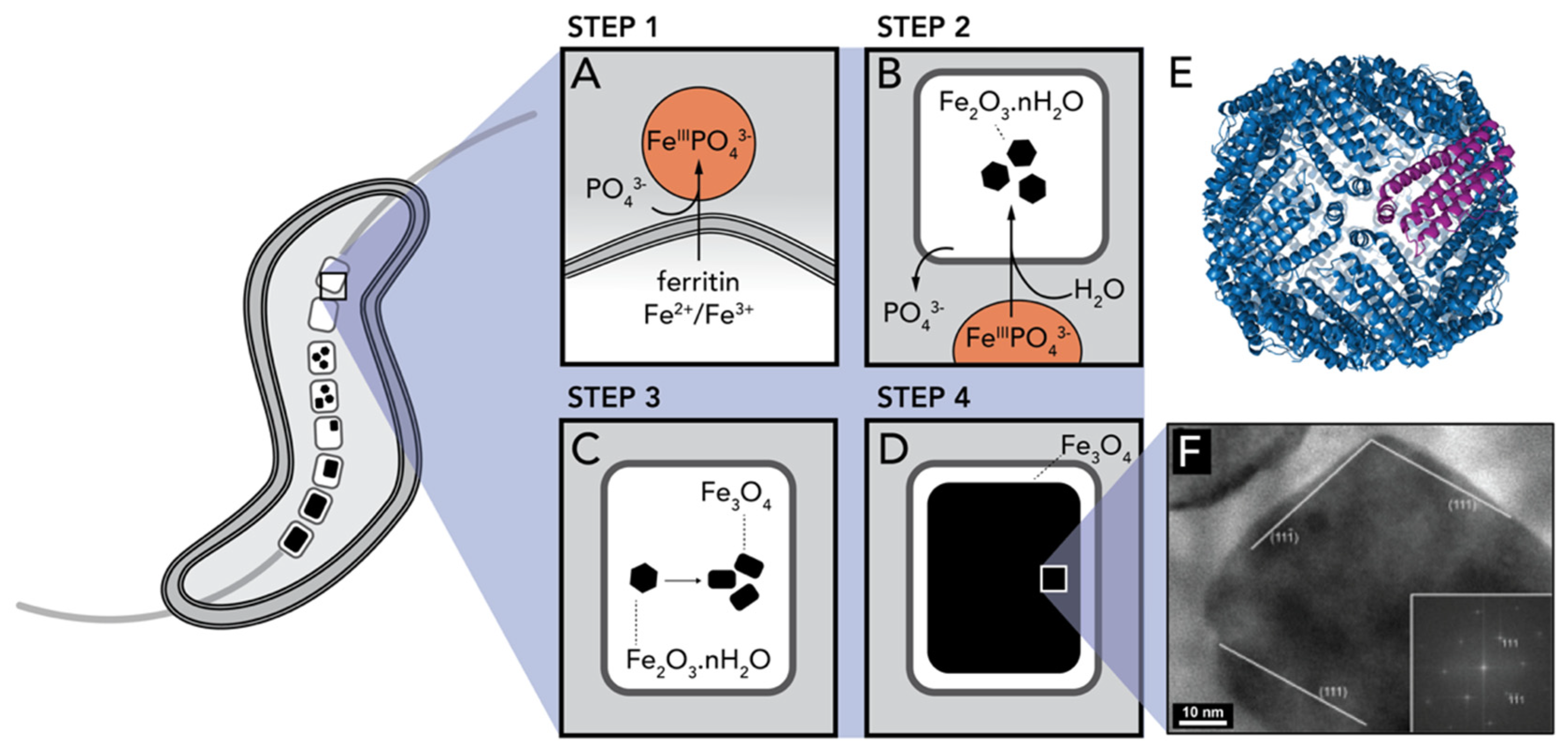
2.3. Iron Oxides
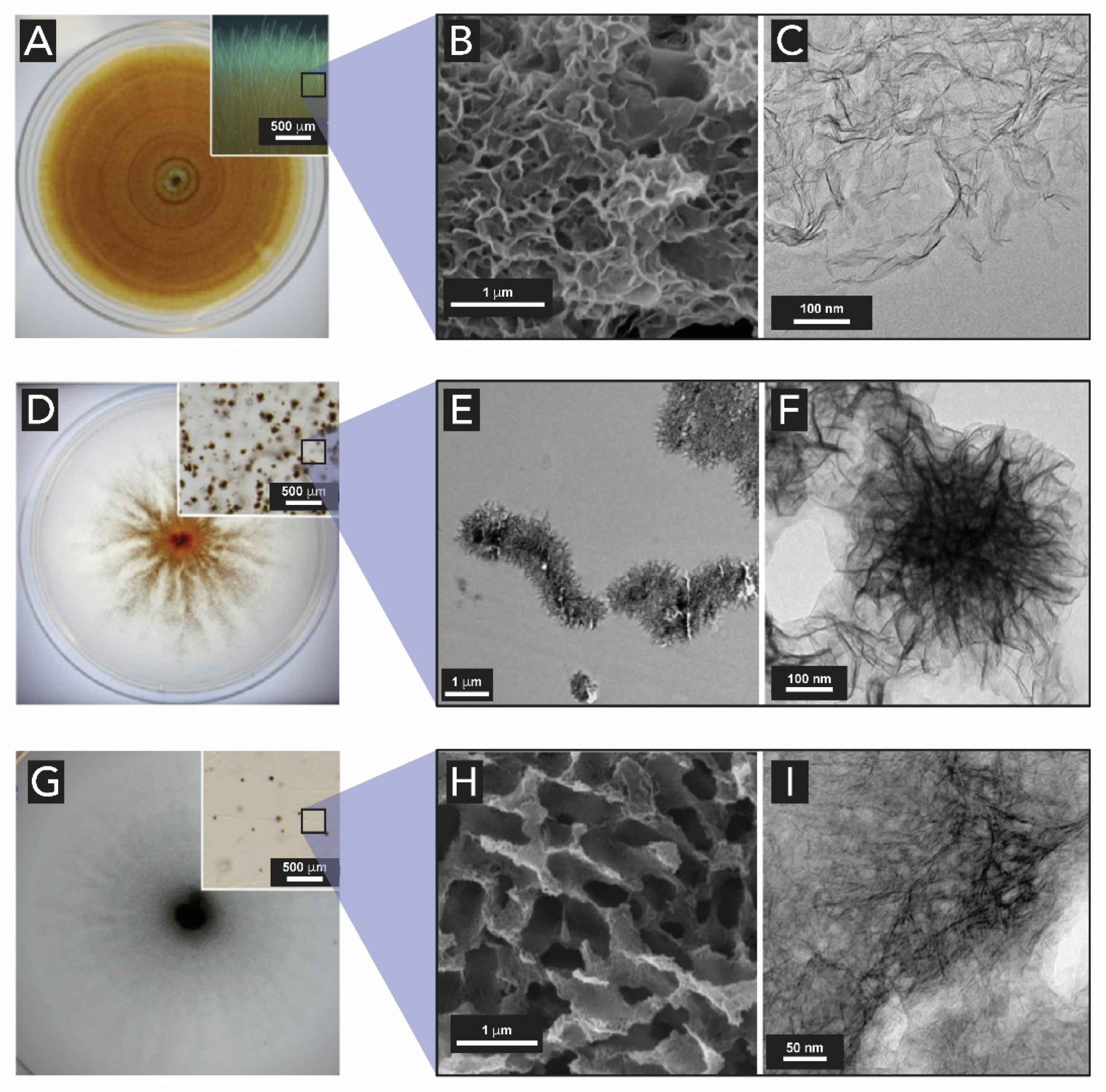
2.4. Manganese Oxides
3. Biomimetics of Biogenic Metal Oxide Structures and Further Inspirations and Perspectives for Materials Synthesis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faivre, D.; Schüler, D. Magnetotactic bacteria and magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.L.; Kirschvink, J.L.; Deffeyes, K.S. Bees have magnetic remanence. Science 1978, 201, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.L.; Volcani, B.E. Silicon and Siliceous Structures in Biological Systems, 1st ed.; Simpson, T.L., Volcani, B.E., Eds.; Springer: New York, NY, USA, 1981; ISBN 9781461259466. [Google Scholar]
- Crichton, R. Biomineralization. In Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function; Elsevier: Amsterdam, The Netherlands, 2019; pp. 517–544. ISBN 9780128117415. [Google Scholar]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unterlass, M. Geomimetics and Extreme Biomimetics Inspired by Hydrothermal Systems—What Can We Learn from Nature for Materials Synthesis? Biomimetics 2017, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoll, A.H. Biomineralization and Evolutionary History. Rev. Mineral. Geochem. 2003, 54, 329–356. [Google Scholar] [CrossRef] [Green Version]
- Lowenstam, H.A.; Weiner, S. On Biomineralization, 1st ed.; Oxford University Press: New York, NY, USA, 1989; ISBN 0-19-504977-2. [Google Scholar]
- Metal Oxides: Chemistry and Applications, 1st ed.; Fierro, J.L.G. (Ed.) Taylor & Francis: Boca Raton, FL, USA, 2006; ISBN 0-8247-2371-6. [Google Scholar]
- Hildebrand, M. Diatoms, biomineralization processes, and genomics. Chem. Rev. 2008, 108, 4855–4874. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.C.; Keeling-Tucker, T. Biosilicification: The role of the organic matrix in structure control. J. Biol. Inorg. Chem. 2000, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Currie, H.A.; Perry, C.C. Silica in plants: Biological, biochemical and chemical studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Fleming, E.J.; McBeth, J.M. Iron-Oxidizing Bacteria: An Environmental and Genomic Perspective. Annu. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Limo, M.J.; Sola-Rabada, A.; Boix, E.; Thota, V.; Westcott, Z.C.; Puddu, V.; Perry, C.C. Interactions between Metal Oxides and Biomolecules: From Fundamental Understanding to Applications. Chem. Rev. 2018, 118, 11118–11193. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, M.B.; Sandhage, K.H.; Naik, R.R. Protein- and peptide-directed syntheses of inorganic materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960. [Google Scholar]
- Gibbs, G.V.; Downs, J.W.; Boisen, M.B. The Elusive SiO Bond. In Silica: Physical Behavior, Geochemistry, and Materials Applications; Heaney, P.J., Prewitt, C.T., Gibbs, G.V., Eds.; De Gruyter: Berlin, Germany, 1994; pp. 331–368. ISBN 978-0-939950-35-5. [Google Scholar]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; John Wiley & Sons, Inc.: New York, NY, USA, 1979; ISBN 0-471-02404-X. [Google Scholar]
- Müller, W.E. (Ed.) Silicon Biomineralization; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-3-642-62451-3. [Google Scholar]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic Manganese Oxides: Properties and Mechanisms of Formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef] [Green Version]
- Riley, J.P.; Sinhaseni, P. Chemical composition of three manganese nodules from the Pacific Ocean. J. Mar. Res. 1958, 17, 466–482. [Google Scholar]
- Hein, J.R.; Spinardi, F.; Okamoto, N.; Mizell, K.; Thorburn, D.; Tawake, A. Critical metals in manganese nodules from the Cook Islands EEZ, abundances and distributions. Ore Geol. Rev. 2015, 68, 97–116. [Google Scholar] [CrossRef]
- Struyf, E.; Smis, A.; van Damme, S.; Meire, P.; Conley, D.J. The global biogeochemical silicon cycle. Silicon 2009, 1, 207–213. [Google Scholar] [CrossRef]
- Liebau, F. Structural Chemistry of Silicates; Springer: Berlin/Heidelberg, Germany, 1985; ISBN 978-3-642-50078-7. [Google Scholar]
- Exley, C. Silicon in Life: Whither Biological Silicification? In Biosilica in Evolution, Morphogenesis, and Nanobiotechnology; Müller, W.E.G., Grachev, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 173–184. ISBN 9783540885511. [Google Scholar]
- Hildebrand, M.; Volcani, B.E.; Gassmann, W.; Schroeder, J.I. A gene family of silicon transporters. Nature 1997, 385, 688–689. [Google Scholar] [CrossRef]
- Matsunaga, S.; Sakai, R.; Jimbo, M.; Kamiya, H. Long-Chain Polyamines (LCPAs) from marine sponge: Possible implication in spicule formation. Chem. Bio. Chem 2007, 8, 1729–1735. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Boreiko, A.; Wang, X.; Belikov, S.I.; Wiens, M.; Grebenjuk, V.A.; Schloßmacher, U.; Schröder, H.C. Silicateins, the major biosilica forming enzymes present in demosponges: Protein analysis and phylogenetic relationship. Gene 2007, 395, 62–71. [Google Scholar] [CrossRef]
- Schröder, H.C.; Wiens, M.; Schloßmacher, U.; Brandt, D.; Müller, W.E.G. Silicatein-Mediated Polycondensation of Orthosilicic Acid: Modeling of a Catalytic Mechanism Involving Ring Formation. Silicon 2012, 4, 33–38. [Google Scholar] [CrossRef]
- Krasko, A.; Lorenz, B.; Batel, R.; Schröder, H.C.; Müller, I.M.; Müller, W.E.G. Expression of silicatein and collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin. Eur. J. Biochem. 2000, 267, 4878–4887. [Google Scholar] [CrossRef] [PubMed]
- Strunz, H.; Nickel, E.H. Strunz Mineralogical Tables: Chemical-Structural Mineral Classification System, 9th ed.; Schweizerbart: Stuttgart, Germany, 2001; ISBN 3-510-65188-X. [Google Scholar]
- Frankel, R.B.; Blakemore, R.P. (Eds.) Iron Biominerals; Springer: New York, NY, USA, 1991; ISBN 978-1-4613-6699-7. [Google Scholar]
- Ferris, F.G.; Beveridge, T.J.; Fyfe, W.S. Iron-silica crystallite nucleation by bacteria in a geothermal sediment. Nature 1986, 320, 609–611. [Google Scholar] [CrossRef]
- Leadbeater, B.S. Ultrastructure and Deposition of Silica in Loricate Choanoflagellates. In Silicon and Siliceous Structures in Biological Systems; Simpson, T.L., Volcani, B.E., Eds.; Springer: New York, NY, USA, 1981; pp. 295–322. ISBN 978-1-4612-5944-2. [Google Scholar]
- Green, J.C.; Hibberd, D.J.; Pienaar, R.N. The taxonomy of Prymnesium (Prymnesiophyceae) including a description of a new cosmopolitan species, P. Patellifera sp. nov., and further observations on P. parvum N. carter. Br. Phycol. J. 1982, 17, 363–382. [Google Scholar] [CrossRef] [Green Version]
- de Barros, H.D.P.; Esquivel, D.M.S.; Danon, J. Magnetotactic Algae. In Anais Academia Brasileira Ciências; Centro Brasileiro de Pesquisas Fisicas: Rio de Janeiro, Brazil, 1982; Volume 54, pp. 257–258. [Google Scholar]
- Schilz-Baldes, M.; Lewin, R.A. Manganese Encrustation of Zygospores of a Chlamydomonas (Chlorophyta: Volvocales). Science 1975, 188, 1119–1120. [Google Scholar] [CrossRef]
- Bovee, E.C. Distribution and Forms of Siliceous Structures Among Protozoa. In Silicon and Siliceous Structures in Biological Systems; Simpson, T.L., Volcani, B.E., Eds.; Springer: New York, NY, USA, 1981; pp. 233–279. ISBN 978-1-4612-5946-6. [Google Scholar]
- Ehrenberg, C.G. Phosphorsaures Kalk in den Zahnen und Kieselerde in dem Panzer von Infusorien. Ann. Phys. Lpz. 1834, 32, 574–576. [Google Scholar]
- Desikachary, T.V.; Dweltz, N.E. The Chemical Composition of the Diatom Frustule. Proc. Indian Acad. Sci. - Sect. B 1961, 53, 157–165. [Google Scholar]
- Round, F.E. Morphology and Phyletic Relationships of the Silicified Algae and the Archetypal Diatom — Monophyly or Polyphyly. In Silicon and Siliceous Structures in Biological Systems; Simpson, T.L., Volcani, B.E., Eds.; Springer: New York, NY, USA, 1981; pp. 97–128. ISBN 978-1-4612-5946-6. [Google Scholar]
- McGrory, C.B.; Leadbeater, B.S.C. Ultrastructure and Deposition of Silica in the Chrysophyceae. In Silicon and Siliceous Structures in Biological Systems; Simpson, T.L., Volcani, B.E., Eds.; Springer: New York, NY, USA, 1981; pp. 201–230. ISBN 978-1-4612-5946-6. [Google Scholar]
- Pringsheim, E.G. On Iron Flagellates. Philos. Trans. R. Soc. London 1946, 232, 311–342. [Google Scholar]
- Blakemore, R. Magnetotatic Bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef]
- De Araujo, F.; Pires, M.; Frankel, R.; Bicudo, C. Magnetite and Magnetotaxis in Algae. Biophys. J. 1986, 50, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Ascaso, C.; Galvan, J.; Ortega, C. The Pedogenic Action of Parmelia Conspersa, Rhizocarpon Geographicum and Umbjlicaria Pustulata. Lichenologist 1976, 8, 151–171. [Google Scholar] [CrossRef] [Green Version]
- Galván, J.; Rodríguez Pascual, C.; Ascaso, C. The Pedogenic Action of Lichens in Metamorphic Rocks. Pedobiologia 1981, 21, 60–73. [Google Scholar]
- Jones, D.; Wilson, M.J.; Tait, J.M. Weathering of a Basalt by Pertusaria Corallina. Lichenologist 1980, 12, 277–290. [Google Scholar] [CrossRef]
- Jackson, T.A.; Keller, W.D. A Comparative Study of the Role of Lichens and “Inorganic” Processes in the Chemical Weathering of Recent Hawaiian Lava Flows. Am. J. Sci. 1970, 269, 446–466. [Google Scholar] [CrossRef]
- Saratovsky, I.; Gurr, S.J.; Hayward, M.A. The Structure of manganese oxide formed by the fungus Acremonium sp. strain KR21-2. Geochim. Cosmochim. Acta 2009, 73, 3291–3300. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Maruo, K.; Tsuno, H.; Sakata, M.; Iwahori, K. Manganese(IV) oxide production by Acremonium sp. strain KR21-2 and extracellular Mn(II) oxidase activity. Appl. Environ. Microbiol. 2006, 72, 6467–6473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, N.; Tani, Y.; Iwahori, K.; Soma, M. Enzymatic formation of manganese oxides by an Acremonium-like hyphomycete fungus, strain KR21-2. FEMS Microbiol. Ecol. 2004, 47, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Benvenuto, M.L.; Fernández Honaine, M.; Osterrieth, M.L. Amorphous silica biomineralizations in Polytrichum strictum Menzies ex Brid. (Bryophyta). J. Bryol. 2013, 35, 112–118. [Google Scholar] [CrossRef]
- Witty, J.E.; Knox, E.G. Grass Opal in Some Chestnut and Forested Soils in North Central Oregon. Soil Sci. Soc. Am. J. 1964, 28, 685–688. [Google Scholar] [CrossRef]
- Frankel, R.B.; Papaefthymiou, G.C.; Blakemore, R.P. Mössbauer Spectroscopy of Iron Biomineralization Products in Magnetotactic Bacteria. In Magnetite Biomineralization and Magnetoreception in Organisms; Kirschvink, J.L., Jones, D.S., MacFadden, B.J., Eds.; Springer: Boston, MA, USA, 1985; pp. 269–287. ISBN 978-1-4613-7992-8. [Google Scholar]
- Kaufman, P.B.; Bigelow, W.C.; Schmid, R.; Ghosheh, N.S. Electron Microprobe Analysis of Silica in Epidermal Cells of Equisetum. Am. J. Bot. 1971, 58, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Brydon, J.E.; Dore, W.G.; Clark, J.S. Silicified Plant Asterosclereids Preserved in Soil. Soil Sci. Soc. Proc. 1963, 27, 476–477. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Dayanandan, P.; Takeoka, Y.; Bigelow, W.C.; Jones, J.D.; Iler, R. Silica in Shoots of Higher Plants. In Silicon and Siliceous Structures in Biological Systems; Simpson, T.L., Volcani, B.E., Eds.; Springer: New York, NY, USA, 1981; pp. 409–449. ISBN 978-1-4612-5946-6. [Google Scholar]
- Sangster, A.G.; Parry, D.W. Ultrastructure of Silica Deposits in Higher Plants. In Silicon and Siliceous Structures in Biological Systems; Simpson, T.L., Volcani, B.E., Eds.; Springer: New York, NY, USA, 1981; pp. 383–408. ISBN 978-1-4612-5946-6. [Google Scholar]
- Voronkov, M.G.; Zelchan, G.L.; Lukevitz, E. Silizium und Leben; Akademie-Verlag: Berlin, Germany, 1975. [Google Scholar]
- Thoulet, J. Étude des spicules siliceux d’ éponges vivantes. Bull. Société Minéralogique Fr. 1884, 7, 147–151. [Google Scholar] [CrossRef]
- Towe, K.M.; Rützler, K. Lepidocrocite Iron Mineralization in Keratose Sponge Granules. Science 1968, 162, 268–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowenstam, H.A.; Rossman, G.R. Amorphous, Hydrous, Ferric Phosphatic Dermal Granules in Molpadia (Holothuroidea): Physical and Chemical Characterization and Ecologic Implications of the Bioinorganic Fraction. Chem. Geol. 1975, 15, 15–51. [Google Scholar] [CrossRef]
- Lowenstam, H.A. Opal Precipitation by Marine Gastropods (Mollusca). Science 1971, 171, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Lowenstam, H.A. Lepidocrocite, an Apatite Mineral, and Magnetite in Teeth of Chitons (Polyplacophora). Science 1967, 156, 1373–1375. [Google Scholar] [CrossRef]
- Ferris, F.G.; Fyfe, W.S.; Beveridge, T.J. Manganese oxide deposition in a hot spring microbial mat. Geomicrobiol. J. 1987, 5, 33–42. [Google Scholar] [CrossRef]
- Lowenstam, H.A. Magnetite in denticle capping in recent chitons (polyplacophora). Geol. Soc. Am. Bull. 1962, 73, 435–438. [Google Scholar] [CrossRef]
- Lowenstram, H.A. Goethite in Radular Teeth of Recent Marine Gastropods. Science 1962, 137, 279–280. [Google Scholar] [CrossRef]
- Sullivan, B.K.; Miller, C.B.; Peterson, W.T.; Soeldner, A.H. A scanning electron microscope study of the mandibular morphology of boreal copepods. Mar. Biol. 1975, 30, 175–182. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Jones, D.S.; MacFadden, B.J. (Eds.) Magnetite Biomineralization and Magnetoreception in Organisms: A new Biomagnetism; Plenum Press: New York, NY, USA, 1985; ISBN 978-1-4613-7992-8. [Google Scholar]
- Kirschvink, J.L.; Kobayashi-Kirschvink, A.; Woodford, B.J. Magnetite biomineralization in the human brain. Proc. Natl. Acad. Sci. USA 1992, 89, 7683–7687. [Google Scholar] [CrossRef] [Green Version]
- Strait, S.G.; Smith, S.C. Elemental Analysis of Soricine Enamel: Pigmentation Variation and Distribution in Molars of Blarina brevicauda. J. Mammal. 2006, 87, 700–705. [Google Scholar] [CrossRef]
- Villalobos, M.; Toner, B.; Bargar, J.; Sposito, G. Characterization of the manganese oxide produced by Pseudomonas putida strain MnB1. Geochim. Cosmochim. Acta 2003, 67, 2649–2662. [Google Scholar] [CrossRef]
- Friedl, G.; Wehrli, B.; Manceau, A. Solid phases in the cycling of manganese in eutrophic lakes: New insights from EXAFS spectroscopy. Geochim. Cosmochim. Acta 1997, 61, 275–290. [Google Scholar] [CrossRef]
- Hariya, Y.; Kikuchi, T. Precipitation of Manganese by Bacteria in Mineral Springs. Nature 1964, 202, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Resig, J.M.; Lowenstam, H.A.; Echols, R.J.; Weiner, S. An Extant Opaline Foraminifer: Test Ultrastructure, Mineralogy and Taxonomy. In Studies in Marine Micropaleontology and Paleoecology: A Memorial Volume to Orville L. Bandy; Sliter, W.V., Ed.; Cushman Foundation for Foraminiferal Research: Lawrence, KS, USA, 1980; pp. 205–214. ISBN 9781970168129. [Google Scholar]
- Orr, W.N.; Conley, S. Siliceous Dinoflagellates in the Northeast Pacific Rim. Micropaleontology 1976, 22, 92. [Google Scholar] [CrossRef]
- Jones, B.; de Ronde, C.E.J.; Renaut, R.W. Mineralized microbes from Giggenbach submarine volcano. J. Geophys. Res. Solid Earth 2008, 113, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Konhauser, K.; Jones, B.; Phoenix, V.; Ferris, F.G.; Renaut, R.W. The Microbial Role in Hot Spring Silicification. Ambio 2004, 33, 552–558. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Fyfe, W.S.; Ferris, F.G.; Beveridge, T.J. Metal sorption and mineral precipitation by bacteria in two Amazonian river systems: Rio Solimoes and Rio Negro, Brazil. Geology 1993, 21, 1103–1106. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Ferris, F.G. Diversity of iron and silica precipitation by microbial mats in hydrothermal waters, Iceland: Implications for Precambrian iron formations. Geology 1996, 24, 323–326. [Google Scholar] [CrossRef] [Green Version]
- Fortin, D.; Beveridge, T.J. Role of the bacterium Thiobacillus in the formation of silicates in acidic mine tailings. Chem. Geol. 1997, 141, 235–250. [Google Scholar] [CrossRef]
- Douglas, S.; Beveridge, T.J. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 1998, 26, 79–88. [Google Scholar] [CrossRef]
- Mera, M.U.; Beveridge, T.J. Mechanism of silicate binding to the bacterial cell wall in Bacillus subtilis. J. Bacteriol. 1993, 175, 1936–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benning, L.G.; Phoenix, V.R.; Mountain, B.W. Biosilicification: The role of cyanobacteria in silica sinter deposition. In Micro-organisms and Earth Systems (Society for General Microbiology Symposia); Gadd, G.M., Semple, K.T., Lappin-Scott, H.M., Eds.; Cambridge University Press: New York, NY, USA, 2005; pp. 131–150. ISBN 9780511754852. [Google Scholar]
- Uriz, M.J.; Turon, X.; Becerro, M.A.; Agell, G. Siliceous spicules and skeleton frameworks in sponges: Origin, diversity, ultrastructural patterns, and biological functions. Microsc. Res. Tech. 2003, 62, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.V. Biomimetic and bioinspired silica: Recent developments and applications. Chem. Commun. 2011, 47, 7567–7582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Schröder, H.C.; Wiens, M.; Schloßmacher, U.; Müller, W.E.G. Biosilica. Molecular Biology, Biochemistry and Function in Demosponges as well as its Applied Aspects for Tissue Engineering. In Advances in Marine Biology; Becerro, M.A., Uriz, M.J., Maldonado, M., Turon, X., Eds.; Elsevier Ltd.: Oxford, UK, 2012; Volume 62, pp. 231–271. ISBN 9780123942838. [Google Scholar]
- Cha, J.N.; Shimizu, K.; Zhou, Y.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D.; Morse, D.E. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Cha, J.; Stucky, G.D.; Morse, D.E. Silicatein α: Cathepsin L-like protein in sponge biosilica. Proc. Natl. Acad. Sci. USA 1998, 95, 6234–6238. [Google Scholar] [CrossRef] [Green Version]
- Jochum, K.P.; Wang, X.; Vennemann, T.W.; Sinha, B.; Müller, W.E.G. Siliceous deep-sea sponge Monorhaphis chuni: A potential paleoclimate archive in ancient animals. Chem. Geol. 2012, 300–301, 143–151. [Google Scholar] [CrossRef]
- Weaver, J.C.; Morse, D.E. Molecular biology of demosponge axial filaments and their roles in biosilicification. Microsc. Res. Tech. 2003, 62, 356–367. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Krasko, A.; Pennec, G.L.; Steffen, R.; Wiens, M.; Ammar, M.S.A.; Müller, I.M.; Schröder, H.C. Molecular Mechanism of Spicule Formation in the Demosponge Suberites domuncula: Silicatein-Collagen-Myotrophin. In Silicon Biomineralization. Progress in Molecular and Subcellular Biology; Müller, W.E.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 33, pp. 195–221. ISBN 978-3-642-62451-3. [Google Scholar]
- Müller, W.E.G.; Kaluzhnaya, O.V.; Belikov, S.I.; Rothenberger, M.; Schröder, H.C.; Reiber, A.; Kaandorp, J.A.; Manz, B.; Mietchen, D.; Volke, F. Magnetic resonance imaging of the siliceous skeleton of the demosponge Lubomirskia baicalensis. J. Struct. Biol. 2006, 153, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Schröder, H.C.; Boreiko, A.; Korzhev, M.; Tahir, M.N.; Tremel, W.; Eckert, C.; Ushijima, H.; Müller, I.M.; Müller, W.E.G. Co-expression and functional interaction of silicatein with galectin: Matrix-guided formation of siliceous spicules in the marine demosponge Suberites domuncula. J. Biol. Chem. 2006, 281, 12001–12009. [Google Scholar] [CrossRef] [Green Version]
- Schröer, H.C.; Krasko, A.; Pennec, G.L.; Adell, T.; Wiens, M.; Hassanein, H.; Müller, I.M.; Müller, W.E.G. Silicase, an Enzyme Which Degrades Biogenous Amorphous Silica: Contribution to the Metabolism of Silica Deposition in the Demosponge Suberites domuncula. In Silicon Biomineralization. Progress in Molecular and Subcellular Biology; Müller, W.E.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 33, pp. 249–268. ISBN 978-3-642-62451-3. [Google Scholar]
- Gross, J.; Sokal, Z.; Rougvie, M. Structural and Chemical Studies on the Connective Tissue of Marine Sponges. J. Histochem. Cytochem. 1956, 4, 227–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, H.; Maldonado, M.; Spindler, K.-D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First Evidence of Chitin as a Component of the Skeletal Fibers of Marine Sponges. J. Exp. Zool. 2007, 308B, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Fan, X.T.; Schröder, H.C.; Müller, W.E.G. Flashing light in sponges through their siliceous fiber network: A new strategy of “neuronal transmission” in animals. Chinese Sci. Bull. 2012, 57, 3300–3311. [Google Scholar] [CrossRef] [Green Version]
- Kooyman, G.L.; Kooyman, T.G.; Horning, M.; Kooyman, C.A. Optical fibres in an Antarctic sponge. Nature 1996, 383, 397–398. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wendt, K.; Geppert, C.; Wiens, M.; Reiber, A.; Schröder, H.C. Novel photoreception system in sponges? Unique transmission properties of the stalk spicules from the hexactinellid Hyalonema sieboldi. Biosens. Bioelectron. 2006, 21, 1149–1155. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Kasueske, M.; Wang, X.; Schröder, H.C.; Wang, Y.; Pisignano, D.; Wiens, M. Luciferase a light source for the silica-based optical waveguides (spicules) in the demosponge Suberites domuncula. Cell. Mol. Life Sci. 2009, 66, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Kul’Chin, Y.N.; Voznesenski, S.S.; Bukin, O.A.; Bezverbnyi, A.V.; Drozdov, A.L.; Nagorny, I.G.; Galkina, A.N. Spicules of glass sponges as a new type of self-organizing natural photonic crystal. Opt. Spectrosc. 2009, 107, 442–447. [Google Scholar] [CrossRef]
- Supercontinuum Generation. Available online: https://www.nature.com/subjects/supercontinuum-generation (accessed on 18 April 2020).
- Ehrlich, H.; Maldonado, M.; Parker, A.R.; Kulchin, Y.N.; Schilling, J.; Köhler, B.; Skrzypczak, U.; Simon, P.; Reiswig, H.M.; Tsurkan, M.V.; et al. Supercontinuum Generation in Naturally Occurring Glass Sponges Spicules. Adv. Opt. Mater. 2016, 4, 1608–1613. [Google Scholar] [CrossRef] [Green Version]
- Müller, W.E.G.; Wang, X.; Schröder, H.C.; Korzhev, M.; Grebenjuk, V.A.; Markl, J.S.; Jochum, K.P.; Pisignano, D.; Wiens, M. A cryptochrome-based photosensory system in the siliceous sponge Suberites domuncula (Demospongiae). FEBS J. 2010, 277, 1182–1201. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wang, X.; Binder, M.; Von Lintig, J.; Wiens, M.; Schroöder, H.C. Differential expression of the demosponge (Suberites domuncula) carotenoid oxygenases in response to light: Protection mechanism against the self-produced toxic protein (suberitine). Mar. Drugs 2012, 10, 177–199. [Google Scholar] [CrossRef] [Green Version]
- Hodson, M.J. The development of phytoliths in plants and its influence on their chemistry and isotopic composition. Implications for palaeoecology and archaeology. J. Archaeol. Sci. 2016, 68, 62–69. [Google Scholar] [CrossRef]
- Bennett, D.M.; Sangster, A.G. The distribution of silicon in the adventitious roots of the bamboo Sasa palmata. Can. J. Bot. 1981, 59, 1680–1684. [Google Scholar] [CrossRef]
- Raven, J.A. The Transport and Function of Silicon in Plants. Biol. Rev. 1983, 58, 179–207. [Google Scholar] [CrossRef]
- Kumar, S.; Soukup, M.; Elbaum, R. Silicification in grasses: Variation between different cell types. Front. Plant Sci. 2017, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Rashid, I.; Mir, S.H.; Zurro, D.; Dar, R.A.; Reshi, Z.A. Phytoliths as proxies of the past. Earth-Science Rev. 2019, 194, 234–250. [Google Scholar] [CrossRef]
- Sangster, A.G.; Hodson, M.J.; Tubb, H.J. Silicon deposition in higher plants. In Studies in Agriculture; Datnoff, L.E., Snyder, G.H., Korndorfer, G.H., Eds.; Elsevier B.V: Amsterdam, The Netherlands, 2001; pp. 85–113. [Google Scholar]
- Kumar, S.; Milstein, Y.; Brami, Y.; Elbaum, M.; Elbaum, R. Mechanism of silica deposition in sorghum silica cells. New Phytol. 2017, 213, 791–798. [Google Scholar] [CrossRef]
- Gao, G.; Jie, D.; Wang, Y.; Liu, L.; Liu, H.; Li, D.; Li, N.; Shi, J.; Leng, C. Phytolith reference study for identifying vegetation changes in the forest−grassland region of northeast China. Boreas 2018, 47, 481–497. [Google Scholar] [CrossRef]
- Kido, N.; Yokoyama, R.; Yamamoto, T.; Furukawa, J.; Iwai, H.; Satoh, S.; Nishitani, K. The matrix polysaccharide (1;3,1;4)-2- d -glucan is involved in silicon-dependent strengthening of rice cell wall. Plant Cell Physiol. 2015, 56, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Pierantoni, M.; Tenne, R.; Brumfeld, V.; Kiss, V.; Oron, D.; Addadi, L.; Weiner, S. Plants and Light Manipulation: The Integrated Mineral System in Okra Leaves. Adv. Sci. 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Bäucker, E.; Dudel, E.G. UV-screening of grasses by plant silica layer? J. Biosci. 2013, 38, 413–416. [Google Scholar] [CrossRef]
- Shakoor, S.A.; Bhat, M.A.; Mir, S.H. Phytoliths in Plants: A Review. J. Bot. Sci. 2014, 3, 10–24. [Google Scholar]
- Sharma, R.; Kumar, V.; Kumar, R. Distribution of phytoliths in plants: A review. Geol. Ecol. Landscapes 2019, 3, 123–148. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.H.P.; Handreck, K.A. Studies of silica in the oat plant—III. Uptake of silica from soils by the plant. Plant Soil 1965, 23, 79–96. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohnishi, Y.; Kitagishi, K. Histochemistry of Silicon in Rice Plant. Soil Sci. Plant Nutr. 1962, 8, 1–5. [Google Scholar]
- He, C.; Ma, J.; Wang, L. A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytol. 2015, 206, 1051–1062. [Google Scholar] [CrossRef]
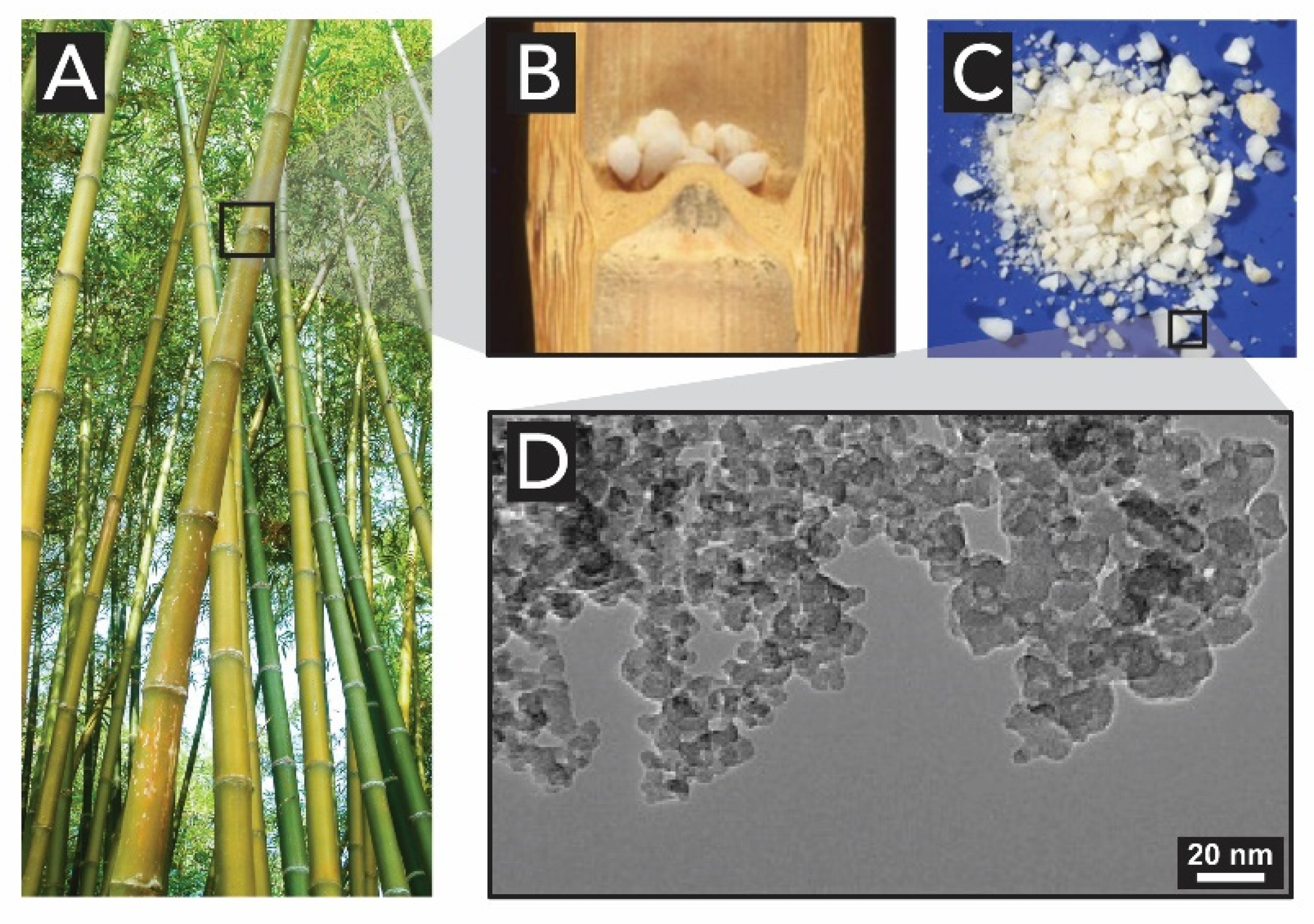
- Jones, L.H.P.; Milne, A.A.; Sanders, J.V. Tabashir: An opal of plant origin. Science 1966, 151, 464–466. [Google Scholar] [CrossRef]
- Liese, W.; Welling, J.; Tang, T.K.H. Utilization of Bamboo. In Bamboo: The Plant and its Uses; Liese, W., Köhl, M., Eds.; Springer: Hamburg, Germany, 2015; pp. 305–346. ISBN 978-3-319-14132-9. [Google Scholar]
- Alshatwi, A.A.; Athinarayanan, J.; Periasamy, V.S. Biocompatibility assessment of rice husk-derived biogenic silica nanoparticles for biomedical applications. Mater. Sci. Eng. C 2015, 47, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sterling, C. Crystalline Silica in Plants. Am. J. Bot. 1967, 54, 840–844. [Google Scholar] [CrossRef]
- Mustafa, A.; Ensikat, H.J.; Weigend, M. Ontogeny and the process of biomineralization in the trichomes of loasaceae. Am. J. Bot. 2017, 104, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.; Ensikat, H.J.; Weigend, M. Stinging hair morphology and wall biomineralization across five plant families: Conserved morphology versus divergent cell wall composition. Am. J. Bot. 2018, 105, 1109–1122. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Chen, S.-J.; Chen, R.-F.; Kuo-Huang, L.-L.; Huang, R.-N. Why do Nettles Sting? About Stinging Hairs Looking Simple but Acting Complex. Funct. Plant Sci. Biotecnol. 2007, 1, 46–55. [Google Scholar]
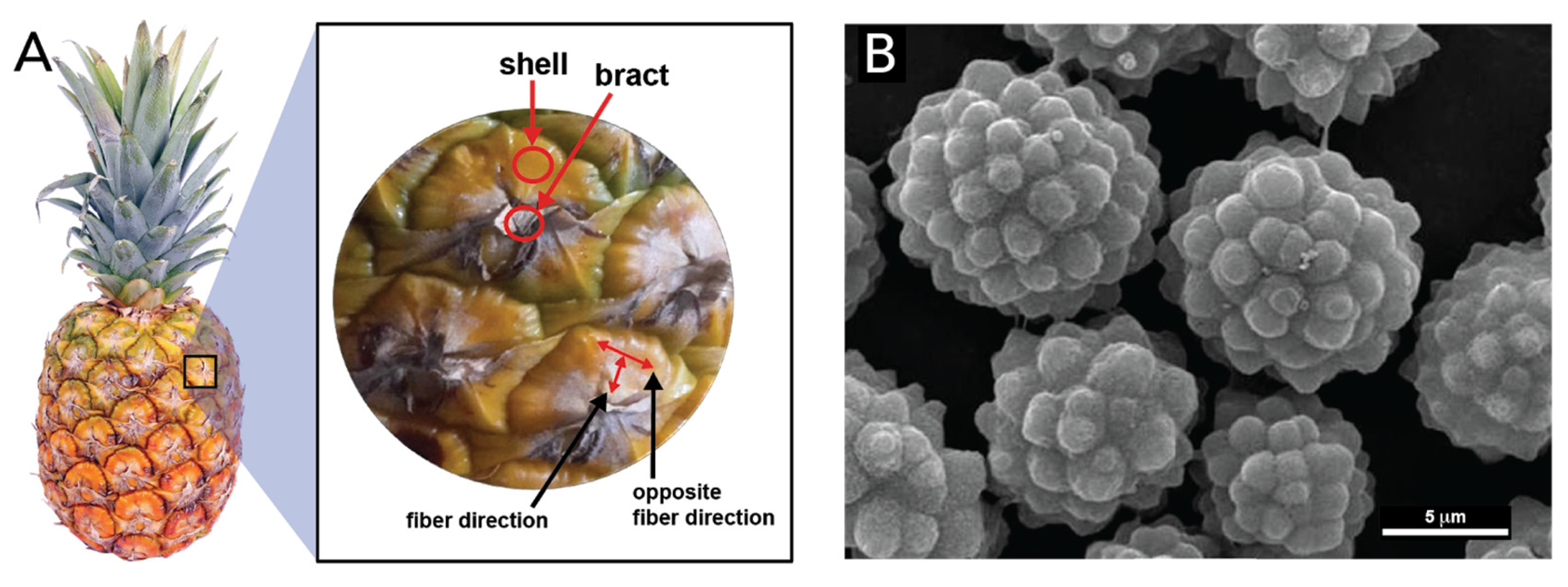
- Corrales-Ureña, Y.R.; Villalobos-Bermúdez, C.; Pereira, R.; Camacho, M.; Estrada, E.; Argüello-Miranda, O.; Vega-Baudrit, J.R. Biogenic silica-based microparticles obtained as a sub-product of the nanocellulose extraction process from pineapple peels. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, D.M. Elements occluded within phytoliths. In Phytoliths:Applications in Earth Science and Human History; Meunier, J.D., Colin, F., Eds.; Swets & Zeitlinger, B.V.: Lisse, The Netherlands, 2001; pp. 313–316. ISBN 90 5809 345 X (HB). [Google Scholar]
- Hodson, M.J.; Evans, D.E. Aluminium/silicon interactions in higher plants. J. Exp. Bot. 1995, 46, 161–171. [Google Scholar] [CrossRef]
- Carnelli, A.L.; Madella, M.; Theurillat, J.P.; Ammann, B. Aluminum in the opal silica reticule of phytoliths: A new tool in palaeoecological studies. Am. J. Bot. 2002, 89, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Scheinost, A.C. Metal Oxides. Encycl. Soils Environ. 2004, 4, 428–438. [Google Scholar]
- Simpson, T.L.; Garrone, R.; Mazzorana, M. Interaction of germanium (Ge) with biosilicification in the freshwater sponge Ephydatia mülleri: Evidence of localized membrane domains in the silicalemma. J. Ultrastruct. Res. 1983, 85, 159–174. [Google Scholar] [CrossRef]
- Nordmann, G.C.; Hochstoeger, T.; Keays, D.A. Magnetoreception: A sense without a receptor. PLoS Biol. 2017, 15, e2003234. [Google Scholar] [CrossRef] [Green Version]
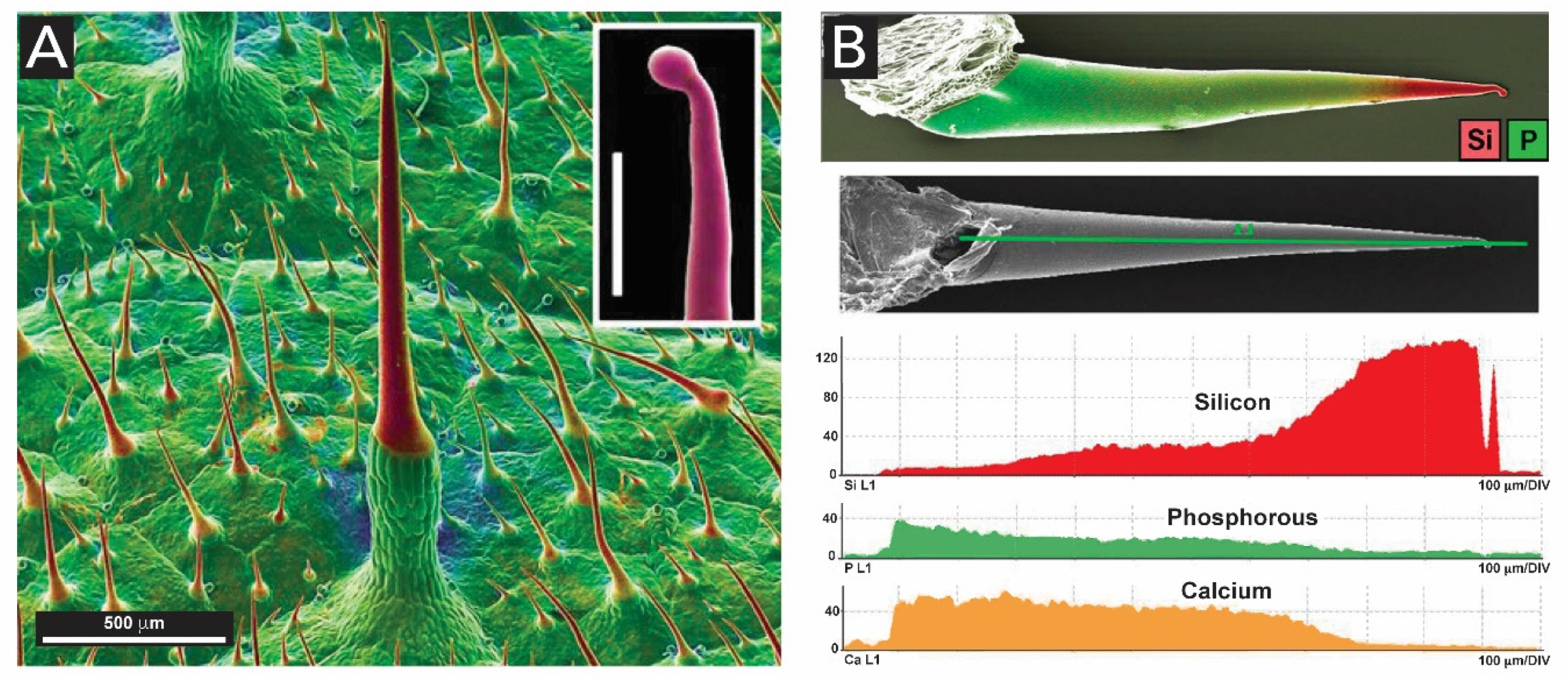
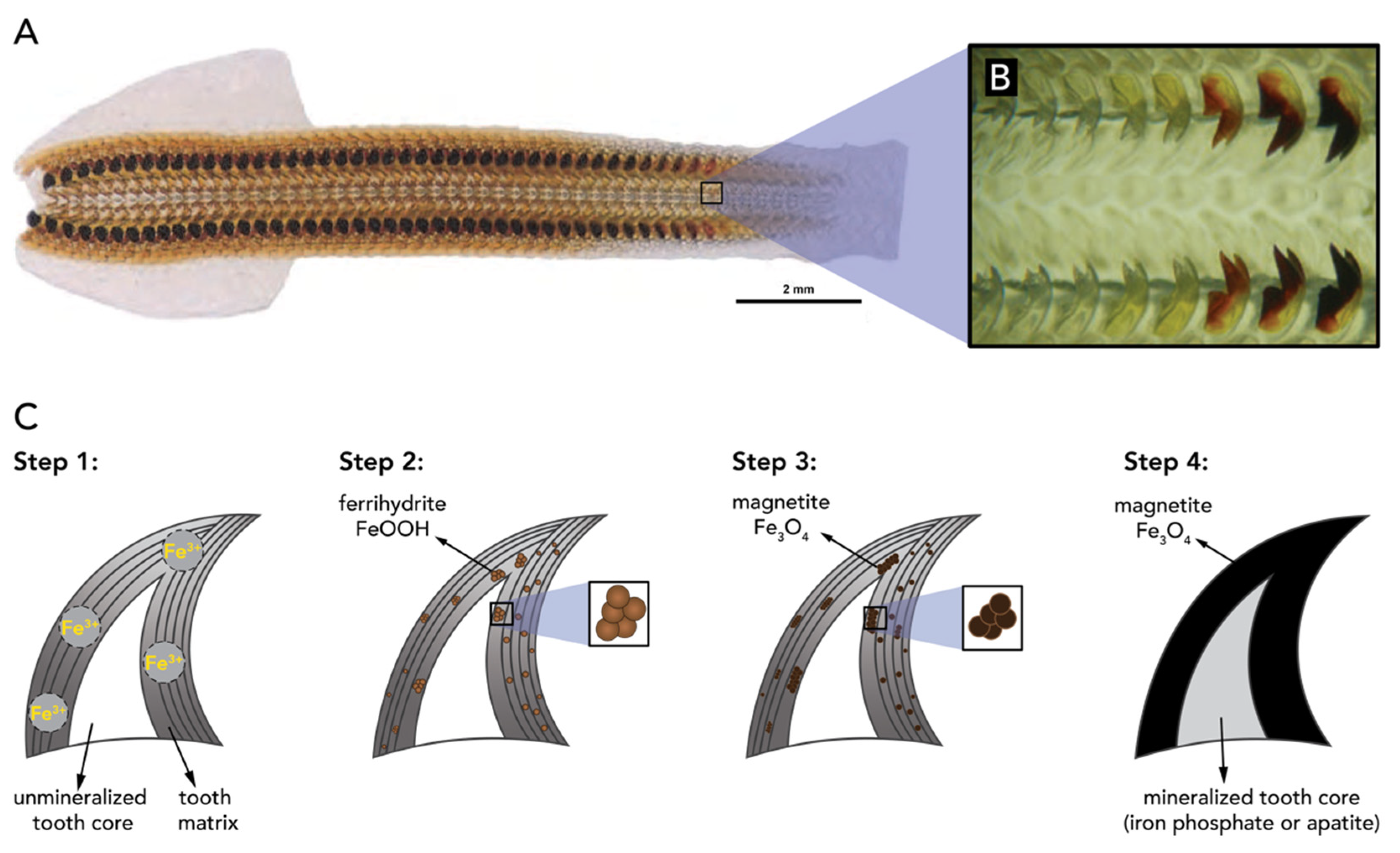
- Shaw, J.A.; Macey, D.J.; Brooker, L.R.; Clode, P.L. Tooth use and wear in three iron-biomineralizing mollusc species. Biol. Bull. 2010, 218, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Towe, K.M.; Lowenstam, H.A. Ultrastructure and development of iron mineralization in the radular teeth of Cryptochiton stelleri (mollusca). J. Ultrastruct. Res. 1967, 17, 1–13. [Google Scholar] [CrossRef]
- Kisailus, D.; Nemoto, M. Structural and Proteomic Analyses of Iron Oxide Biomineralization in Chiton Teeth. In Biological Magnetic Materials and Applications; Matsunaga, T., Tanaka, T., Kisailus, D., Eds.; Springer: Singapore, 2018; pp. 54–73. ISBN 978-981-10-8068-5. [Google Scholar]
- Brooker, L.R.; Shaw, J.A. The Chiton Radula: A Unique Model for Biomineralization Studies. In Advanced Topics in Biomineralization; InTech Europe: Rijeka, Croatia, 2012; pp. 65–84. ISBN 978-953-51-0045-4. [Google Scholar]
- Taoka, A.; Fukumori, Y. Structure and Function of Aligned Magnetic Crystals in Magnetotactic Bacteria. In Biological Magnetic Materials and Applications; Matsunaga, T., Tanaka, T., Kisailus, D., Eds.; Springer: Singapore, 2018; pp. 3–22. ISBN 978-981-10-8068-5. [Google Scholar]
- Monteil, C.L.; Lefevre, C.T. Magnetoreception in Microorganisms. Trends Microbiol. 2020, 28, 266–275. [Google Scholar] [CrossRef]
- Schüler, D.; Baeuerlein, E. Iron-limited growth and kinetics of iron uptake in Magnetospirillum gryphiswaldense. Arch. Microbiol. 1996, 166, 301–307. [Google Scholar] [CrossRef]
- Frankel, R.B.; Papaefthymiou, G.C.; Blakemore, R.P.; O’Brien, W. Fe3O4 precipitation in magnetotactic bacteria. Biochim. Biophys. Acta 1983, 763, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Frankel, R.B.; Blakemore, R.P.; Wolfe, R.S. Magnetite in freshwater magnetotactic bacteria. Science 1979, 203, 1355–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faivre, D.; Böttger, L.H.; Matzanke, B.F.; Schüler, D. Intracellular magnetite biomineralization in bacteria proceeds by a distinct pathway involving membrane-bound ferritin and an iron(II) species. Angew. Chemie Int. Ed. 2007, 46, 8495–8499. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Elia, L.; Poli, M. Ferritin, cellular iron storage and regulation. Int. Union Biochem. Mol. Biol. 2017, 69, 414–422. [Google Scholar] [CrossRef]
- Gorobets, O.; Gorobets, S.; Koralewski, M. Physiological origin of biogenic magnetic nanoparticles in health and disease: From bacteria to humans. Int. J. Nanomedicine 2017, 12, 4371–4395. [Google Scholar] [CrossRef] [Green Version]
- Faivre, D.; Godec, T.U. From bacteria to mollusks: The principles underlying the biomineralization of iron oxide materials. Angew. Chemie Int. Ed. 2015, 54, 4728–4747. [Google Scholar] [CrossRef]
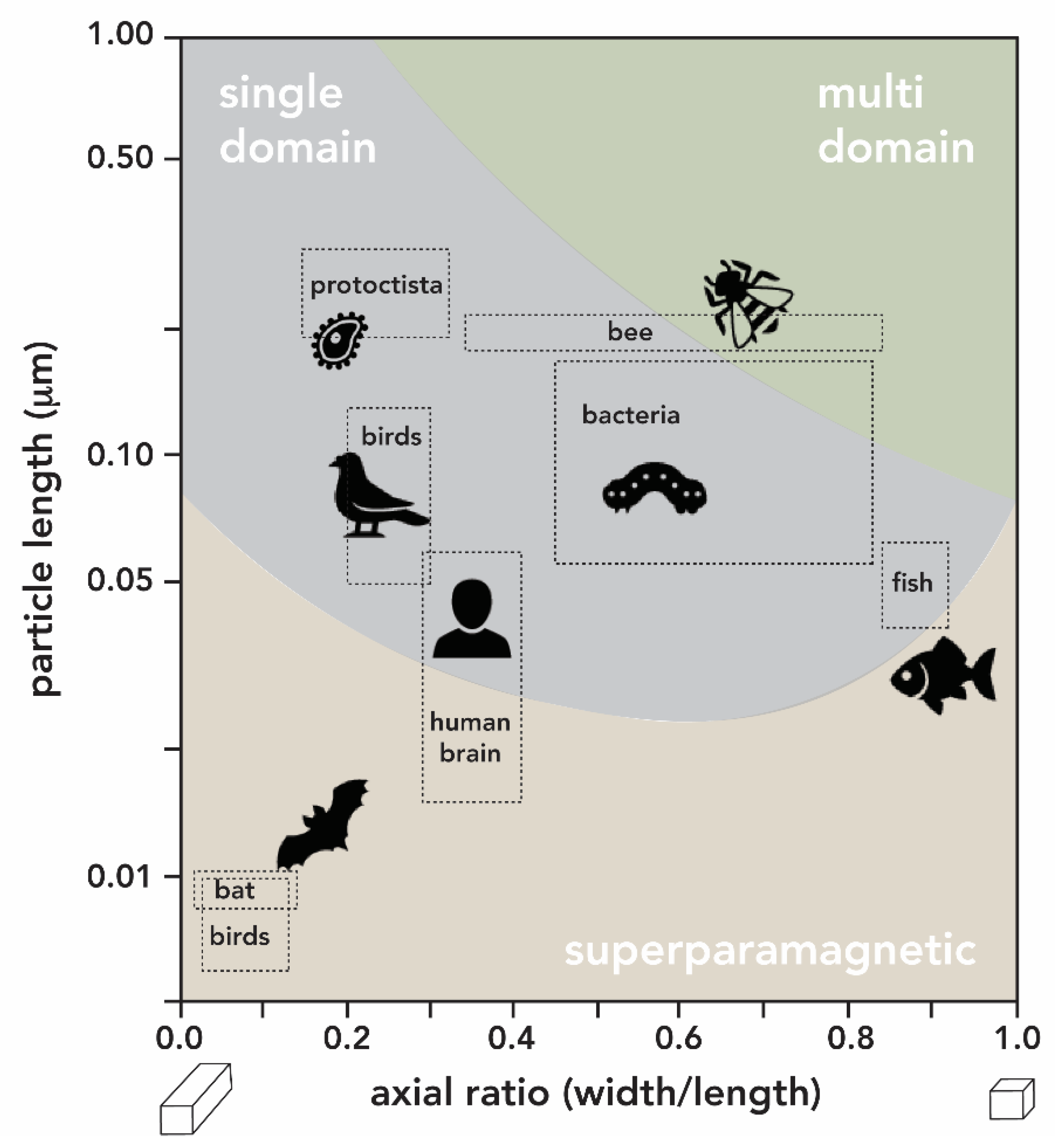
- Mather, J.G. Magnetoreception and the Search for Magnetic Material in Rodents. In Magnetite Biomineralization and Magnetoreception in Organisms; Kirschvink, J.L., Jones, D.S., MacFadden, B.J., Eds.; Springer: Boston, MA, USA, 1985; pp. 509–533. ISBN 978-1-4613-7992-8. [Google Scholar]
- Holland, R.A.; Kirschvink, J.L.; Doak, T.G.; Wikelski, M. Bats use magnetite to detect the earth’s magnetic field. PLoS ONE 2008, 3, e1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walcott, C.; Goulu, J.L.; Kirschvink, J.L. Pigeons have magnets. Science 1979, 205, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Q.; Dickman, J.D. Neural Correlates of a Magnetic Sense. Science 2012, 336, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.M.; Kirshcvink, J.L.; Chang, S.-B.R.; Dizon, A.E. A Candidate Magnetic Sense Organ in the Yellowfin Tuna, Thunnus albacares. Science 1984, 224, 751–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoeger, J.; Dunn, J.R.; Fuller, M. Magnetic Material in the Head of the Common Pacific Dolphin. Science 1981, 213, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Gilder, S.A.; Wack, M.; Kaub, L.; Roud, S.C.; Petersen, N.; Heinsen, H.; Hillenbrand, P.; Milz, S.; Schmitz, C. Distribution of magnetic remanence carriers in the human brain. Sci. Rep. 2018, 8, 11363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murros, K.; Wasiljeff, J.; Macías-Sánchez, E.; Faivre, D.; Soinne, L.; Valtonen, J.; Pohja, M.; Saari, P.; Pesonen, L.J.; Salminen, J.M. Magnetic Nanoparticles in Human Cervical Skin. Front. Med. 2019, 6, 123. [Google Scholar] [CrossRef]
- Maher, B.A.; Ahmed, I.A.M.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.A.; Torres-Jardón, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [Green Version]
- Grassi-Schultheiss, P.P.; Heller, F.; Dobson, J. Analysis of magnetic material in the human heart, spleen and liver. BioMetals 1997, 10, 351–355. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Gould, J.L. Biogenic magnetite as a basis for magnetic field detection in animals. BioSystems 1981, 13, 181–201. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A 2005, 191, 675–693. [Google Scholar] [CrossRef]
- Shaw, J.; Boyd, A.; House, M.; Woodward, R.; Mathes, F.; Cowin, G.; Saunders, M.; Baer, B. Magnetic particle-mediated magnetoreception. J. R. Soc. Interface 2015, 12, 20150499. [Google Scholar] [CrossRef] [Green Version]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [Green Version]
- Hansel, C.M. Manganese in Marine Microbiology. In Advances in Microbial Physiology, 1st ed.; Poole, R.K., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 70, pp. 37–83. ISBN 978-0-12-812386-7. [Google Scholar]
- Ghiorse, W.C. Biology of Iron- and Manganese-Depositing Bacteria. Ann. Rev. Microbiol. 1984, 38, 515–550. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.A.; Co, E.M.; Tebo, B.M. Enzymatic Manganese(II) Oxidation by a Marine α-Proteobacterium. Appl. Environ. Microbiol. 2001, 67, 4024–4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crerar, D.A.; Barnes, H.L. Deposition of deep-sea manganese nodules. Geochim. Cosmochim. Acta 1974, 38, 279–300. [Google Scholar] [CrossRef]
- Corstjens, P.L.A.M.; Devrind, J.P.M.; Goosen, T.; de Vrind-de Jong, E.W. Identification and molecular analysis of the leptothrix discophora SS-1 mofA gene, a gene putatively encoding a manganese-oxidizing protein with copper domains. Geomicrobiol. J. 1997, 14, 91–108. [Google Scholar] [CrossRef]
- van Waasbergen, L.G.; Hildebrand, M.; Tebo, B.M. Identification and Characterization of a Gene Cluster Involved in Manganese Oxidation by Spores of the Marine Bacillus sp. Strain SG-1. J. Bacteriol. 1996, 178, 3517–3530. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, G.J.; De Vrind, J.P.M.; Corstjens, P.L.A.M.; Cornelis, P.; Baysse, C.; De Vrind-De Jong, E.W. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 1999, 65, 1762–1768. [Google Scholar] [CrossRef] [Green Version]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2605. [Google Scholar] [CrossRef]
- Kim, H.S.; Stair, P.C. Bacterially produced manganese oxide and todorokite: UV Raman spectroscopic comparison. J. Phys. Chem. B 2004, 108, 17019–17026. [Google Scholar] [CrossRef]
- Kim, H.S.; Pastén, P.A.; Gaillard, J.F.; Stair, P.C. Nanocrystalline Todorokite-Like Manganese Oxide Produced by Bacterial Catalysis. J. Am. Chem. Soc. 2003, 125, 14284–14285. [Google Scholar] [CrossRef]
- Jürgensen, A.; Widmeyer, J.R.; Gordon, R.A.; Bendell-Young, L.I.; Moore, M.M.; Crozier, E.D. The structure of the manganese oxide on the sheath of the bacterium Leptothrix discophora: An XAFS study. Am. Mineral. 2004, 89, 1110–1118. [Google Scholar] [CrossRef]
- Saratovsky, I.; Wightman, P.G.; Pastén, P.A.; Gaillard, J.F.; Poeppelmeier, K.R. Manganese oxides: Parallels between abiotic and biotic structures. J. Am. Chem. Soc. 2006, 128, 11188–11198. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, Q.; Huang, A.; Giraldo, O.; Suib, S.L. Double-aging method for preparation of stabilized Na-buserite and transformations to todorokites incorporated with various metals. Inorg. Chem. 1999, 38, 6106–6113. [Google Scholar] [CrossRef] [PubMed]
- Atkins, A.L.; Shaw, S.; Peacock, C.L. Nucleation and growth of todorokite from birnessite: Implications for trace-metal cycling in marine sediments. Geochim. Cosmochim. Acta 2014, 144, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Tiquia-arashiro, S.M.; Grube, M. (Eds.) Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-19029-3. [Google Scholar]
- Santelli, C.M.; Pfister, D.H.; Lazarus, D.; Sun, L.; Burgos, W.D.; Hansel, C.M. Promotion of Mn(II) oxidation and remediation of coal mine drainage in passive treatment systems by diverse fungal and bacterial communities. Appl. Environ. Microbiol. 2010, 76, 4871–4875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, I.A.; Huber, D.M.; Guest, C.A.; Schulze, D.G. Fungal manganese oxidation in a reduced soil. Environ. Microbiol. 2005, 7, 1480–1487. [Google Scholar] [CrossRef]
- Miyata, N.; Sugiyama, D.; Tani, Y.; Tsuno, H.; Seyama, H.; Sakata, M.; Iwahori, K. Production of biogenic manganese oxides by repeated-batch cultures of laboratory microcosms. J. Biosci. Bioeng. 2007, 103, 432–439. [Google Scholar] [CrossRef]
- Petkov, V.; Ren, Y.; Saratovsky, I.; Pastén, P.; Gurr, S.J.; Hayward, M.A.; Poeppelmeier, K.R.; Gaillard, J.F. Atomic-scale structure of biogenic materials by total X-ray diffraction: A study of bacterial and fungal MnOx. ACS Nano 2009, 3, 441–445. [Google Scholar] [CrossRef]
- Santelli, C.M.; Webb, S.M.; Dohnalkova, A.C.; Hansel, C.M. Diversity of Mn oxides produced by Mn(II)-oxidizing fungi. Geochim. Cosmochim. Acta 2011, 75, 2762–2776. [Google Scholar] [CrossRef]
- Remucal, C.K.; Ginder-Vogel, M. A critical review of the reactivity of manganese oxides with organic contaminants. Environ. Sci. Process. Impacts 2014, 16, 1247–1266. [Google Scholar] [CrossRef]
- Banh, A.; Chavez, V.; Doi, J.; Nguyen, A.; Hernandez, S.; Ha, V.; Jimenez, P.; Espinoza, F.; Johnson, H.A. Manganese (Mn) Oxidation Increases Intracellular Mn in Pseudomonas putida GB-1. PLoS ONE 2013, 8, e77835. [Google Scholar] [CrossRef] [Green Version]
- Zerfaß, C.; Christie-Oleza, J.A.; Soyer, O.S. Manganese Oxide Biomineralization Provides Protection against Nitrite Toxicity in a Cell-Density-Dependent Manner. Appl. Environ. Microbiol. 2019, 85, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Asayama, T.; Shiraki, N.; Inoue, S.; Okuda, E.; Hayashi, C.; Nishida, K.; Hasegawa, H.; Harada, E. Mn accumulation in a submerged plant Egeria densa (Hydrocharitaceae) is mediated by epiphytic bacteria. Plant Cell Environ. 2017, 40, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Cheng, J.; Sun, N.; Ma, C.; Dai, M. Two endophytic bacterial strains modulate Mn oxidation and accumulation in the wetland plant Suaeda salsa pall. Plant Soil 2019, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Crookes-Goodson, W.J.; Slocik, J.M.; Naik, R.R. Bio-directed synthesis and assembly of nanomaterials. Chem. Soc. Rev. 2008, 37, 2403–2412. [Google Scholar] [CrossRef]
- Deravi, L.F.; Swartz, J.D.; Wright, D.W. The Biomimetic Synthesis of Metal Oxide Nanomaterials. In Nanotechnologies for the Life Sciences; Kumar, C.S.S.R., Ed.; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2010; Volume 2, pp. 3–54. ISBN 9783527321520. [Google Scholar]
- Nudelman, F.; Sommerdijk, N.A.J.M. Biomineralization as an inspiration for materials chemistry. Angew. Chemie Int. Ed. 2012, 51, 6582–6596. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, L.; Zhang, L.C.; Su, Y.-D.; Yu, S.H. Biogenic and biomimetic magnetic nanosized assemblies. Nano Today 2012, 7, 297–315. [Google Scholar] [CrossRef]
- Kröger, N.; Dickerson, M.B.; Ahmad, G.; Cai, Y.; Haluska, M.S.; Sandhage, K.H.; Poulsen, N.; Sheppard, V.C. Bioenabled synthesis of rutile (TiO2) at ambient temperature and neutral pH. Angew. Chemie Int. Ed. 2006, 45, 7239–7243. [Google Scholar] [CrossRef]
- Sumerel, J.L.; Yang, W.; Kisailus, D.; Weaver, J.C.; Choi, J.H.; Morse, D.E. Biocatalytically Templated Synthesis of Titanium Dioxide. Chem. Mater. 2003, 15, 4804–4809. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Mukherjee, N.; Steinitz-Kannan, M.; Clarson, S.J. Bioinspired synthesis of new silica structures. Chem. Commun. 2003, 3, 1122–1123. [Google Scholar] [CrossRef]
- Kisailus, D.; Choi, J.H.; Weaver, J.C.; Yang, W.; Morse, D.E. Enzymatic synthesis and nanostructural control of gallium oxide at low temperature. Adv. Mater. 2005, 17, 314–318. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Naik, R.R.; Stone, M.O.; Cai, Y.; Sandhage, K.H. Identification of peptides that promote the rapid precipitation of germania nanoparticle networks via use of a peptide display library. Chem. Commun. 2004, 10, 1776–1777. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, K.; Yamashita, I. Bio-template synthesis of nanoparticle by cage-shaped protein supramolecule, apoferritin. J. Clust. Sci. 2007, 18, 358–370. [Google Scholar] [CrossRef]
- Umetsu, M.; Mizuta, M.; Tsumoto, K.; Ohara, S.; Takami, S.; Watanabe, H.; Kumagai, I.; Adschiri, T. Bioassisted room-temperature immobilization and mineralization of zinc oxide—The structural ordering of ZnO nanoparticles into a flower-type morphology. Adv. Mater. 2005, 17, 2571–2575. [Google Scholar] [CrossRef]
- Klem, M.T.; Resnick, D.A.; Gilmore, K.; Young, M.; Idzerda, Y.U.; Douglas, T. Synthetic control over magnetic moment and exchange bias in all-oxide materials encapsulated within a spherical protein cage. J. Am. Chem. Soc. 2007, 129, 197–201. [Google Scholar] [CrossRef]
- Brutchey, R.L.; Yoo, E.S.; Morse, D.E. Biocatalytic synthesis of a nanostructured and crystalline bimetallic perovskite-like barium oxofluorotitanate at low temperature. J. Am. Chem. Soc. 2006, 128, 10288–10294. [Google Scholar] [CrossRef]
- Unterlass, M.M. Green Synthesis of Inorganic—Organic Hybrid Materials: State of the Art and Future Perspectives. Eur. J. Inorg. Chem. 2016, 1135–1156. [Google Scholar] [CrossRef]
- Ramachandran, R.; Jung, D.; Spokoyny, A.M. Cross-linking dots on metal oxides. NPG Asia Mater. 2019, 11, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Wysokowski, M.; Piasecki, A.; Bazhenov, V.V.; Paukszta, D.; Born, R.; Schupp, P.; Petrenko, I.; Jesionowski, T. Poriferan Chitin as the Scaffold for Nanosilica Deposition under Hydrothermal Synthesis Conditions. J. Chitin Chitosan Sci. 2013, 1, 26–33. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Stöcker, H.; Bazhenov, V.V.; Langer, E.; Dobrowolska, A.; Czaczyk, K.; Galli, R.; Stelling, A.L.; Behm, T.; et al. An extreme biomimetic approach: Hydrothermal synthesis of β-chitin/ZnO nanostructured composites. J. Mater. Chem. B 2013, 1, 6469–6476. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Bazhenov, V.V.; Stawski, D.; Petrenko, I.; Ehrlich, A.; Behm, T.; Kljajic, Z.; Stelling, A.L.; Jesionowski, T.; et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013, 7, 248–260. [Google Scholar] [CrossRef]
- Leimhofer, L.; Baumgartner, B.; Puchberger, M.; Prochaska, T.; Konegger, T.; Unterlass, M.M. Green one-pot synthesis and processing of polyimide-silica hybrid materials. J. Mater. Chem. A 2017, 5, 16326–16335. [Google Scholar] [CrossRef] [Green Version]
- Unterlass, M.M. Creating geomimetic polymers. Mater. Today 2015, 18, 242–243. [Google Scholar] [CrossRef]
- Baumgartner, B.; Bojdys, M.J.; Unterlass, M.M. Geomimetics for green polymer synthesis: Highly ordered polyimides via hydrothermal techniques. Polym. Chem. 2014, 5, 3771–3776. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, B.; Puchberger, M.; Unterlass, M.M. Towards a general understanding of hydrothermal polymerization of polyimides. Polym. Chem. 2015, 6, 5773–5781. [Google Scholar] [CrossRef]
- Baumgartner, B.; Bojdys, M.J.; Skrinjar, P.; Unterlass, M.M. Design strategies in hydrothermal polymerization of polyimides. Macromol. Chem. Phys. 2016, 217, 485–500. [Google Scholar] [CrossRef]
- Taublaender, M.J.; Reiter, M.; Unterlass, M.M. Exerting Additive-Assisted Morphological Control during Hydrothermal Polymerization. Macromol. Chem. Phys. 2018, 219, 1700397. [Google Scholar] [CrossRef] [Green Version]
- Taublaender, M.J.; Reiter, M.; Unterlass, M.M. Highly Crystalline, Nanostructured Polyimide Microparticles via Green and Tunable Solvothermal Polymerization. Macromolecules 2019, 52, 6318–6329. [Google Scholar] [CrossRef]
- Taublaender, M.J.; Glöcklhofer, F.; Marchetti-Deschmann, M.; Unterlass, M.M. Green and Rapid Hydrothermal Crystallization and Synthesis of Fully Conjugated Aromatic Compounds. Angew. Chemie Int. Ed. 2018, 57, 12180. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, B.; Svirkova, A.; Bintinger, J.; Hametner, C.; Marchetti-Deschmann, M.; Unterlass, M.M. Green and highly efficient synthesis of perylene and naphthalene bisimides in nothing but water. Chem. Commun. 2017, 53, 1229–1232. [Google Scholar] [CrossRef] [Green Version]
- Taublaender, M.J.; Mezzavilla, S.; Thiele, S.; Glöcklhofer, F.; Unterlass, M.M. Hydrothermal Generation of Conjugated Polymers Using the Example of Pyrrone Polymers and Polybenzimidazoles. Angew. Chemie Int. Ed. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asuncion, M.Z.; Hasegawa, I.; Kampf, J.W.; Laine, R.M. The selective dissolution of rice hull ash to form [OSiO1.5]8[R4N]8 (R= Me, CH2CH2OH) octasilicates. Basic nanobuilding blocks and possible models of intermediates formed during biosilicification processes. J. Mater. Chem. 2005, 15, 2114–2121. [Google Scholar] [CrossRef]
- Bansal, V.; Ahmad, A.; Sastry, M. Fungus-mediated biotransformation of amorphous silica in rice husk to nanocrystalline silica. J. Am. Chem. Soc. 2006, 128, 14059–14066. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Martin, J.C.; Fan, X.; Han, A.; Luo, Z.; Sun, L. Silica nanoparticles and frameworks from rice husk biomass. ACS Appl. Mater. Interfaces 2012, 4, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.L.; Rylott, E.L.; Hunt, A.J.; Dodson, J.R.; Taylor, A.F.; Bruce, N.C.; Clark, J.H. Supported palladium nanoparticles synthesized by living plants as a catalyst for Suzuki-Miyaura reactions. PLoS ONE 2014, 9, e87192. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, H.M.; Unterlass, M.M. Biogenic Metal Oxides. Biomimetics 2020, 5, 29. https://doi.org/10.3390/biomimetics5020029
Moura HM, Unterlass MM. Biogenic Metal Oxides. Biomimetics. 2020; 5(2):29. https://doi.org/10.3390/biomimetics5020029
Chicago/Turabian StyleMoura, Hipassia M., and Miriam M. Unterlass. 2020. "Biogenic Metal Oxides" Biomimetics 5, no. 2: 29. https://doi.org/10.3390/biomimetics5020029
APA StyleMoura, H. M., & Unterlass, M. M. (2020). Biogenic Metal Oxides. Biomimetics, 5(2), 29. https://doi.org/10.3390/biomimetics5020029




