The Effect of Chloride Anions on Charge Transfer in Dye-Sensitized Photoanodes for Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photoanode Preparation
2.2. Electrolyte Preparation
2.3. Stationary Absorption Characterization
2.4. Photoelectrochemical Cell Setup
3. Results and Discussion
3.1. Spectral Measurements
3.2. Chronoamperometry Studies
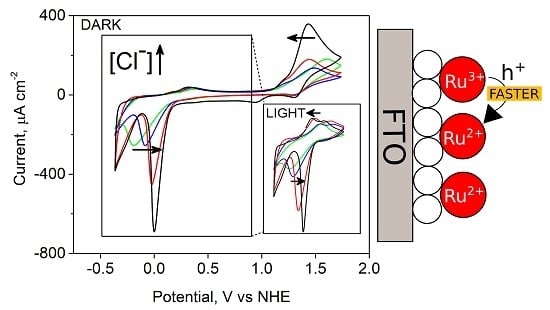
3.3. Cyclic Voltammetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, Z.; Li, F.; Sun, L. Recent advances in dye-sensitized photoelectrochemical cells for solar hydrogen production based on molecular components. Energy Environ. Sci. 2015, 8, 760–775. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- House, R.L.; Iha, N.Y.M.; Coppo, R.L.; Alibabaei, L.; Sherman, B.D.; Kang, P.; Brennaman, M.K.; Hoertz, P.G.; Meyer, T.J. Artificial photosynthesis: Where are we now? Where can we go? J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Ashford, D.L.; Gish, M.K.; Vannucci, A.K.; Brennaman, M.K.; Templeton, J.L.; Papanikolas, J.M.; Meyer, T.J. Molecular chromophore-catalyst assemblies for solar fuel applications. Chem. Rev. 2015, 115, 13006–13049. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Swierk, J.R.; Mallouk, T.E. Design and development of photoanodes for water-splitting dye-sensitized photoelectrochemical cells. Chem. Soc. Rev. 2013, 42, 2357–2387. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; McCool, N.S.; Mallouk, T.E. Water splitting dye-sensitized solar cells. Nano Today 2017, 14, 42–58. [Google Scholar] [CrossRef]
- Materna, K.L.; Crabtree, R.H.; Brudvig, G.W. Anchoring groups for photocatalytic water oxidation on metal oxide surfaces. Chem. Soc. Rev. 2017, 46, 6099–6110. [Google Scholar] [CrossRef]
- Meyer, T.J.; Sheridan, M.V.; Sherman, B.D. Mechanisms of molecular water oxidation in solution and on oxide surfaces. Chem. Soc. Rev. 2017, 46, 6148–6169. [Google Scholar] [CrossRef]
- Chen, Z.; Concepcion, J.J.; Hu, X.; Yang, W.; Hoertz, P.G.; Meyer, T.J. Concerted O atom-proton transfer in the O—O bond forming step in water oxidation. Proc. Natl. Acad. Sci. USA 2010, 107, 7225–7229. [Google Scholar] [CrossRef]
- Spadavecchia, F.; Ardizzone, S.; Cappelletti, G.; Falciola, L.; Ceotto, M.; Lotti, D. Investigation and optimization of photocurrent transient measurements on nano-TiO2. J. Appl. Electrochem. 2013, 43, 217–225. [Google Scholar] [CrossRef]
- Hanson, K.; Brennaman, M.K.; Luo, H.; Glasson, C.R.K.; Concepcion, J.J.; Song, W.; Meyer, T.J. Photostability of phosphonate-derivatized, RuII polypyridyl complexes on metal oxide surfaces. ACS Appl. Mater. Interfaces 2012, 4, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, F.; Wang, L.; Daniel, Q.; Gabrielsson, E.; Sun, L. Pt-free tandem molecular photoelectrochemical cells for water splitting driven by visible light. Phys. Chem. Chem. Phys. 2014, 16, 25234–25240. [Google Scholar] [CrossRef]
- Li, F.; Fan, K.; Xu, B.; Gabrielsson, E.; Daniel, Q.; Li, L.; Sun, L. Organic dye-sensitized tandem photoelectrochemical cell for light driven total water splitting. J. Am. Chem. Soc. 2015, 137, 9153–9159. [Google Scholar] [CrossRef]
- Li, L.; Duan, L.; Xu, Y.; Gorlov, M.; Hagfeldt, A.; Sun, L. A photoelectrochemical device for visible light driven water splitting by a molecular ruthenium catalyst assembled on dye-sensitized nanostructured TiO2. Chem. Commun. 2010, 46, 7307. [Google Scholar] [CrossRef]
- Swierk, J.R.; McCool, N.S.; Saunders, T.P.; Barber, G.D.; Mallouk, T.E. Effects of electron trapping and protonation on the efficiency of water-splitting dye-sensitized solar cells. J. Am. Chem. Soc. 2014, 136, 10974–10982. [Google Scholar] [CrossRef]
- Sheridan, M.V.; Sherman, B.D.; Fang, Z.; Wee, K.R.; Coggins, M.K.; Meyer, T.J. Electron transfer mediator effects in the oxidative activation of a ruthenium dicarboxylate water oxidation catalyst. ACS Catal. 2015, 5, 4404–4409. [Google Scholar] [CrossRef]
- Gierszewski, M.; Grądzka, I.; Glinka, A.; Ziółek, M. Insights into the limitations of solar cells sensitized with ruthenium dyes revealed in time-resolved spectroscopy studies. Phys. Chem. Chem. Phys. 2017, 19, 20463–20473. [Google Scholar] [CrossRef]
- Grądzka, I.; Gierszewski, M.; Karolczak, J.; Ziółek, M. Comparison of charge transfer dynamics in polypyridyl ruthenium sensitizers for solar cells and water splitting systems. Phys. Chem. Chem. Phys. 2018, 20, 7710–7720. [Google Scholar] [CrossRef]
- Qu, P.; Meyer, G.J. Proton-controlled electron injection from molecular excited states to the empty states in nanocrystalline TiO2. Langmuir 2001, 17, 6720–6728. [Google Scholar] [CrossRef]
- Imanishi, A.; Okamura, K.T.; Ohashi, N.; Nakamura, R.; Nakato, Y. Mechanism of water photooxidation reaction at atomically flat TiO2 (rutile) (110) and (100) surfaces: Dependence on solution pH. J. Am. Chem.Soc. 2007, 129, 11569. [Google Scholar] [CrossRef] [PubMed]
- Gillaizeau-Gauthier, I.; Odobel, F.; Alebbi, M.; Argazzi, R.; Costa, E.; Bignozzi, C.A.; Qu, P.; Meyer, G.J.; Nantes, T.D.; Cedex, N.; et al. Phosphonate-based bipyridine dyes for stable photovoltaic devices. Inorg. Chem. 2001, 40, 6073–6079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zakeeruddin, S.M.; Nazeeruddin, K.; Humphry-Baker, R. Molecular wiring of nanocrystals: NCS-enhanced cross-surface charge transfer in self-assembled Ru-complex monolayer on mesoscopic oxide films. J. Am. Chem. Soc. 2006, 128, 4446–4452. [Google Scholar] [CrossRef] [PubMed]
- Ardo, S.; Meyer, G.J. Direct observation of photodriven intermolecular hole transfer across TiO2 nanocrystallites: Lateral self-exchange reactions and catalyst oxidation. J. Am. Chem. Soc. 2010, 132, 9283–9285. [Google Scholar] [CrossRef]
- Song, W.; Ito, A.; Binstead, R.A.; Hanson, K.; Luo, H.; Brennaman, M.K.; Concepcion, J.J.; Meyer, T.J. Accumulation of multiple oxidative equivalents at a single site by cross-surface electron transfer on TiO2. J. Am. Chem. Soc. 2013, 135, 11587–11594. [Google Scholar] [CrossRef]





| Electrolyte | pH | J0 (μA/cm2) | Jstab (μA/cm2) | Jdark (μA/cm2) | Jstab/J0 |
|---|---|---|---|---|---|
| 0.07 M Phosphate buffer | ≈7 | 240 | 50 | −20 | 0.21 |
| 0.1 M KCl | ≈5 | 320 | 10 | −20 | 0.03 |
| 0.001 M HCl | ≈3 | 640 | 5 | −140 | 0.01 |
| EPA | ≈3 | 140 | 7 | −40 | 0.05 |
| EPA + 0.001 M KCl | 120 | 6 | −40 | 0.05 | |
| EPA + 0.01 M KCl | 200 | 7 | −40 | 0.04 | |
| EPA + 0.1 M KCl | 390 | 7 | −40 | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grądzka, I.; Gierszewski, M.; Ziółek, M. The Effect of Chloride Anions on Charge Transfer in Dye-Sensitized Photoanodes for Water Splitting. Biomimetics 2019, 4, 5. https://doi.org/10.3390/biomimetics4010005
Grądzka I, Gierszewski M, Ziółek M. The Effect of Chloride Anions on Charge Transfer in Dye-Sensitized Photoanodes for Water Splitting. Biomimetics. 2019; 4(1):5. https://doi.org/10.3390/biomimetics4010005
Chicago/Turabian StyleGrądzka, Iwona, Mateusz Gierszewski, and Marcin Ziółek. 2019. "The Effect of Chloride Anions on Charge Transfer in Dye-Sensitized Photoanodes for Water Splitting" Biomimetics 4, no. 1: 5. https://doi.org/10.3390/biomimetics4010005