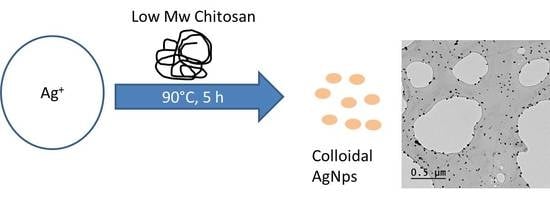
On the Ability of Low Molecular Weight Chitosan Enzymatically Depolymerized to Produce and Stabilize Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzymatic Chitosan Depolymerization
2.2. Polymer Characterization
2.3. Silver Nanoparticles Production and Characterization
3. Results
3.1. Polymer Characterization
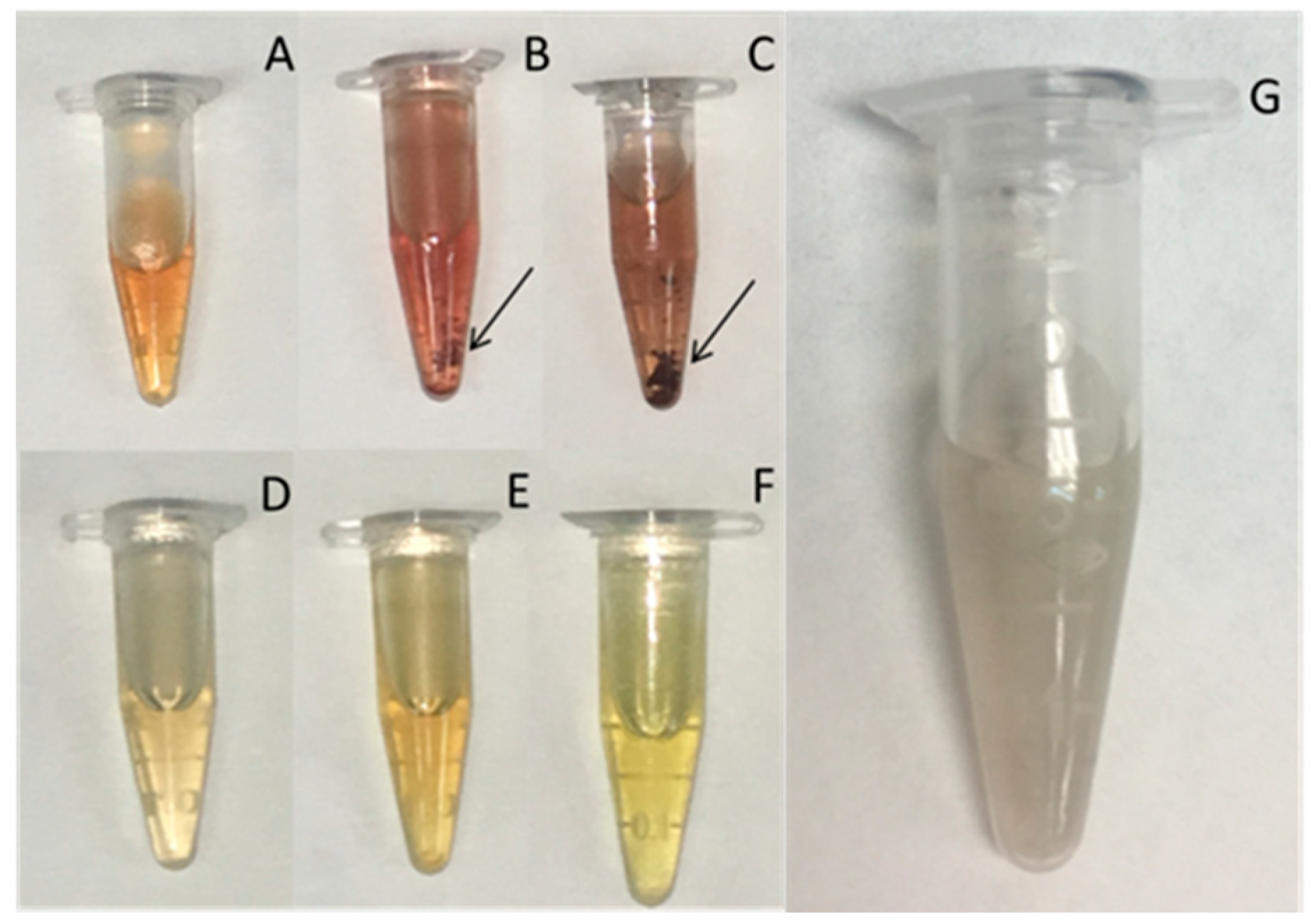
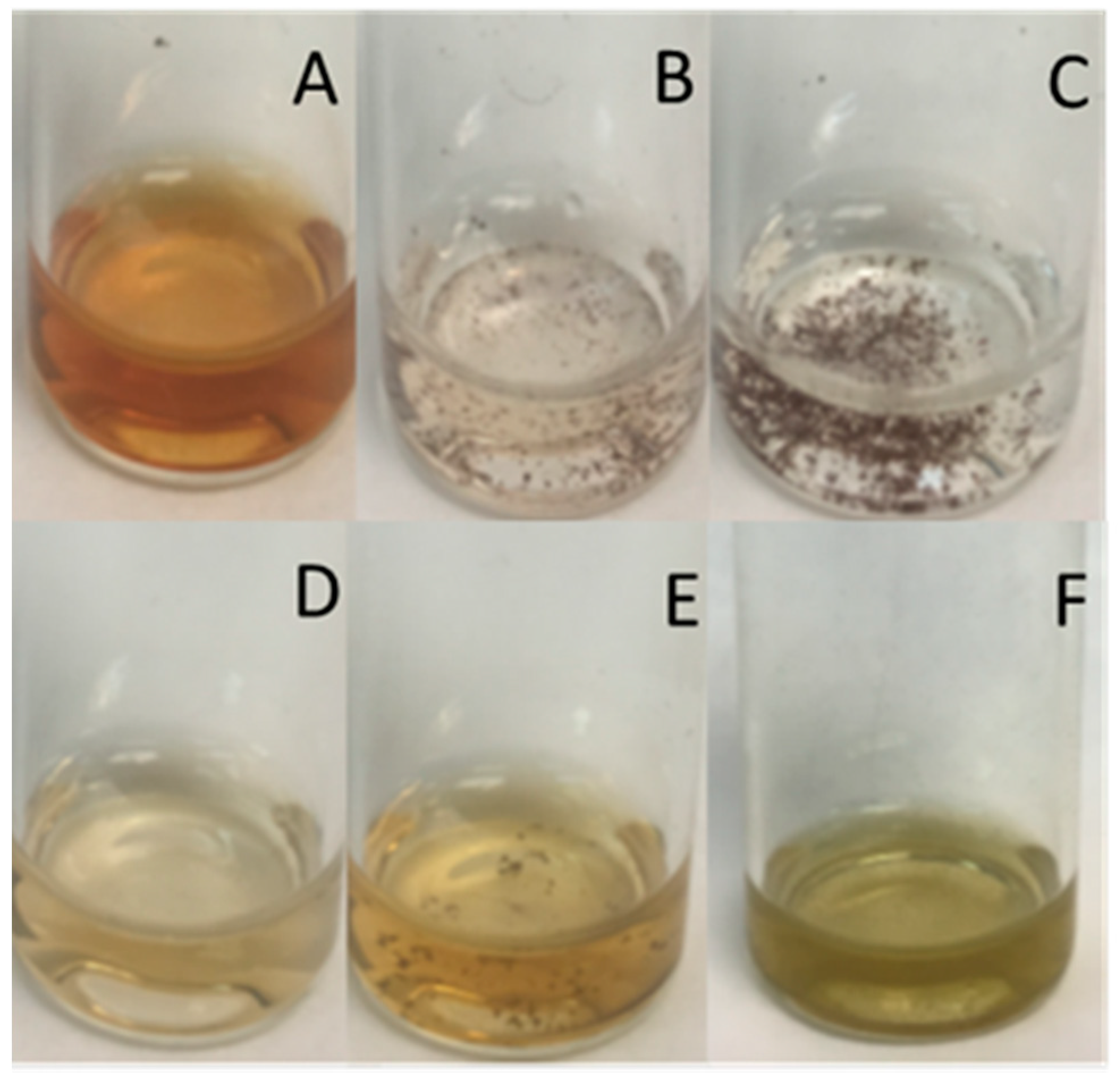
3.2. Silver Nanoparticle Production
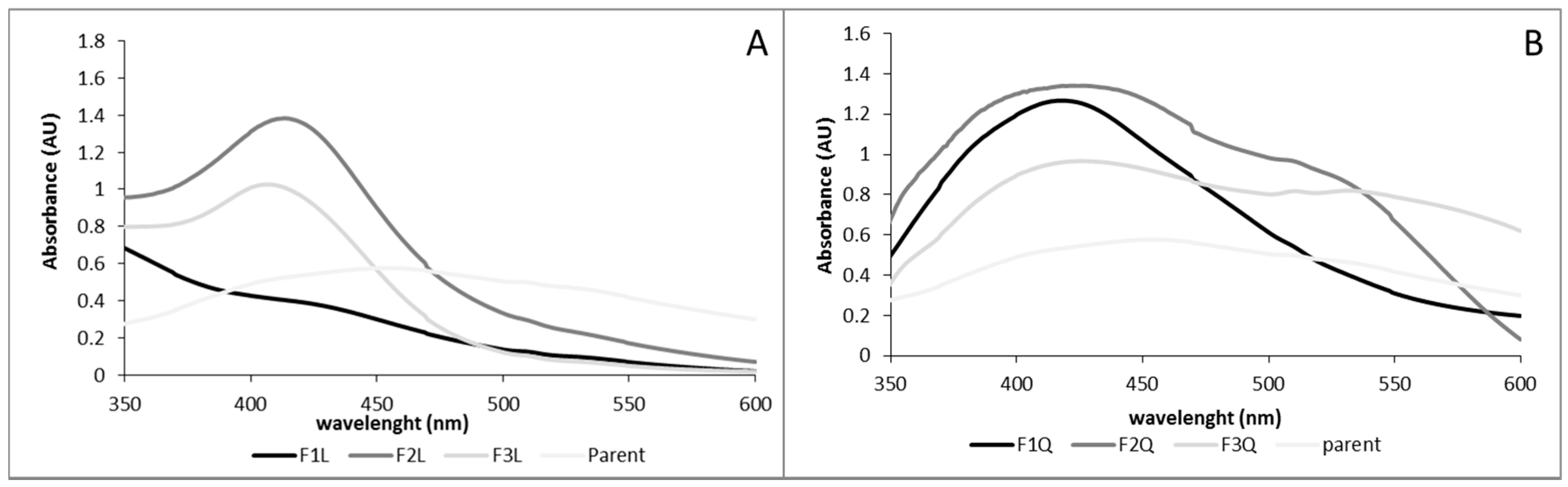
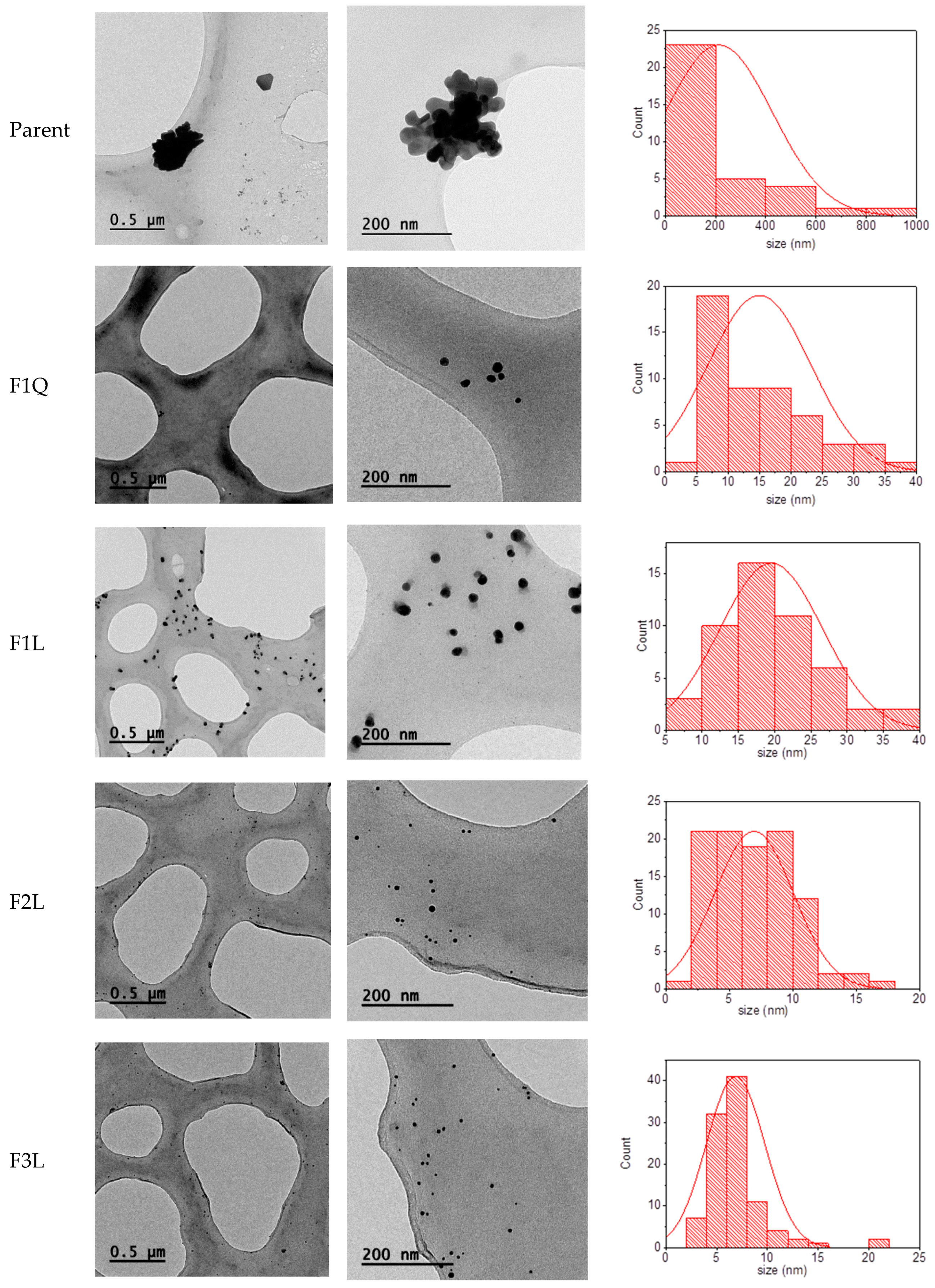
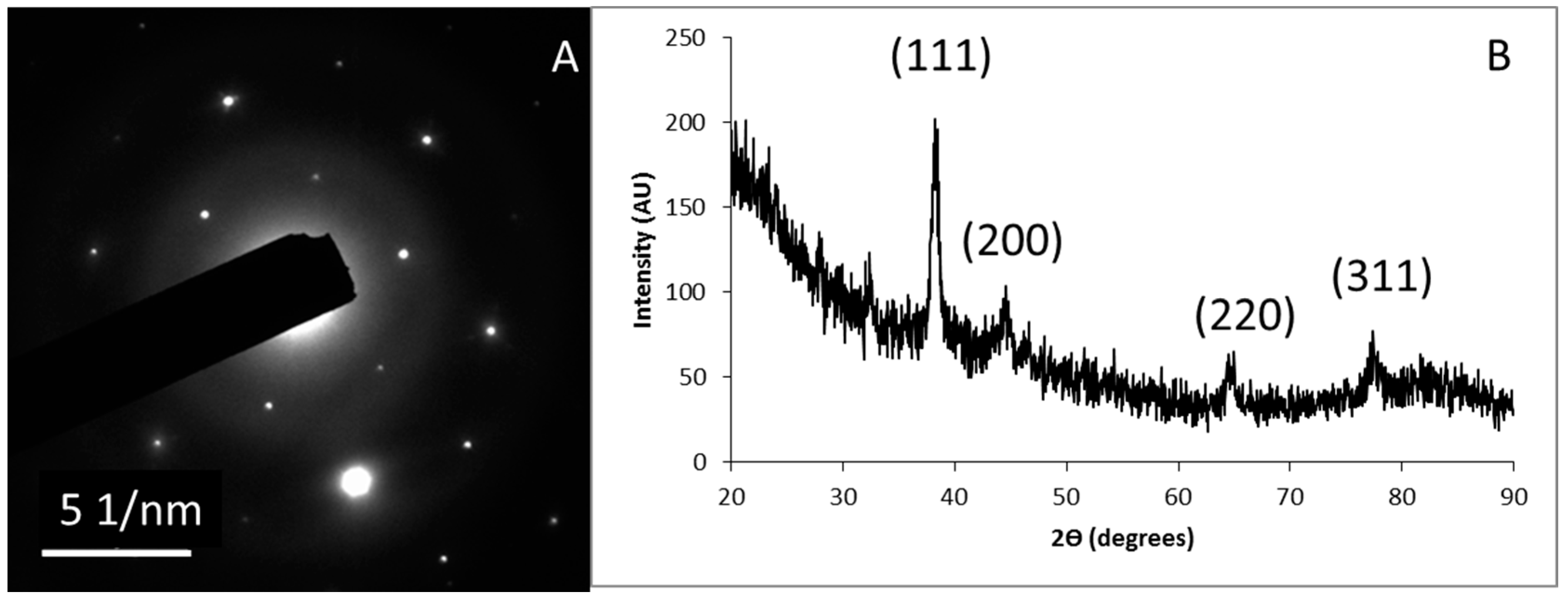
3.3. Nanoparticles Characterization
3.4. Nanoparticles Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Azizi-Toupkanloo, H. Silver colloid nanoparticles: Ultrasound-assisted synthesis, electrical and rheological properties. Powder Technol. 2013, 237, 97–101. [Google Scholar] [CrossRef]
- Mănoiu, V.S.; Aloman, A. Obtaining silver nanoparticles by sonochemical methods. UPB Sci. Bull. Ser. B 2010, 72, 179. [Google Scholar]
- Yamamoto, H.; Kozawa, T.; Tagawa, S.; Naito, M.; Marignier, J.L.; Mostafavi, M.; Belloni, J. Radiation-induced synthesis of metal nanoparticles in ethers THF and PGMEA. Radiat. Phys. Chem. 2013, 91, 148–155. [Google Scholar] [CrossRef]
- Chen, P.; Song, L.; Liu, Y.; Fang, Y.E. Synthesis of silver nanoparticles by γ-ray irradiation in acetic water solution containing chitosan. Radiat. Phys. Chem. 2007, 76, 1165–1168. [Google Scholar] [CrossRef]
- Muthu, K.; Priya, S. Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 179, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Hong, J. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Ghaseminezhad, S.M.; Hamedi, S.; Shojaosadati, S.A. Green synthesis of silver nanoparticles by a novel method: Comparative study of their properties. Carbohydr. Polym. 2012, 89, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of palladium nanoparticles. Mater. Lett. 2010, 64, 1951–1953. [Google Scholar] [CrossRef]
- Valencia, G.A.; de Oliveira Vercik, L.C.; Ferrari, R.; Vercik, A. Synthesis and characterization of silver nanoparticles using water-soluble starch and its antibacterial activity on Staphylococcus aureus. Stärke 2013, 65, 931–937. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Twu, Y.K.; Chen, Y.W.; Shih, C.M. Preparation of silver nanoparticles using chitosan suspensions. Powder Technol. 2008, 185, 251–257. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, Q.; Yang, X. Preparation and characterization of metal–chitosan nanocomposites. Colloids Surf. B 2004, 39, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, A.; Chattopadhyay, A. A ‘green’ chitosan–silver nanoparticle composite as a heterogeneous as well as micro-heterogeneous catalyst. Nanotechnology 2007, 19, 015603. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Qian, W. Facile synthesis of Ag and Au nanoparticles utilizing chitosan as a mediator agent. Colloids Surf. B 2008, 62, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Hameedha Beevi, A.; Anand, M. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front. Lab. Med. 2018, 2, 30–35. [Google Scholar] [CrossRef]
- Mengíbar, M.; Gananl, M.; Miralles, B.; Carrascosa, A.V.; Martínez-Rodriguez, A.J.; Peter, M.G.; Heras, A. Antibacterial activity of products of depolymerization of chitosans with lysozyme and chitosanase against Campylobacter jejuni. Carbohydr. Polym. 2011, 84, 844–848. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Rocchetti, R. Determination of the degree of acetylation of chitosans by first derivative ultraviolet spectrophotometry. Carbohydr. Polym. 1985, 5, 461–472. [Google Scholar] [CrossRef]
- Henglein, A. Colloidal silver nanoparticles: Photochemical preparation and interaction with O2, CCl4, and some metal ions. Chem. Mater. 1998, 10, 444–450. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Vårum, K.M.; Holme, H.K.; Izume, M.; Stokke, B.T.; Smidsrød, O. Determination of enzymatic hydrolysis specificity of partially N-acetylated chitosans. Biochim. Biophys. Acta 1996, 1291, 5–15. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Fong, D. Immunological responses to chitosan for biomedical applications. In Chitosan Based Biomaterials; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Volume 1, pp. 45–79. [Google Scholar]

| Polymer Characteristics | AgNPs Characteristics | ||||
|---|---|---|---|---|---|
| Mw (kDa) | FA | Dispersion | Shape | Size (nm) | Ref. |
| 1240 | 0.13 | Polydisperse | Spherical | 10–150 | [13] |
| 400 | 0 | Polydisperse | Spherical, Clusters | 20–100 | [14] |
| High Mw | 0.25 | Monodisperse | Spherical | 4–8 | [15] |
| High Mw | 0.15 | Polydisperse | Spherical, Fractal | 20–200 | [16] |
| - | - | Monodisperse | Spherical | 4–5 | [6] |
| - | - | Polydisperse | Spherical | 10–80 | [17] |
| Sample 1 | FA | Mn (kDa) | Mw (kDa) | PDI |
|---|---|---|---|---|
| F1Q | 38.54 ± 0.78 | 16,048 | 42,196 | 2.63 |
| F2Q | 31.07 ± 0.71 | 6207 | 10,312 | 1.66 |
| F3Q | 26.03 ± 0.27 | 5123 | 7020 | 1.37 |
| F1L | 38.43 ± 1.48 | 11,768 | 30,933 | 2.63 |
| F2L | 42.29 ± 1.63 | 5793 | 8171 | 1.41 |
| F3L | 42.67 ± 1.18 | 3578 | 4232 | 1.18 |
| Parent | 48.34 ± 1.83 | 128,008 | 538,448 | 4.21 |
| Sample 1 | Zeta Potential (mV) | Size (nm) 2 | Size (nm) 3 | <10 nm (%) |
|---|---|---|---|---|
| F1Q | +24.0 ± 6.5 | 202.5 | 15 | 40 |
| F2Q | +12.4 ± 7.3 | 153.8 | - | - |
| F3Q | +20.8 ± 4.6 | 169.8 | - | - |
| F1L | +23.7 ± 7.6 | 291.6 | 20 | 6 |
| F2L | +15.4 ± 7.0 | 131.3 | 7 | 80 |
| F3L | +20.6 ± 4.3 | 273.9 | 7 | 90 |
| Parent | +40.6 ± 4.6 | 909.1 | 200 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranaz, I.; Castro, C.; Heras, A.; Acosta, N. On the Ability of Low Molecular Weight Chitosan Enzymatically Depolymerized to Produce and Stabilize Silver Nanoparticles. Biomimetics 2018, 3, 21. https://doi.org/10.3390/biomimetics3030021
Aranaz I, Castro C, Heras A, Acosta N. On the Ability of Low Molecular Weight Chitosan Enzymatically Depolymerized to Produce and Stabilize Silver Nanoparticles. Biomimetics. 2018; 3(3):21. https://doi.org/10.3390/biomimetics3030021
Chicago/Turabian StyleAranaz, Inmaculada, Carolina Castro, Angeles Heras, and Niuris Acosta. 2018. "On the Ability of Low Molecular Weight Chitosan Enzymatically Depolymerized to Produce and Stabilize Silver Nanoparticles" Biomimetics 3, no. 3: 21. https://doi.org/10.3390/biomimetics3030021