State-of-Charge Monitoring and Battery Diagnosis of NiCd Cells Using Impedance Spectroscopy
Abstract
:1. Introduction
1.1. State-of-Charge Indicators
1.2. Aging Phenomena
1.3. Impedance Spectroscopy
1.4. Capacitance and Time Constant
2. Experimental Setup
- Successive discharge. Pack #1 (fabricated in 2017) was cycled between SOC = 100% and SOC = 0% with 1C rate. Pack #4 (2018) was cycled between SOC = 100% and SOC = 80% at 0.5C rate. After every 400 cycles, the batteries were fully charged and then successively discharged by 2% steps, each characterized by impedance spectroscopy, until SOC = 70% was reached. Capacity was measured at the end of the endurance test, so that no memory effect was eliminated.
- Rapid test. Pack #2 (2017) was cycled between SOC = 100% and SOC = 80% at 1C rate, and pack #5 of 2018 suffered at 0.5C. Every 400 cycles the batteries received (i) full charge, (ii) impedance measurement, (iii) discharge by 0.19 Ah, (iv) again impedance measurement, and (v) capacity measurement by ampere-hour counting. The memory effect was studied between successive tests.
- Extensive test. Pack #3 (2017) was cycled between SOC = 100% and SOC = 80% at 0.1C rate and pack #6 (of 2018) at 0.5C. The analysis combined the above methods: (i) full charge, (ii) successive discharge by 2% and impedance measurement, until SOC = 70% was reached, (iii) full charge, and (iv) capacity determination by ampere-hour counting. The loss of capacity was studied for a large number of charge–discharge cycles.
3. Results and Discussion
3.1. State-of-Charge Monitoring
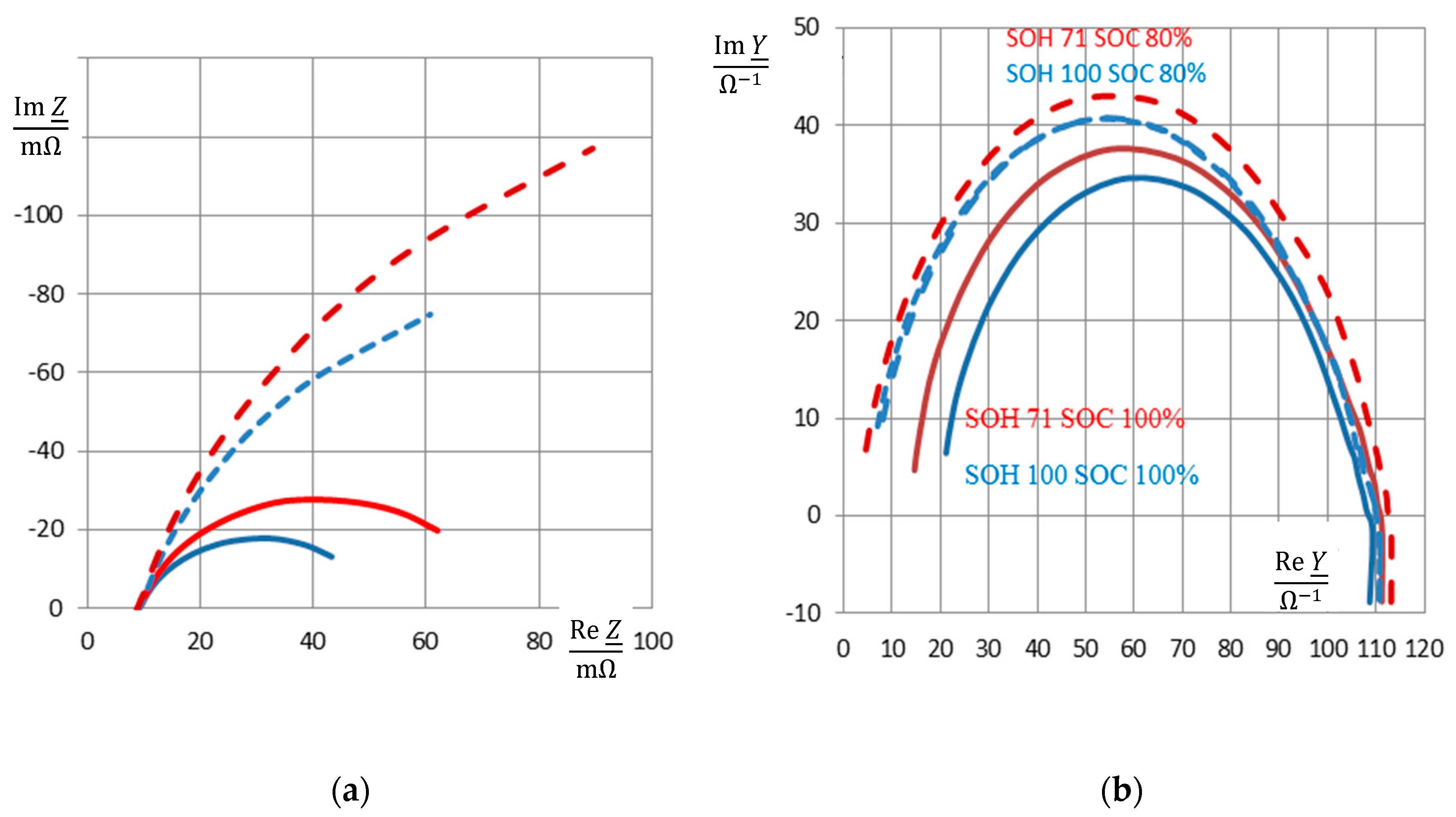
3.2. Reactance during Aging and the Memory Effect
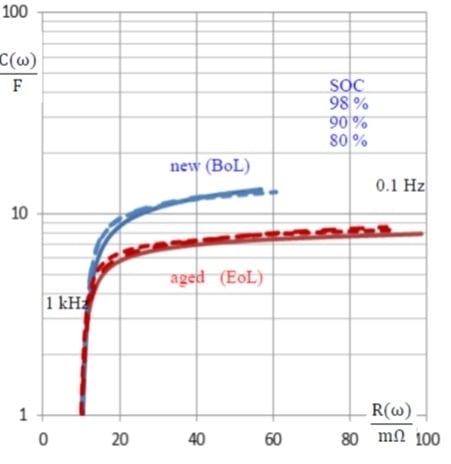
3.3. Capacitance as an Aging Indicator
3.4. Pseudo-Charge as a SOH Indicator
3.5. Separation of SOC and SOH
3.6. Impedance-Based Aging Indicators
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kurzweil, P.; Shamonin, M. State-of-Charge Monitoring by Impedance Spectroscopy during Long-Term Self-Discharge of Supercapacitors and Lithium-Ion Batteries. Batteries 2018, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model. J. Power Sources 2012, 219, 204–216. [Google Scholar] [CrossRef]
- Hammouche, A.; Karden, E.; De Doncker, R.W. Monitoring state-of-charge of Ni–MH and Ni–Cd batteries using impedance spectroscopy. J. Power Sources 2004, 127, 105–111. [Google Scholar] [CrossRef]
- Xiong, R.; Cao, J.; Yu, Q.; He, H.; Sun, F. Critical Review on the Battery State of Charge Estimation Methods for Electric Vehicles. IEEE Access 2017, 6, 1832–1843. [Google Scholar] [CrossRef]
- Sarmah, S.B.; Kalita, P.; Garg, A.; Niu, X.; Zhang, X.-W.; Peng, X.; Bhattacharjee, D. A Review of State of Health Estimation of Energy Storage Systems: Challenges and Possible Solutions for Futuristic Applications of Li-Ion Battery Packs in Electric Vehicles. J. Electrochem. Energy Convers. Storage 2019, 16, 040801. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Hu, X.; Ma, H.; Li, S. Combined State of Charge and State of Health estimation over lithium-ion battery cell cycle lifespan for electric vehicles. J. Power Sources 2015, 273, 793–803. [Google Scholar] [CrossRef]
- Rodrigues, S.; Munichandraiah, N.; Shukla, A.K. A review of state-of-charge indication of batteries by means of a.c. impedance measurements. J. Power Sources 2000, 87, 12–20. [Google Scholar] [CrossRef]
- Bounaga, A. Device and Method for Measuring the Charge State of a Nickel-Cadmium Accumulator. U.S. Patent 5,650,937A, 22 July 1997. [Google Scholar]
- Latner, N. Method for Determining the State of Charge of Nickel Cadmium Batteries by Measuring the Farad Capacitance Thereof. U.S. Patent 3,562,634A, 9 February 1971. Available online: https://worldwide.espacenet.com/searchResults?ST=singleline&locale=de_EP&submitted=true&DB=&query=US3562634 (accessed on 8 January 2020).
- Sathyanarayana, S.; Venugopalan, S.; Gopikanth, M.L. Impedance parameters and the state-of charge. I. Nickel-cadmium battery. J. Appl. Electrochem. 1979, 9, 125–139. [Google Scholar] [CrossRef]
- Wenzl, H. Capacity. In Encyclopedia of Electrochemical Power Sources; Garche, J., Dyer, C.K., Moseley, P., Ogumi, Z., Rand, D., Scrosati, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 1, pp. 395–400. [Google Scholar]
- Waag, W.; Sauer, D.U. State-of-Charge/Health. In Encyclopedia of Electrochemical Power Sources; Garche, J., Dyer, C.K., Moseley, P., Ogumi, Z., Rand, D., Scrosati, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 4, pp. 793–804. [Google Scholar]
- Piller, S.; Perrin, M.; Jossen, A. Methods for state-of-charge determination and their applications. J. Power Sources 2001, 96, 113–120. [Google Scholar] [CrossRef]
- Bergveld, H.J.; Danilov, D.; Notten, P.H.L.; Pop, V.; Regtien, P.P.L. Adaptive State-of-charge determination. In Encyclopedia of Electrochemical Power Sources; Garche, J., Dyer, C.K., Moseley, P., Ogumi, Z., Rand, D., Scrosati, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 1, pp. 450–477. [Google Scholar]
- Dowgiallo, E.J. Method for Determining Battery State of Charge by Measuring A.C. Electrical Phase Angle Change. U.S. Patent 3,984,762A, 5 October 1975. [Google Scholar]
- Finger, E.P.; Sands, E.A. Method and Apparatus for Measuring the State of Charge of a Battery by Monitoring Reductions in Voltage. U.S. Patent 4,193,026A, 11 March 1978. [Google Scholar]
- Kikuoka, T.; Yamamoto, H.; Sasaki, N.; Wakui, K.; Murakami, K.; Ohnishi, K.; Kawamura, G.; Noguchi, H.; Ukigaya, F. System for Measuring State of Charge of Storage Battery. U.S. Patent 4,377,787A, 22 March 1983. [Google Scholar]
- Seyfang, G.R. Battery State of Charge Indicator. U.S. Patent 4,949,046, 14 August 1990. [Google Scholar]
- Peled, E.; Yamin, H.; Reshef, I.; Kelrich, D.; Rozen, S. Method and Apparatus for Determining the State-of-Charge of Batteries Particularly Lithium Batteries. U.S. Patent 4,725,784A, 16 February 1988. [Google Scholar]
- Hahn, M.; Schindler, S.; Triebs, L.C.; Danzer, M.A. Optimized Process Parameters for a Reproducible Distribution of Relaxation Times Analysis of Electrochemical Systems. Batteries 2019, 5, 43. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Takeuchi, S.; Kobayakawa, K. Cause of the memory effect observed in alkaline secondary batteries using nickel electrode. J. Power Sources 2001, 93, 20–24. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Handbook of Batteries; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Kurzweil, P.; Ober, J.; Wabner, D.W. Method for Correction and Analysis of Impedance Spectra. Electrochim. Acta 1989, 34, 1179–1185. [Google Scholar] [CrossRef]
- Kurzweil, P.; Fischle, H.J. A new monitoring method for electrochemical aggregates by impedance spectroscopy. J. Power Sources 2004, 127, 331–340. [Google Scholar] [CrossRef]
- Kurzweil, P.; Hildebrand, A.; Weiss, M. Accelerated Life Testing of Double-Layer Capacitors: Reliability and safety under Excess Voltage and Temperature. ChemElectroChem 2015, 2, 150–159. [Google Scholar] [CrossRef]










| Test Method | Battery Pack: 7.5 V, 1.7 Ah, 5 Single Cells | A. Cycling (SOC) at 50 °C | B. Impedance Measurements During Discharge (SOC 1 → 0.7) After 400, 800, 1200 Cycles | C. Capacity After Full 0.5C Charge (Ah Counting) |
|---|---|---|---|---|
| 1 Full discharge | (a) old (#1) (b) new (#4) | 1C (1→ 0) 0.5C (1→ 0) | by 2% voltage steps | at cycle 400, 800, and 1200 |
| 2 Partial discharge | (a) old (#2) (b) new (#5) | 1C (1→ 0.8) 0.5C (1→ 0.8) | by 0.19 Ah steps | at cycle 400, 800, and 1200 |
| 3 Partial discharge | (a) old (#3) (b) new (#6) | 0.1C (1→ 0.8) 0.5C (1→ 0.8) | by 2% voltage steps | at cycle 400, 800 and 1200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzweil, P.; Scheuerpflug, W. State-of-Charge Monitoring and Battery Diagnosis of NiCd Cells Using Impedance Spectroscopy. Batteries 2020, 6, 4. https://doi.org/10.3390/batteries6010004
Kurzweil P, Scheuerpflug W. State-of-Charge Monitoring and Battery Diagnosis of NiCd Cells Using Impedance Spectroscopy. Batteries. 2020; 6(1):4. https://doi.org/10.3390/batteries6010004
Chicago/Turabian StyleKurzweil, Peter, and Wolfgang Scheuerpflug. 2020. "State-of-Charge Monitoring and Battery Diagnosis of NiCd Cells Using Impedance Spectroscopy" Batteries 6, no. 1: 4. https://doi.org/10.3390/batteries6010004