Parameterization and Validation of an Electrochemical Thermal Model of a Lithium-Ion Battery
Abstract
:1. Introduction
2. Theory
2.1. Lithium Ion Battery
2.2. Heat Transfer
2.3. Coupling of the Physics
3. Experimental Techniques
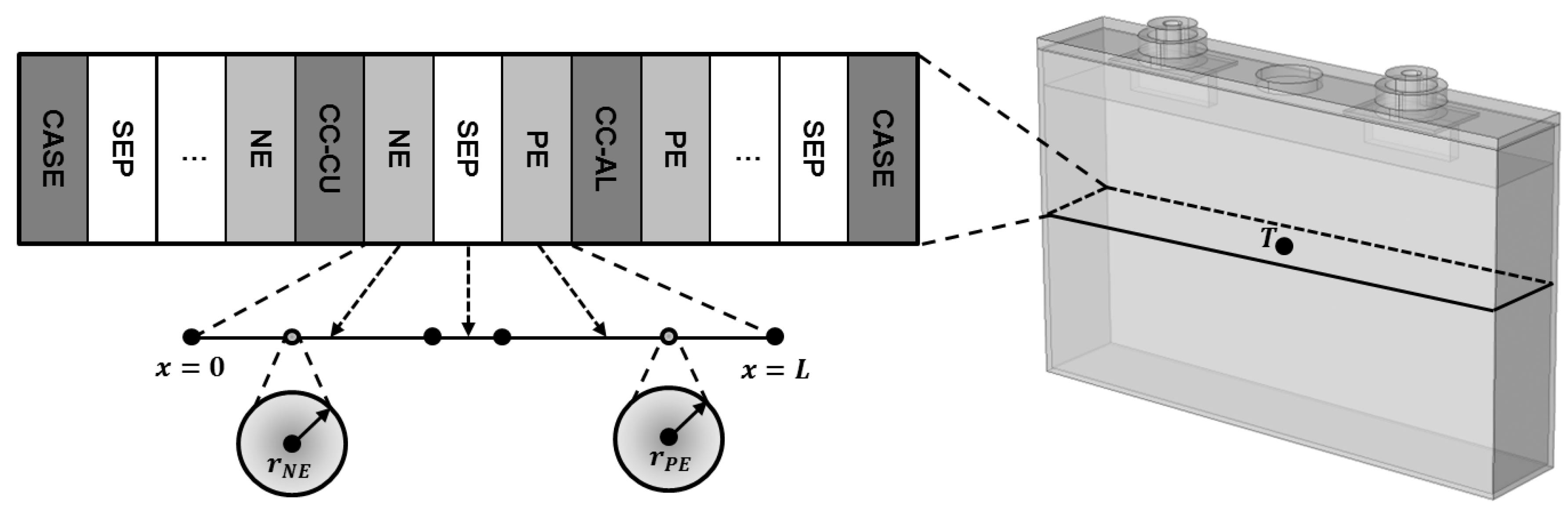
3.1. Battery Description
3.2. Pre-Mortem Techniques
3.2.1. Quasi-Open-Circuit Voltage (qOCV)—Low Current Method
3.2.2. Open-Circuit Voltage (OCV)—Rest Method
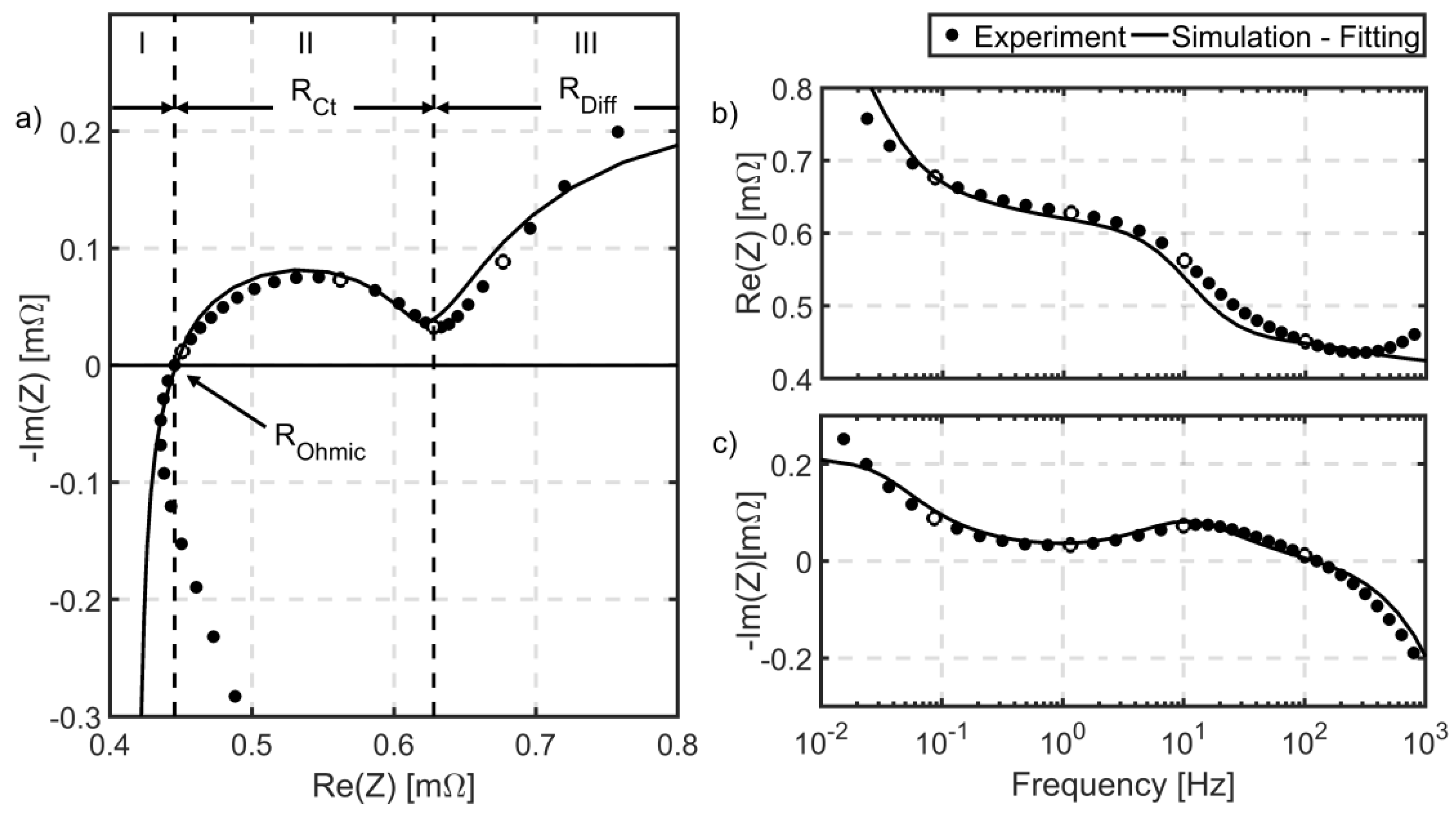
3.2.3. Electrochemical Impedance Spectroscopy (EIS)
3.2.4. Internal Resistance Measurement by Hybrid Pulse Power Characterization (HPPC)
3.2.5. Electrical/Thermal Validation
3.3. Post-Mortem Techniques
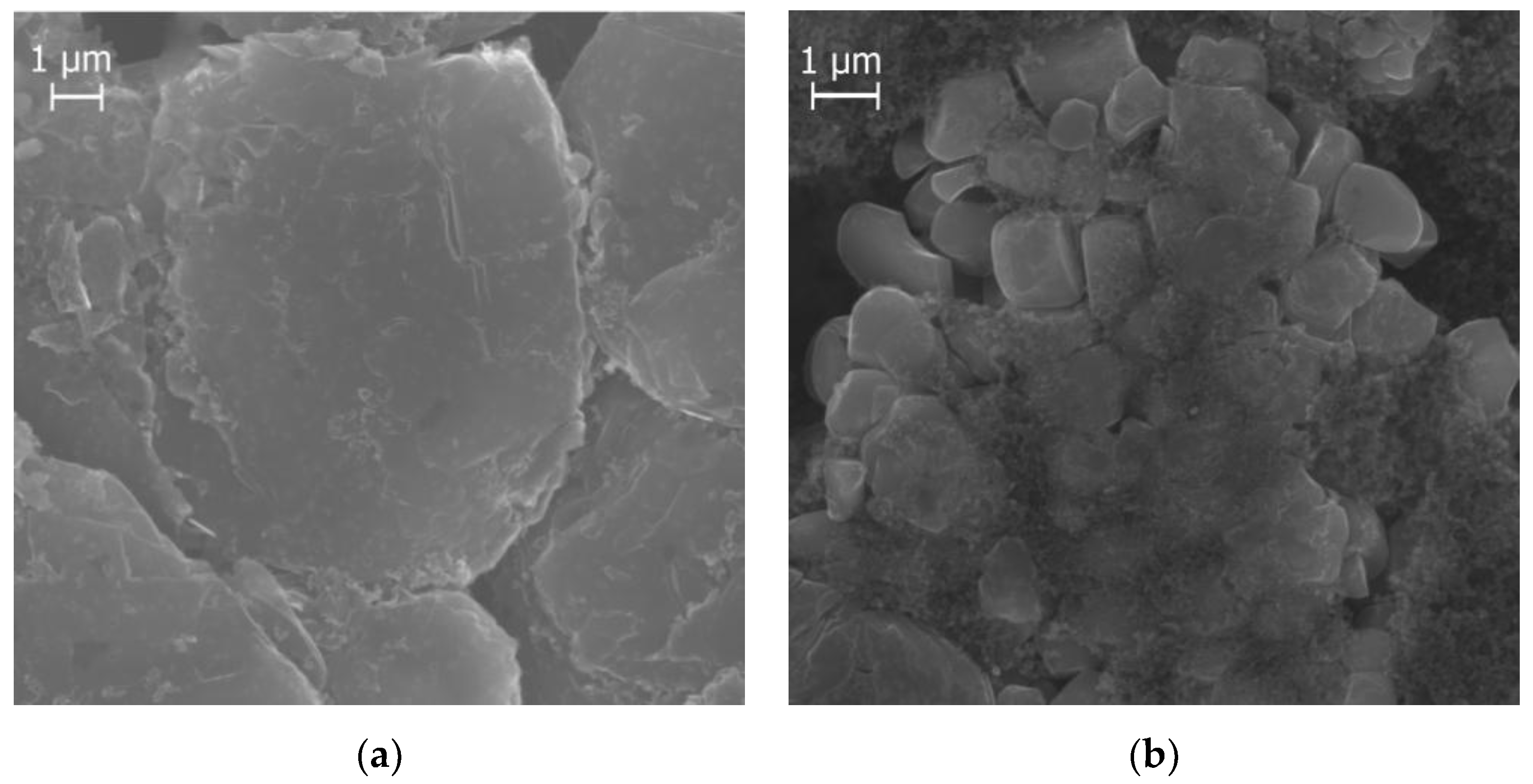
3.3.1. Cell Opening and Sample Preparation
3.3.2. Geometrical Data Measurement
- NRecon: The obtained datasets of recorded images are reconstructed to a 3D cross-Section image stack. The ring artefact and beam hardening values are kept constant for all the samples.
- Data Viewer: The reconstructed images are adjusted parallel to their respective viewing plane, coronal and sagittal, and the VOI is defined.
- CTAn: For the 3D morphometric analysis, the shape and sheet number of ROI are defined.
3.3.3. Thermal Parameter Measurement
4. Simulation Techniques
4.1. Cell and Electrode Examination
- The qOCV potential for the cell is measured during a charge and discharge phase to investigate the actual capacity . The OCV potential of the cell is approximated by the average of the qOCV profiles at normalized time, since the overvoltage error is canceled with respect to the direction of the energy transfer:The overvoltage error cancels out with respect to the direction of each energy transfer.
- The positive electrodes potential in dependence of the electrode SoC is chosen to be taken from literature [25]:The positive electrode potential profile is known to show no extreme characteristic slopes in comparison to the negative electrode potential within the mean SoC range.
- By applying Equations (4) and (5) to Equation (3), the negative electrodes potential is calculated as follows:
- The inactive fraction of the electrodes SoC windows is calculated by:
- The actual electrode capacities and are calculated based on the fact that the usable capacity represents the active material loading, while the counterparts in each electrode remain inactive:
- The theoretical electrode capacities are calculated as follows:
- The electrodes active material fraction is received by calculating the ratio of the actual electrode capacity of the theoretical electrode capacity:
- The specific surface area is calculated as follows:
- Similarly, the actual surface area is calculated as follows:
4.2. Reaction Kinetics Examination
- : The impedance spectrum crosses the -axis at the frequency, when the inductive behavior is compensated by capacitive behavior of battery components. Hence, the battery impedance at this point is nearly the pure ohmic resistance of the battery.
- : The negative imaginary part of the impedance spectrum shows a local minimum at the end of a large semicircle at low frequencies. The semicircle is usually associated with interfacial charge transfer reaction combined with double layer capacitance.
- The time-dependent model variables, here represented by , are modified by a sinusoidal perturbation and solved in the complex-valued domain for predefined frequencies
- Where is the initial time-dependent value at time zero and is the complex-valued extension of . The particle concentration time derivative mentioned in Table 1 is exchanged by a frequency mode as follows:where incorporates the double layer capacitance at each electrode:Correspondingly, instead of in the governing equations in Table 1.
- Thus, the cell impedance is calculated dependent on the perturbed boundary flux variables and and incorporated conditions defined at the positive current collector:Therein and are a resistance and inductance component, is equal to the applied current density in the solid domain, is the potential within the solid domain, and are the initial time-dependent values at time zero, is the normal boundary flux vector, is the amplitude of the potential perturbation and is the amplitude of the current perturbation induced by .
- The temperature dependence of model variables is considered by applying the Arrhenius relation as considered in [25]:where is the variable, is the variables value at the reference temperature , is the activation energy, is the universal gas constant, and is the temperature variable.
- Each electrode’s exchange current density is defined temperature-dependent with respect to [25]:
- Each electrode’s diffusion coefficient is dependent on the electrodes SoC and temperature. Therefore, the following equations are defined with respect to [25]:whereand
- The Li+ transference number is defined as follows, as taken from [50]:
- The effective diffusional electrolyte conductivity is defined as follows, similar to [3]:
4.3. Thermal Behaviour Examination
5. Results and Discussion
5.1. Electrode Geometry Evaluation
5.2. Battery Thermal Parameter Examination
5.3. Cell Balancing Evaluation
- Due to the difficulty to set up the initial cell SoC at 0%, as described in Section 3.2, an off-set within the experiment is realistic.
- An overhang effect at the negative electrode during a prior charge-phase lead to an increased discharge capacity after relaxation [54].
5.4. Reaction Kinetics Evaluation
- Each nominal SoC level of the batteries could comprise a deviation in their real capacity that cannot be captured by the experiment setup.
- Each battery contains ninety cells gathered in a parallelized electrode stack. Non-uniformity of the cell SoCs within the stack would indicate a variation in duration and time until the slopes S2 or S4 are passed. Since the batteries’ internal resistance comprises the contributions of each of the cell’s contribution, a slight shift within the peak’s potential would shave the peak magnitude and move its location.
5.5. Model Parameterization
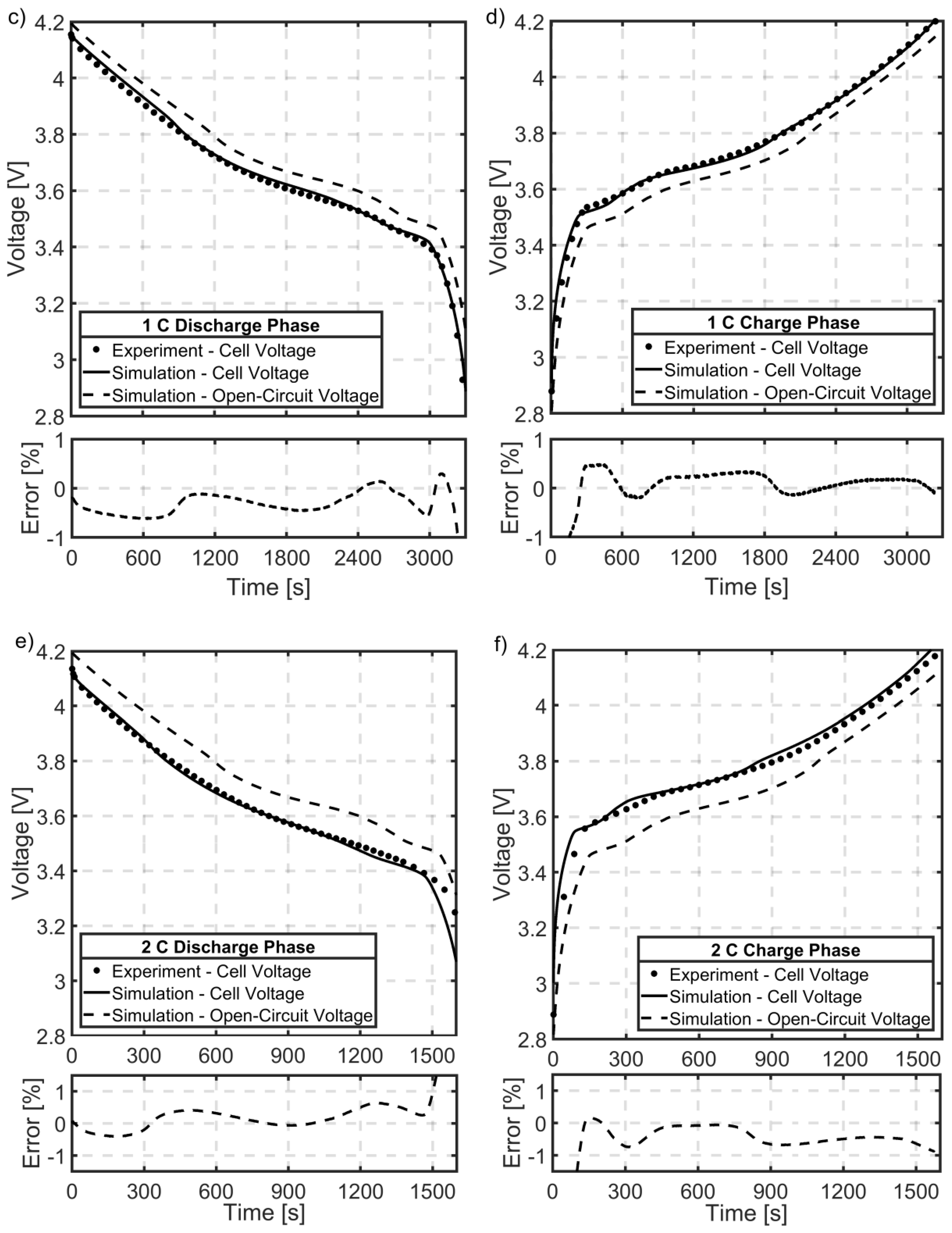
5.6. Cell Voltage Validation
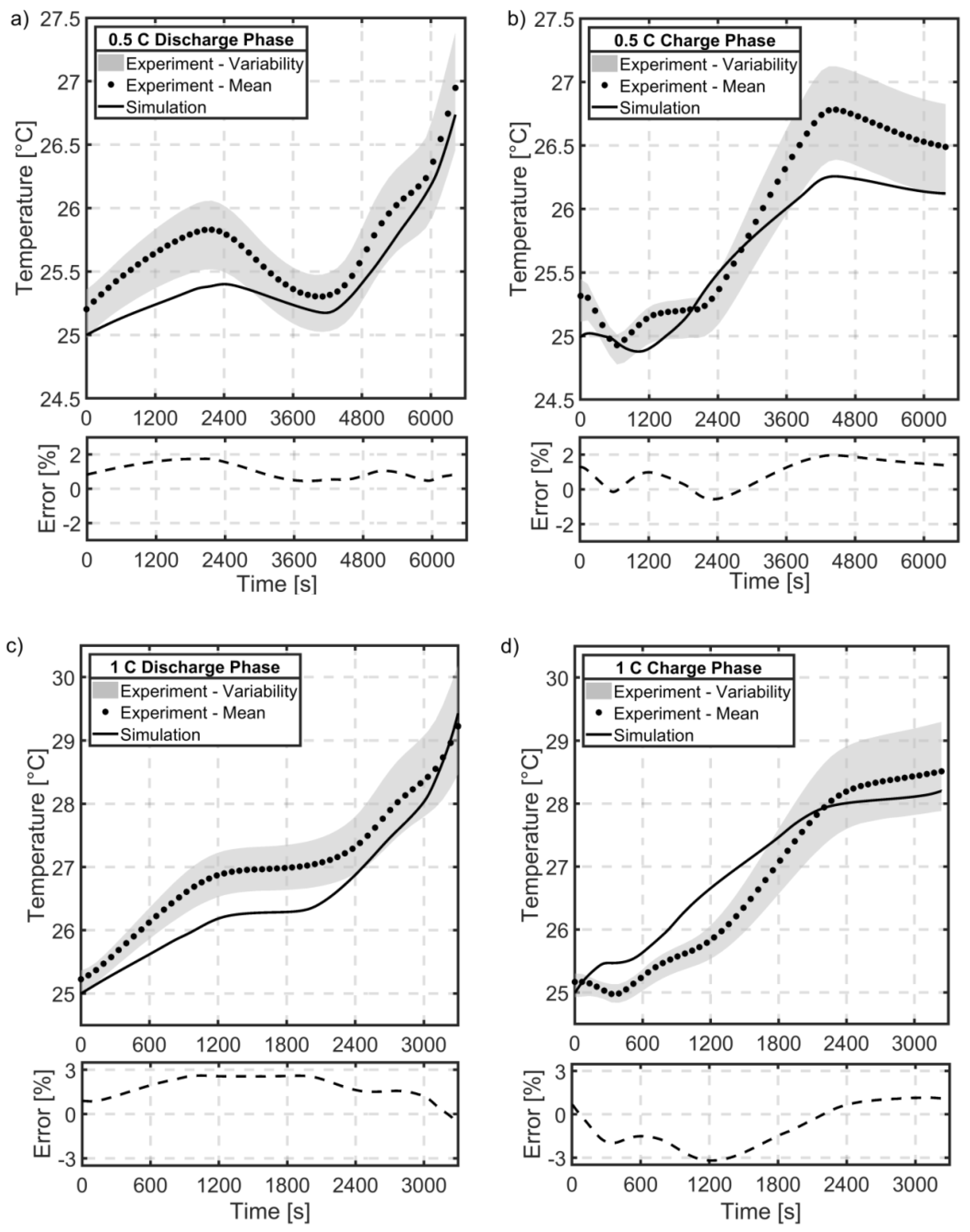
5.7. Temperature Validation
- No spatial resolution of the battery cell is considered. Hence, no thermal gradient based on the interior cell component connection is created.
- The environment conditions of the battery could affect the experimental results. The climate chamber was turned off, when the experiment was initialized at a constant temperature to prevent weak forced cooling, but fluctuations could still arise by the buoyancy effect within air [64]. The wires and fastener connected to the battery cell could influence the experimental thermal behavior of the battery cell by inducing a thermal outflow [2].
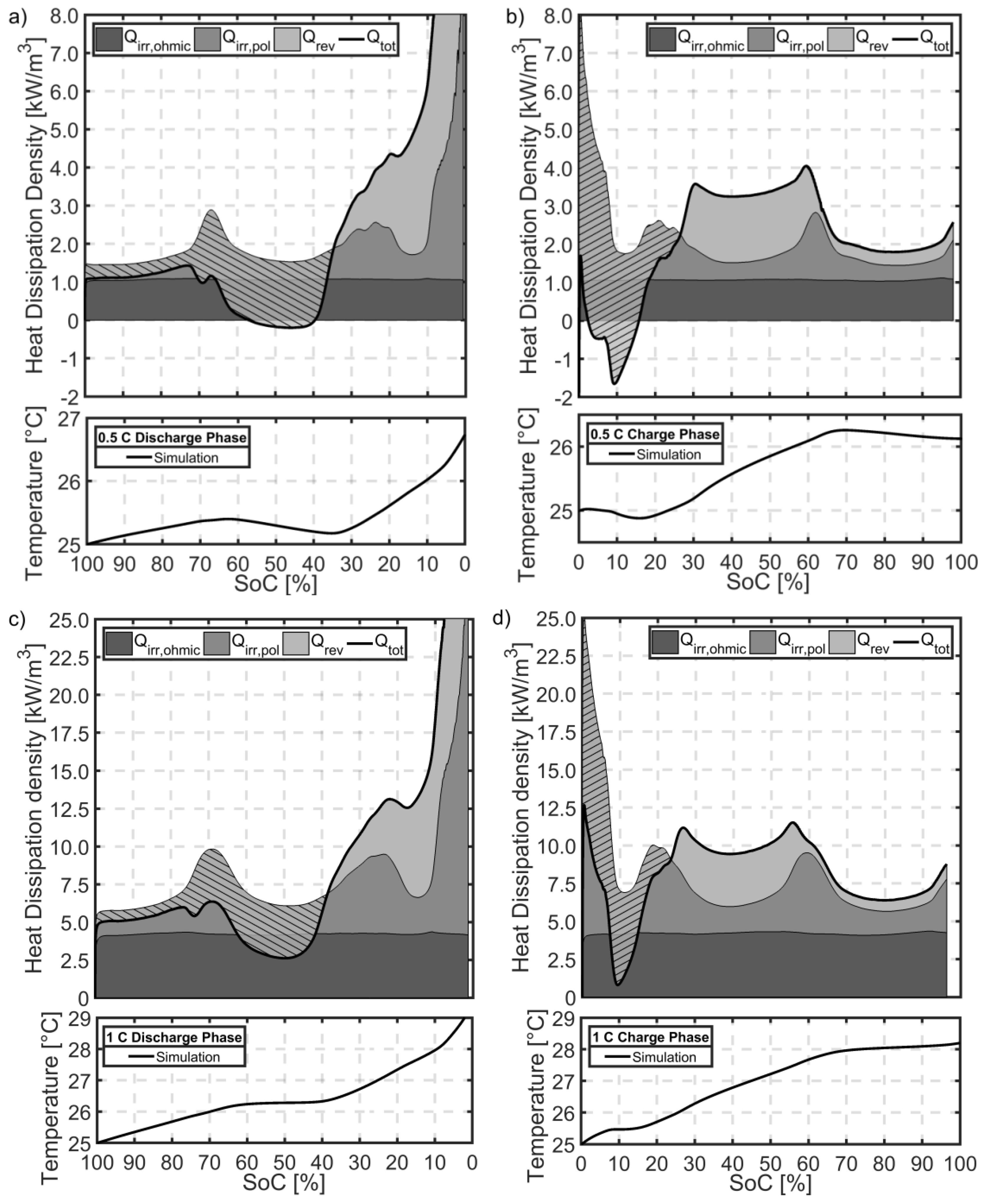
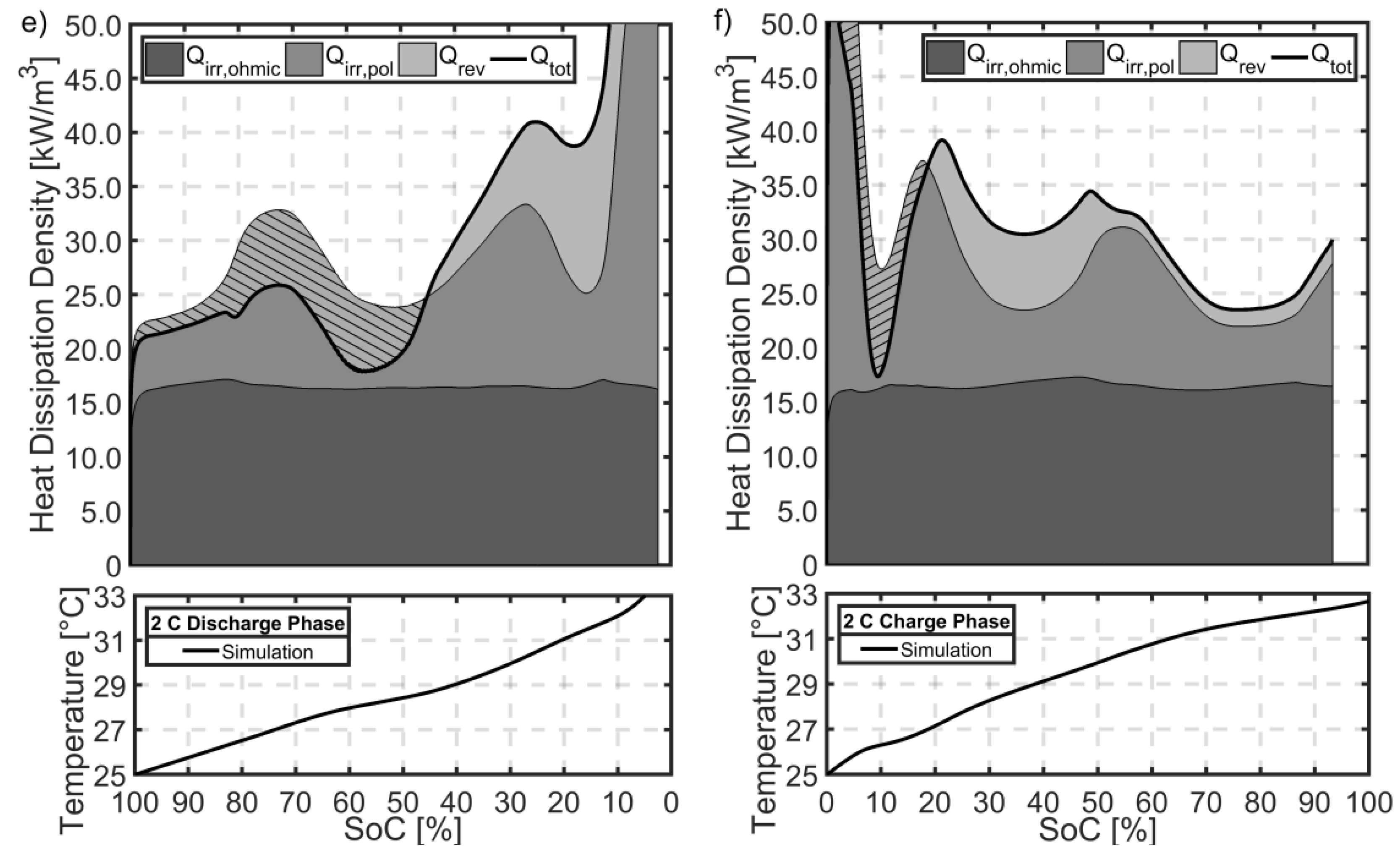
5.8. The Effect of Heat Dissipation on Thermal Behaviour
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
OCV Profiles
| 0.0100 | 0.8391 | 0.4150 | 4.2754 |
| 0.0178 | 0.6452 | 0.4204 | 4.2626 |
| 0.0255 | 0.5368 | 0.4258 | 4.2497 |
| 0.0333 | 0.4587 | 0.4312 | 4.2369 |
| 0.0410 | 0.3983 | 0.4366 | 4.2242 |
| 0.0488 | 0.3493 | 0.4420 | 4.2116 |
| 0.0565 | 0.3082 | 0.4474 | 4.1993 |
| 0.0643 | 0.2736 | 0.4528 | 4.1873 |
| 0.0720 | 0.2447 | 0.4582 | 4.1754 |
| 0.0798 | 0.2271 | 0.4636 | 4.1636 |
| 0.0875 | 0.2226 | 0.4690 | 4.1519 |
| 0.0953 | 0.2211 | 0.4744 | 4.1404 |
| 0.1030 | 0.2200 | 0.4798 | 4.1290 |
| 0.1108 | 0.2190 | 0.4852 | 4.1177 |
| 0.1185 | 0.2178 | 0.4906 | 4.1066 |
| 0.1263 | 0.2163 | 0.4960 | 4.0956 |
| 0.1340 | 0.2142 | 0.5014 | 4.0847 |
| 0.1418 | 0.2114 | 0.5068 | 4.0739 |
| 0.1495 | 0.2070 | 0.5122 | 4.0633 |
| 0.1573 | 0.2007 | 0.5176 | 4.0527 |
| 0.1650 | 0.1941 | 0.5230 | 4.0423 |
| 0.1728 | 0.1884 | 0.5284 | 4.0320 |
| 0.1805 | 0.1821 | 0.5338 | 4.0219 |
| 0.1883 | 0.1754 | 0.5392 | 4.0119 |
| 0.1960 | 0.1699 | 0.5446 | 4.0022 |
| 0.2038 | 0.1658 | 0.5500 | 3.9926 |
| 0.2115 | 0.1617 | 0.5554 | 3.9832 |
| 0.2193 | 0.1574 | 0.5608 | 3.9738 |
| 0.2270 | 0.1528 | 0.5662 | 3.9646 |
| 0.2348 | 0.1500 | 0.5716 | 3.9555 |
| 0.2425 | 0.1480 | 0.5770 | 3.9465 |
| 0.2503 | 0.1459 | 0.5824 | 3.9378 |
| 0.2580 | 0.1440 | 0.5878 | 3.9294 |
| 0.2658 | 0.1426 | 0.5932 | 3.9212 |
| 0.2735 | 0.1413 | 0.5986 | 3.9133 |
| 0.2813 | 0.1399 | 0.6040 | 3.9057 |
| 0.2890 | 0.1391 | 0.6094 | 3.8982 |
| 0.2968 | 0.1383 | 0.6148 | 3.8910 |
| 0.3045 | 0.1378 | 0.6202 | 3.8839 |
| 0.3123 | 0.1374 | 0.6256 | 3.8770 |
| 0.3200 | 0.1369 | 0.6310 | 3.8703 |
| 0.3278 | 0.1367 | 0.6364 | 3.8639 |
| 0.3355 | 0.1363 | 0.6418 | 3.8577 |
| 0.3433 | 0.1358 | 0.6472 | 3.8519 |
| 0.3510 | 0.1354 | 0.6526 | 3.8464 |
| 0.3588 | 0.1350 | 0.6580 | 3.8410 |
| 0.3665 | 0.1347 | 0.6634 | 3.8359 |
| 0.3743 | 0.1343 | 0.6688 | 3.8309 |
| 0.3820 | 0.1339 | 0.6742 | 3.8261 |
| 0.3898 | 0.1333 | 0.6796 | 3.8215 |
| 0.3975 | 0.1329 | 0.6850 | 3.8171 |
| 0.4053 | 0.1323 | 0.6904 | 3.8128 |
| 0.4130 | 0.1316 | 0.6958 | 3.8088 |
| 0.4208 | 0.1310 | 0.7012 | 3.8048 |
| 0.4285 | 0.1301 | 0.7066 | 3.8011 |
| 0.4363 | 0.1293 | 0.7120 | 3.7975 |
| 0.4440 | 0.1282 | 0.7174 | 3.7941 |
| 0.4518 | 0.1269 | 0.7228 | 3.7907 |
| 0.4595 | 0.1254 | 0.7282 | 3.7875 |
| 0.4673 | 0.1242 | 0.7336 | 3.7844 |
| 0.4750 | 0.1225 | 0.7390 | 3.7813 |
| 0.4828 | 0.1205 | 0.7444 | 3.7783 |
| 0.4905 | 0.1180 | 0.7498 | 3.7753 |
| 0.4983 | 0.1141 | 0.7552 | 3.7724 |
| 0.5060 | 0.1092 | 0.7606 | 3.7696 |
| 0.5138 | 0.1037 | 0.7660 | 3.7669 |
| 0.5215 | 0.0989 | 0.7714 | 3.7642 |
| 0.5293 | 0.0954 | 0.7768 | 3.7616 |
| 0.5370 | 0.0931 | 0.7822 | 3.7589 |
| 0.5448 | 0.0915 | 0.7876 | 3.7563 |
| 0.5525 | 0.0905 | 0.7930 | 3.7537 |
| 0.5603 | 0.0894 | 0.7984 | 3.7510 |
| 0.5680 | 0.0886 | 0.8038 | 3.7483 |
| 0.5758 | 0.0879 | 0.8092 | 3.7456 |
| 0.5835 | 0.0873 | 0.8146 | 3.7429 |
| 0.5913 | 0.0864 | 0.8200 | 3.7402 |
| 0.5990 | 0.0857 | 0.8254 | 3.7375 |
| 0.6068 | 0.0851 | 0.8308 | 3.7347 |
| 0.6145 | 0.0848 | 0.8362 | 3.7319 |
| 0.6223 | 0.0846 | 0.8416 | 3.7291 |
| 0.6300 | 0.0844 | 0.8470 | 3.7262 |
| 0.6378 | 0.0843 | 0.8524 | 3.7232 |
| 0.6455 | 0.0843 | 0.8578 | 3.7201 |
| 0.6533 | 0.0840 | 0.8632 | 3.7171 |
| 0.6610 | 0.0841 | 0.8686 | 3.7140 |
| 0.6688 | 0.0840 | 0.8740 | 3.7109 |
| 0.6765 | 0.0838 | 0.8794 | 3.7076 |
| 0.6843 | 0.0840 | 0.8848 | 3.7043 |
| 0.6920 | 0.0838 | 0.8902 | 3.7008 |
| 0.6998 | 0.0838 | 0.8956 | 3.6971 |
| 0.7075 | 0.0837 | 0.9010 | 3.6933 |
| 0.7153 | 0.0838 | 0.9064 | 3.6893 |
| 0.7230 | 0.0836 | 0.9118 | 3.6852 |
| 0.7308 | 0.0835 | 0.9172 | 3.6810 |
| 0.7385 | 0.0834 | 0.9226 | 3.6765 |
| 0.7463 | 0.0833 | 0.9280 | 3.6718 |
| 0.7540 | 0.0835 | 0.9334 | 3.6669 |
| 0.7618 | 0.0834 | 0.9388 | 3.6616 |
| 0.7695 | 0.0832 | 0.9442 | 3.6560 |
| 0.7773 | 0.0829 | 0.9496 | 3.6500 |
| 0.7850 | 0.0808 | 0.9550 | 3.6434 |
References
- Bohn, P.; Liebig, G.; Komsiyska, L.; Wittstock, G. Temperature propagation in prismatic lithium-ion-cells after short term thermal stress. J. Power Sources 2016, 313, 30–36. [Google Scholar] [CrossRef]
- Mei, W.; Chen, H.; Sun, J.; Wang, Q. The effect of electrode design parameters on battery performance and optimization of electrode thickness based on the electrochemical–thermal coupling model. Sustain. Energy Fuels 2019, 3, 148–165. [Google Scholar] [CrossRef]
- Bucci, G.; Swamy, T.; Chiang, Y.-M.; Carter, W.C. Modeling of internal mechanical failure of all-solid-state batteries during electrochemical cycling, and implications for battery design. J. Mater. Chem. A 2017, 5, 19422–19430. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Peabody, C.; Arnold, C.B. The role of mechanically induced separator creep in lithium-ion battery capacity fade. J. Power Sources 2011, 196, 8147–8153. [Google Scholar] [CrossRef]
- Shim, J.; Kostecki, R.; Richardson, T.; Song, X.; Striebel, K.A. Electrochemical analysis for cycle performance and capacity fading of a lithium-ion battery cycled at elevated temperature. J. Power Sources 2002, 112, 222–230. [Google Scholar] [CrossRef]
- Von Srbik, M.-T.; Marinescu, M.; Martinez-Botas, R.F.; Offer, G.J. A physically meaningful equivalent circuit network model of a lithium-ion battery accounting for local electrochemical and thermal behaviour, variable double layer capacitance and degradation. J. Power Sources 2016, 325, 171–184. [Google Scholar] [CrossRef]
- Bizeray, A.M.; Zhao, S.; Duncan, S.R.; Howey, D.A. Lithium-ion battery thermal-electrochemical model-based state estimation using orthogonal collocation and a modified extended Kalman filter. J. Power Sources 2015, 296, 400–412. [Google Scholar] [CrossRef]
- Safari, M.; Morcrette, M.; Teyssot, A.; Delacourt, C. Life-Prediction Methods for Lithium-Ion Batteries Derived from a Fatigue Approach. J. Electrochem. Soc. 2010, 157, A713. [Google Scholar] [CrossRef]
- Behrou, R.; Maute, K. Numerical Modeling of Damage Evolution Phenomenon in Solid-State Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A2573–A2589. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L. Optimal Design of Li-Ion Batteries through Multi-Physics Modeling and Multi-Objective Optimization. J. Electrochem. Soc. 2017, 164, E3254–E3264. [Google Scholar] [CrossRef]
- Jokar, A.; Rajabloo, B.; Désilets, M.; Lacroix, M. Review of simplified Pseudo-two-Dimensional models of lithium-ion batteries. J. Power Sources 2016, 327, 44–55. [Google Scholar] [CrossRef]
- Cai, L.; White, R.E. Mathematical modeling of a lithium ion battery with thermal effects in COMSOL Inc. Multiphysics (MP) software. J. Power Sources 2011, 196, 5985–5989. [Google Scholar] [CrossRef]
- Arora, P.; Doyle, M.; Gozdz, A.S.; White, R.E.; Newman, J. Comparison between computer simulations and experimental data for high-rate discharges of plastic lithium-ion batteries. J. Power Sources 2000, 88, 219–231. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Wang, C.-Y. Li-Ion Cell Operation at Low Temperatures. J. Electrochem. Soc. 2013, 160, A636–A649. [Google Scholar] [CrossRef]
- Newman, J.; Tiedemann, W. Porous-electrode theory with battery applications. AIChE J. 1975, 21, 25–41. [Google Scholar] [CrossRef]
- Guo, M.; Sikha, G.; White, R.E. Single-Particle Model for a Lithium-Ion Cell: Thermal Behavior. J. Electrochem. Soc. 2011, 158, A122. [Google Scholar] [CrossRef]
- Kim, G.-H.; Smith, K.; Lee, K.-J.; Santhanagopalan, S.; Pesaran, A. Multi-Domain Modeling of Lithium-Ion Batteries Encompassing Multi-Physics in Varied Length Scales. J. Electrochem. Soc. 2011, 158, A955. [Google Scholar] [CrossRef]
- Meyer, M.; Komsiyska, L.; Lenz, B.; Agert, C. Study of the local SOC distribution in a lithium-ion battery by physical and electrochemical modeling and simulation. Appl. Math. Model. 2013, 37, 2016–2027. [Google Scholar] [CrossRef]
- Lundgren, H.; Svens, P.; Ekström, H.; Tengstedt, C.; Lindström, J.; Behm, M.; Lindbergh, G. Thermal Management of Large-Format Prismatic Lithium-Ion Battery in PHEV Application. J. Electrochem. Soc. 2015, 163, A309–A317. [Google Scholar] [CrossRef]
- Reimers, J.N.; Shoesmith, M.; Lin, Y.S.; Valoen, L.O. Simulating High Current Discharges of Power Optimized Li-Ion Cells. J. Electrochem. Soc. 2013, 160, A1870–A1884. [Google Scholar] [CrossRef]
- Wang, C.Y.; Gu, W.B.; Liaw, B.Y. Micro-Macroscopic Coupled Modeling of Batteries and Fuel Cells: I. Model Development. J. Electrochem. Soc. 1998, 145, 3407–3417. [Google Scholar] [CrossRef]
- Ecker, M.; Tran, T.K.D.; Dechent, P.; Kabitz, S.; Warnecke, A.; Sauer, D.U. Parameterization of a Physico-Chemical Model of a Lithium-Ion Battery: I. Determination of Parameters. J. Electrochem. Soc. 2015, 162, A1836–A1848. [Google Scholar] [CrossRef]
- Ecker, M.; Kabitz, S.; Laresgoiti, I.; Sauer, D.U. Parameterization of a Physico-Chemical Model of a Lithium-Ion Battery: II. Model Validation. J. Electrochem. Soc. 2015, 162, A1849–A1857. [Google Scholar] [CrossRef]
- Schmalstieg, J. Physikalisch-elektrochemische Simulation von Lithium-Ionen-Batterien: Implementierung, Parametrierung und Anwendung. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2017. [Google Scholar] [CrossRef]
- Reimers, J.N. Algorithmic Improvements and PDE Decoupling, for the Simulation of Porous Electrode Cells. J. Electrochem. Soc. 2013, 160, A811–A818. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Uddin, K.; Perera, S.; Widanage, W.; Somerville, L.; Marco, J. Characterising Lithium-Ion Battery Degradation through the Identification and Tracking of Electrochemical Battery Model Parameters. Batteries 2016, 2, 13. [Google Scholar] [CrossRef]
- Dao, T.-S.; Vyasarayani, C.P.; McPhee, J. Simplification and order reduction of lithium-ion battery model based on porous-electrode theory. J. Power Sources 2012, 198, 329–337. [Google Scholar] [CrossRef]
- Hadigol, M.; Maute, K.; Doostan, A. On uncertainty quantification of lithium-ion batteries: Application to an LiC 6/LiCoO 2 cell. J. Power Sources 2015, 300, 507–524. [Google Scholar] [CrossRef]
- Zhu, C.; Li, X.; Song, L.; Xiang, L. Development of a theoretically based thermal model for lithium ion battery pack. J. Power Sources 2013, 223, 155–164. [Google Scholar] [CrossRef]
- Thomas, K.E.; Newman, J. Heats of mixing and of entropy in porous insertion electrodes. J. Power Sources 2003, 119–121, 844–849. [Google Scholar] [CrossRef]
- Liu, G.; Ouyang, M.; Lu, L.; Li, J.; Han, X. Analysis of the heat generation of lithium-ion battery during charging and discharging considering different influencing factors. J. Therm. Anal. Calorim. 2014, 116, 1001–1010. [Google Scholar] [CrossRef]
- Schuster, E.; Ziebert, C.; Melcher, A.; Rohde, M.; Seifert, H.J. Thermal behavior and electrochemical heat generation in a commercial 40 Ah lithium ion pouch cell. J. Power Sources 2015, 286, 580–589. [Google Scholar] [CrossRef]
- Ecker, M. Lithium Plating in Lithium-Ion Batteries: An Experimental and Simulation Approach. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2016. [Google Scholar]
- Erhard, S. Mehrdimensionale Elektrochemisch-Thermische Modellierung von Lithium-Ionen-Batterien. Ph.D. Thesis, Technische Universität München, München, Germany, 2017. [Google Scholar]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Williard, N.; Sood, B.; Osterman, M.; Pecht, M. Disassembly methodology for conducting failure analysis on lithium–ion batteries. J. Mater. Sci. Mater. Electron. 2011, 22, 1616–1630. [Google Scholar] [CrossRef]
- Höhne, G.W.H.; Hemminger, W.F.; Flammersheim, H.-J. Theoretical Fundamentals of Differential Scanning Calorimeters. In Differential Scanning Calorimetry; Springer: Berlin/Heidelberg, Germany, 2003; pp. 31–63. [Google Scholar] [CrossRef]
- Piłatowicz, G.; Marongiu, A.; Drillkens, J.; Sinhuber, P.; Sauer, D.U. A critical overview of definitions and determination techniques of the internal resistance using lithium-ion, lead-acid, nickel metal-hydride batteries and electrochemical double-layer capacitors as examples. J. Power Sources 2015, 296, 365–376. [Google Scholar] [CrossRef]
- Jalkanen, K.; Aho, T.; Vuorilehto, K. Entropy change effects on the thermal behavior of a LiFePO 4/graphite lithium-ion cell at different states of charge. J. Power Sources 2013, 243, 354–360. [Google Scholar] [CrossRef]
- Dai, H.; Jiang, B.; Wei, X. Impedance Characterization and Modeling of Lithium-Ion Batteries Considering the Internal Temperature Gradient. Energies 2018. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Uddin, K.; Chouchelamane, G.H.; Widanage, W.D.; Marco, J. A Comparison between Electrochemical Impedance Spectroscopy and Incremental Capacity-Differential Voltage as Li-ion Diagnostic Techniques to Identify and Quantify the Effects of Degradation Modes within Battery Management Systems. J. Power Sources 2017, 360, 301–318. [Google Scholar] [CrossRef]
- Tröltzsch, U.; Kanoun, O.; Tränkler, H.-R. Characterizing aging effects of lithium ion batteries by impedance spectroscopy. Electrochim. Acta 2006, 51, 1664–1672. [Google Scholar] [CrossRef]
- Maryam Ghalkhania, M.M. Modeling the Impedance Characterization of Prismatic Lithium Ion Batteries. Procedia Manuf. 2019, 32, 762–767. [Google Scholar] [CrossRef]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental investigation of the lithium-ion battery impedance characteristic at various conditions and aging states and its influence on the application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
- COMSOL Multiphysics®. Electrochemical Impedance Spectroscopy. Available online: https://www.comsol.de/model/download/538831/models.edecm.impedance_spectroscopy.pdf (accessed on 16 August 2019).
- Schweiger, H.G.; Obeidi, O.; Komesker, O.; Raschke, A.; Schiemann, M.; Zehner, C.; Gehnen, M.; Keller, M.; Birke, P. Comparison of several methods for determining the internal resistance of lithium ion cells. Sensors 2010, 10, 5604–5625. [Google Scholar] [CrossRef]
- Ye, Y.; Shi, Y.; Cai, N.; Lee, J.; He, X. Electro-thermal modeling and experimental validation for lithium ion battery. J. Power Sources 2012, 199, 227–238. [Google Scholar] [CrossRef]
- Nyman, A.; Behm, M.; Lindbergh, G. Electrochemical characterisation and modelling of the mass transport phenomena in LiPF6–EC–EMC electrolyte. Electrochim. Acta 2008, 53, 6356–6365. [Google Scholar] [CrossRef]
- Zavalis, T.G.; Behm, M.; Lindbergh, G. Investigation of Short-Circuit Scenarios in a Lithium-Ion Battery Cell. J. Electrochem. Soc. 2012, 159, A848–A859. [Google Scholar] [CrossRef]
- Kim, S.U.; Albertus, P.; Cook, D.; Monroe, C.W.; Christensen, J. Thermoelectrochemical simulations of performance and abuse in 50-Ah automotive cells. J. Power Sources 2014, 268, 625–633. [Google Scholar] [CrossRef]
- Optris. Basic Principles of Non-Contact Temperature Measurement. Available online: https://www.optris.de/lexikon?file=tl_files/pdf/Downloads/Zubehoer/IR-Grundlagen.pdf (accessed on 3 July 2019).
- Gyenes, B.; Stevens, D.A.; Chevrier, V.L.; Dahn, J.R. Understanding Anomalous Behavior in Coulombic Efficiency Measurements on Li-Ion Batteries. J. Electrochem. Soc. 2014, 162, A278–A283. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novák, P. Insertion Electrode Materials for Rechargeable Lithium Batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Dahn, J.R. Phase diagram ofLixC6. Phys. Rev. B 1991, 44, 9170–9177. [Google Scholar] [CrossRef]
- Dahn, H.M.; Smith, A.J.; Burns, J.C.; Stevens, D.A.; Dahn, J.R. User-Friendly Differential Voltage Analysis Freeware for the Analysis of Degradation Mechanisms in Li-Ion Batteries. J. Electrochem. Soc. 2012, 159, A1405–A1409. [Google Scholar] [CrossRef]
- Alavi, S.M.M.; Birkl, C.R.; Howey, D.A. Time-domain fitting of battery electrochemical impedance models. J. Power Sources 2015, 288, 345–352. [Google Scholar] [CrossRef]
- Momma, T.; Matsunaga, M.; Mukoyama, D.; Osaka, T. Ac impedance analysis of lithium ion battery under temperature control. J. Power Sources 2012, 216, 304–307. [Google Scholar] [CrossRef]
- Baker, D.R.; Li, C.; Verbrugge, M.W. Similarities and Differences between Potential-Step and Impedance Methods for Determining Diffusion Coefficients of Lithium in Active Electrode Materials. J. Electrochem. Soc. 2013, 160, A1794–A1805. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamonds and Fullerenes-Processing, Properties and Applications; Noyes Publications: Park Ridge, NJ, USA, 1993. [Google Scholar]
- Villars, P.; Cenzual, K. Li[Ni1/3Co1/3Mn1/3]O2 Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition—2012”. SpringerMaterials. 2016. Available online: https://materials.springer.com/isp/crystallographic/docs/sd_1125611 (accessed on 5 September 2019).
- Viswanathan, V.V.; Choi, D.; Wang, D.; Xu, W.; Towne, S.; Williford, R.E.; Zhang, J.-G.; Liu, J.; Yang, Z. Effect of entropy change of lithium intercalation in cathodes and anodes on Li-ion battery thermal management. J. Power Sources 2010, 195, 3720–3729. [Google Scholar] [CrossRef]
- Ye, B.; Rubel, M.; Li, H. Design and Optimization of Cooling Plate for Battery Module of an Electric Vehicle. Appl. Sci. 2019, 9, 754. [Google Scholar] [CrossRef]




| Domain/Meaning | Governing Equation | Boundary Condition |
|---|---|---|
| Solid Phase/Electrodes | ||
| Mass Conservation | ||
| Charge Conservation | ||
| Liquid-Phase/Electrolyte | ||
| Mass Conservation | ||
| Charge Conservation | ||
| Reaction Kinetics | ||
| Reaction Rate Pore Wall Flux | ||
| Over-Potential | ||
| Battery | ||
| Terminal Voltage | ||
| Domain/Meaning | Equation |
|---|---|
| Electrode | |
| Reversible Heat | |
| Irreversible Polarization Heat | |
| Irreversible Ohmic Heat | |
| Separator | |
| Irreversible Ohmic Heat | |
| Terminal/Current Collector | |
| Irreversible Ohmic Heat | |
| Battery | |
| Total Heat Dissipation | |
| Parameter | Symbol | Unit | Anode | Separator | Cathode |
|---|---|---|---|---|---|
| Number of electrode sheets | # | (pc.) | 91 | 90 | |
| Total thickness of one sheet *1 | (µm) | 110.0 | 24.0 | 129.0 | |
| Height for one electrode sheet | (cm) | 8.0 | 8.0 | 8.0 | |
| Width for one electrode sheet | (cm) | 14.6 | 14.6 | ||
| Electrode surface area | (cm2) | 21,258 | 21,024 *2 | ||
| Thickness of current collector *2 | (µm) | 19.01 ± 0.12 *3 | 17.78 ± 1.20 *3 | ||
| Coating thickness | (µm) | 47.5 | 24.7 | 54.5 | |
| Particle Size | (µm) | 9.89 ± 3.31 *3 | 1.72 ± 0.54 *4 | ||
| Porosity | (%) | 30.79 *3 | 19.73 *4 |
| Material | Density ρ (kg·m−3) | Heat Capacity Cp (J·kg−1K−1) | Volume Fraction (%) |
|---|---|---|---|
| Positive Electrode Coating | 4670 [25] | 940 | 29.29 |
| Negative Electrode Coating | 2260 [25] | 1040 | 25.53 |
| Aluminum [1] | 2700 | 900 | 4.03 *1 |
| 0.26 *2 | |||
| Copper [1] | 8700 | 385 | 5.37 *1 |
| 0.26 *2 | |||
| Separator | 1009 | 1907 | 12.9 |
| Steel [52] | 8030 | 502.48 | 13 |
| Synthetic (Acrylic Plastic) [20] | 1190 | 1470 | 9.36 |
| Material | Emissivity ϵ | Surface Area (cm2) |
|---|---|---|
| Isolating Tape | 0.97 | 399.20 |
| Steel | 0.74 | 11.11 |
| Synthetic (Acrylic Plastic) | 0.95 | 0.695 |
| Definition | Symbol | Unit | Negative Electrode | Separator | Positive Electrode | Reference |
|---|---|---|---|---|---|---|
| Design Specifications | ||||||
| Domain Thickness | L | (µm) | 47.5 | 24.7 | 54.5 | Table 3 |
| Electrode Plate Area | A | (m2) | 2.1024 | Table 3 | ||
| Particle Radius | (µm) | 9.89 | 1.72 | Table 3 | ||
| Actual Capacity | (Ah) | 48.17 | 69.20 | Equation (10) | ||
| Active Electrode Volume | (cm3) | 99.86 | 114.58 | |||
| Molar Mass | (g/mol) | 72.0 | 96.5 | [25] | ||
| Density | (kg/m3) | 2260 [61] | 4670 [62] | [25] | ||
| Theoretical Capacity | (Ah) | 84.01 | 148.61 | Equation (11) | ||
| Lower Electrode SoC | 0.01 | 0.415 | Fitted: OCV | |||
| Upper Electrode SoC | 0.785 | 0.955 | Fitted: OCV | |||
| Active Material Fraction | 0.548 | 0.457 | Equation (12) | |||
| Specific Surface Area | (m-1) | 172,730 | 825,880 | Equation (13) | ||
| Surface Area | (m2) | 17.25 | 94.63 | Equation (14) | ||
| Electrolyte Volume Fraction | 0.308 | 0.395 [25] | 0.191 | |||
| Inactive Volume Fraction | 0.189 | 0.45 | Equation (9) | |||
| Kinetic and transport properties | ||||||
| Open-Circuit Potential | U | (V) | Equation (6) | Equation (5) | Fitted OCV, [25] | |
| Temperature derivative of Open-Circuit Potential | (V/K) | Taken from [32] | [63] | [32,63] | ||
| Charge Transfer Symmetry Factor | 0.5 | 0.5 | [36] | |||
| Maximum Lithium Intercalation Concentration | (mol/m3) | 31,389 | 48396 | [25] | ||
| Equilibrium Electrolyte Concentration | (mol/m3) | 1000 | 1000 | 1000 | [25] | |
| Effective Electrode Diffusion Coefficient | (m2/s) | Equation (25)/(26) | Equation (25)/(27) | [25] | ||
| Reference Electrode Diffusion Coefficient | (m2/s) | Equation (26) | Equation (27) | [25] | ||
| Effective Electrode Electronic Conductivity | (s/m) | Equation (34) | Equation (34) | [25] | ||
| Reference Electrode Electronic Conductivity | (s/m) | 100 | 10 | [25] | ||
| Effective Electrolyte Conductivity | (s/m) | Equation (32) | [50] | |||
| Reference Electrolyte Conductivity | (s/m) | Equation (33) | [50] | |||
| Bruggeman Exponent | 1.5 | 1.5 | 1.5 | [3] | ||
| Effective Diffusional Electrolyte conductivity | (s/m) | Equation (35) | [52] | |||
| Effective Electrolyte Diffusion Coefficient | (m2/s) | Equation (28) | [50,51] | |||
| Reference Electrolyte Diffusion Coefficient | (m2/s) | Equation (29) | [50] | |||
| Li-transference Number | Equation (30) | [50] | ||||
| Effective Electrolyte Activity coefficient | Equation (31) | [50,51] | ||||
| Reaction Rate Coefficient | (m2.5/mol0.5s) | Fitted: EIS | ||||
| Double Layer Capacitance | (F/m2) | 5.18 | 0.96 | Fitted: EIS | ||
| Ohmic Resistance | (mΩ) | 1.24 | Fitted: EIS | |||
| Inductance | (H) | Fitted: EIS | ||||
| Exchange Current Density Activation Energy | (kJ/mol) | 48.9 | 78.1 | [25] | ||
| Electrode Diffusion Activation Energy | (kJ/mol) | 28.8 | 49.6 | [25] | ||
| Electrolyte Diffusion Activation Energy | (kJ/mol) | 16.5 | [51] | |||
| Electrolyte Conductivity Activation Energy | (kJ/mol) | 4.0 | [51] | |||
| Electrolyte Activity Coefficient Activation Energy | (kJ/mol) | −1.0 | [51] | |||
| Thermal parameters | ||||||
| Heat Capacity | (J/kgK) | 1055.1 | Equation (37) | |||
| Density | (kg/m3) | 3179.66 | Equation (36) | |||
| Battery Volume | (cm3) | 371.08 | Measured | |||
| Electrode Stack Volume | (cm3) | 286.16 | Measured | |||
| Convective Heat-Transfer Coefficient | (W/m2K) | 6.9 | [36] | |||
| Convective Cooling Surface Area | (cm2) | 415.99 | Measured | |||
| Area-specific Emissivity | (cm2) | 402.05 | Equation (38) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebig, G.; Gupta, G.; Kirstein, U.; Schuldt, F.; Agert, C. Parameterization and Validation of an Electrochemical Thermal Model of a Lithium-Ion Battery. Batteries 2019, 5, 62. https://doi.org/10.3390/batteries5030062
Liebig G, Gupta G, Kirstein U, Schuldt F, Agert C. Parameterization and Validation of an Electrochemical Thermal Model of a Lithium-Ion Battery. Batteries. 2019; 5(3):62. https://doi.org/10.3390/batteries5030062
Chicago/Turabian StyleLiebig, Gerd, Gaurav Gupta, Ulf Kirstein, Frank Schuldt, and Carsten Agert. 2019. "Parameterization and Validation of an Electrochemical Thermal Model of a Lithium-Ion Battery" Batteries 5, no. 3: 62. https://doi.org/10.3390/batteries5030062






