Enhancing the Cycle Life of a Zinc–Air Battery by Means of Electrolyte Additives and Zinc Surface Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Aqueous Alkaline Electrolyte Systems
2.2. Zinc Powder and Ionomer-Coated Zinc Particles (ICZP)
2.3. Electrodes Preparation
2.4. Electrochemical Characterization
3. Results and Discussion
3.1. Coating of Zn Powder with Nafion® as the Protective Ionomer Layer
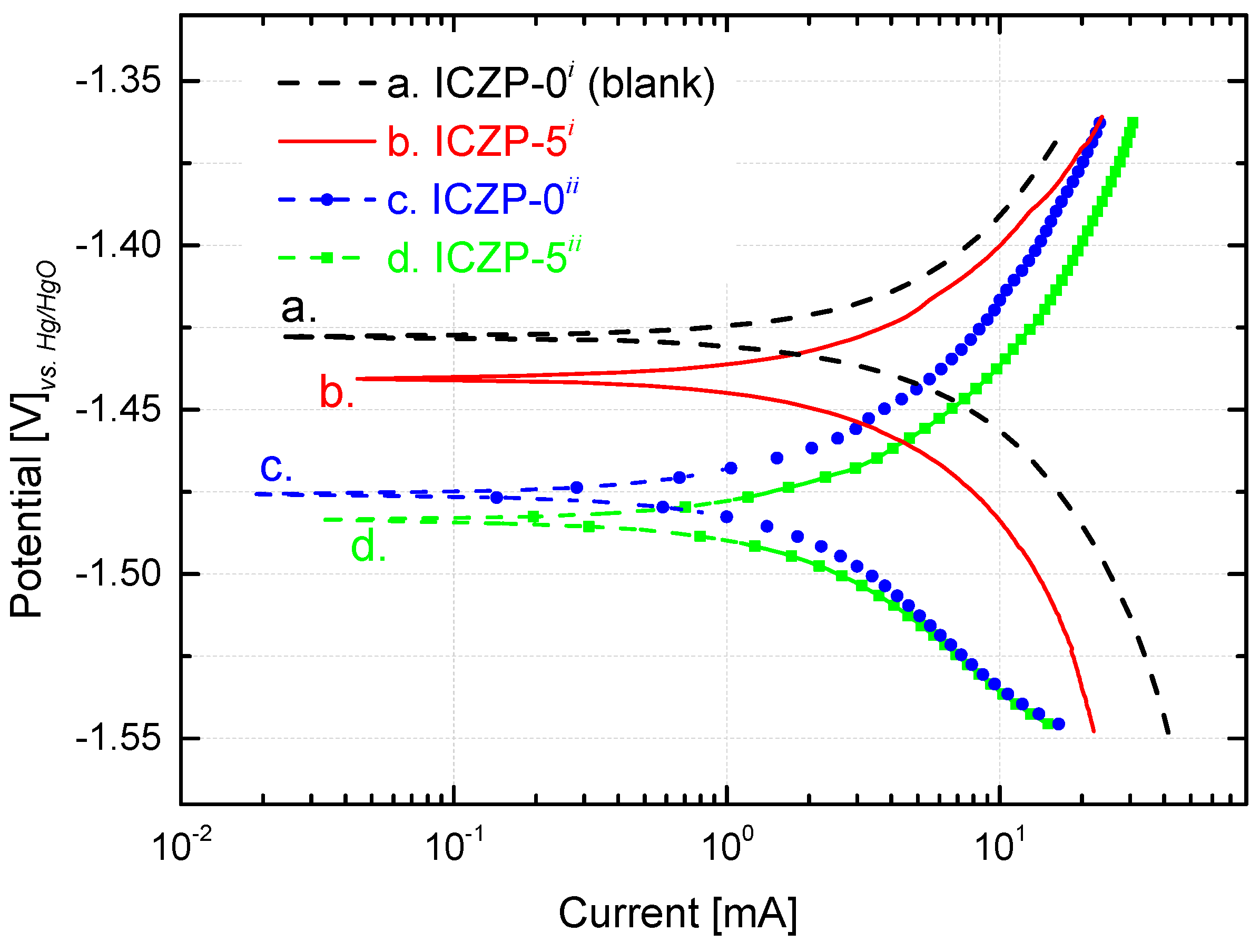
3.2. Polarization Behavior of Uncoated and Coated Zinc Particles
3.3. Cyclic Voltammetry and Reversibility of Zn Electrodes
3.3.1. Zn-Pellet Electrodes
3.3.2. Zn-Paste Electrodes
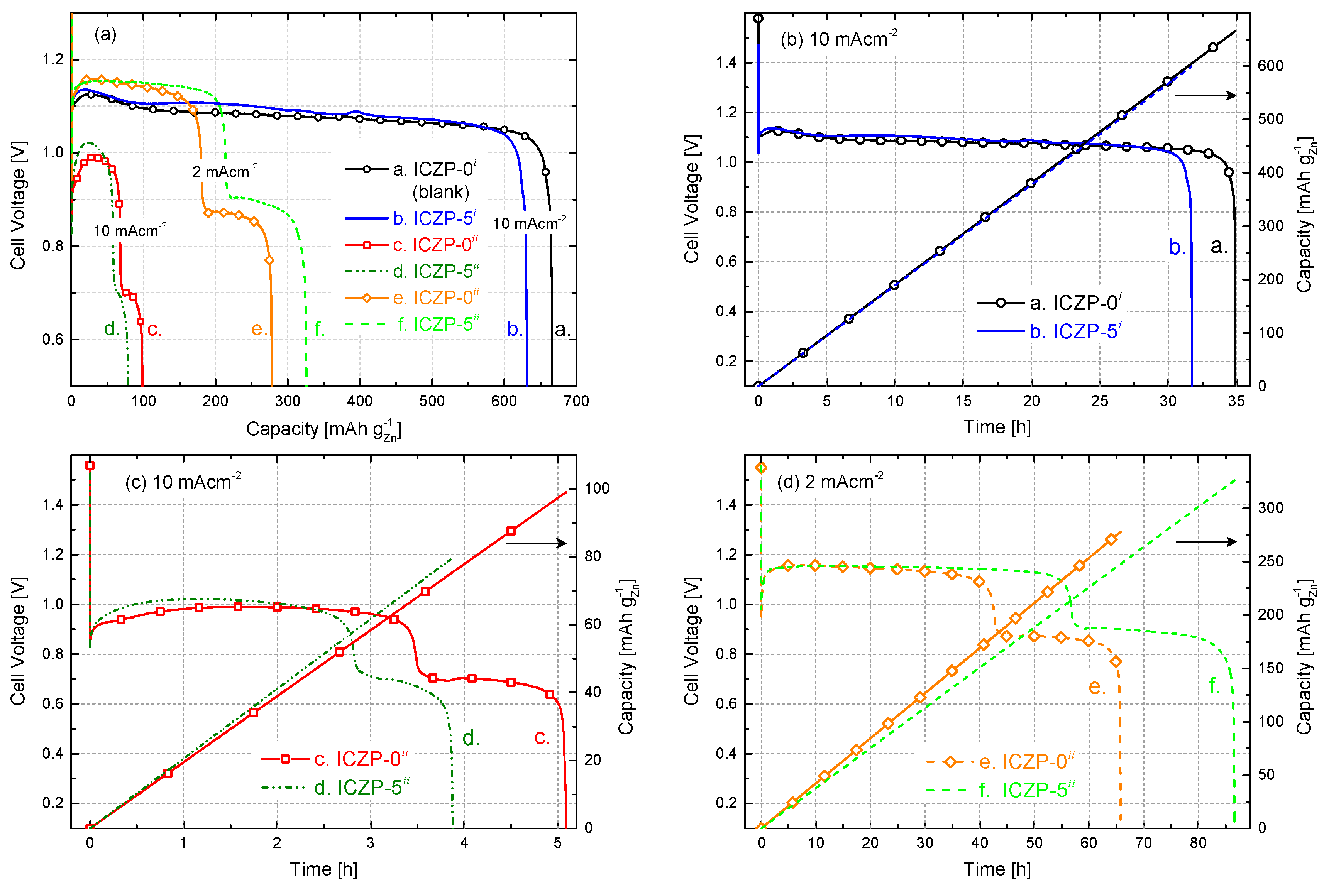
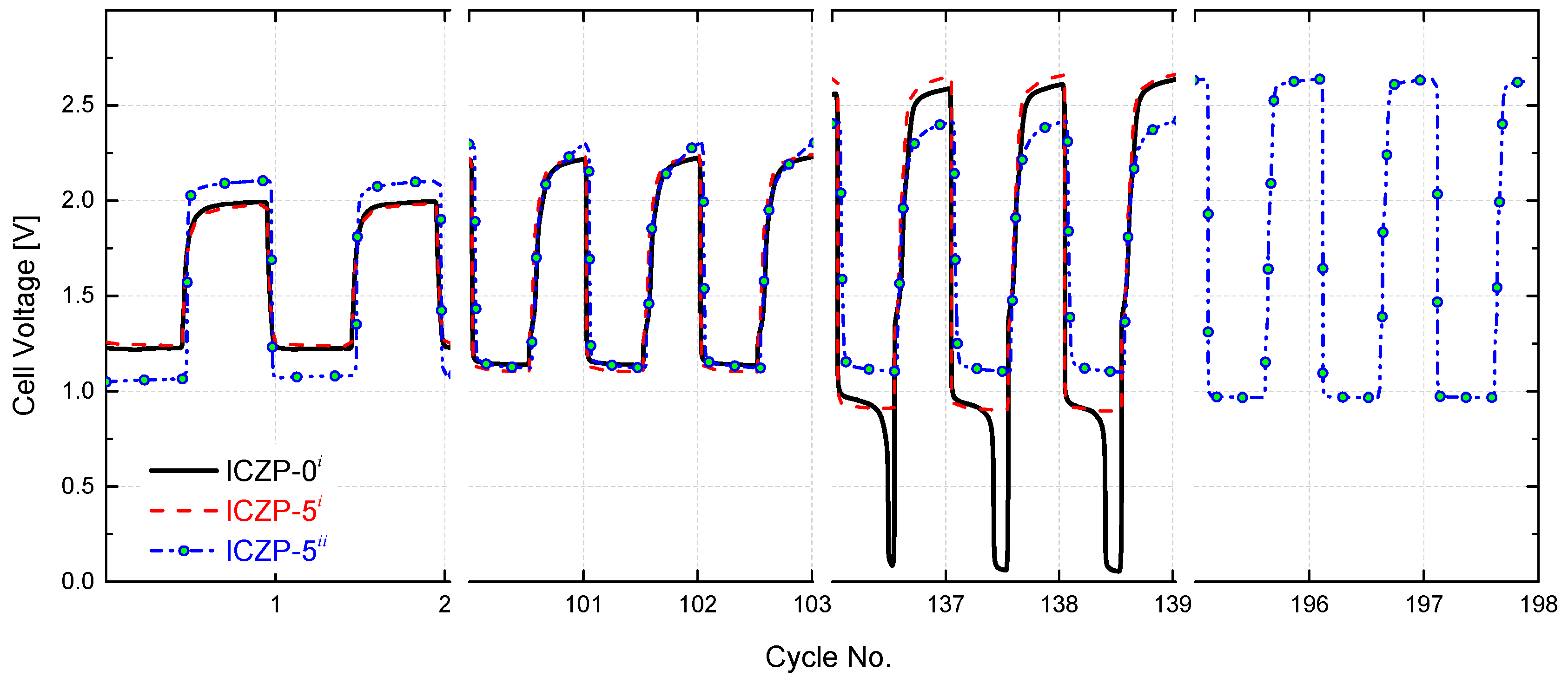
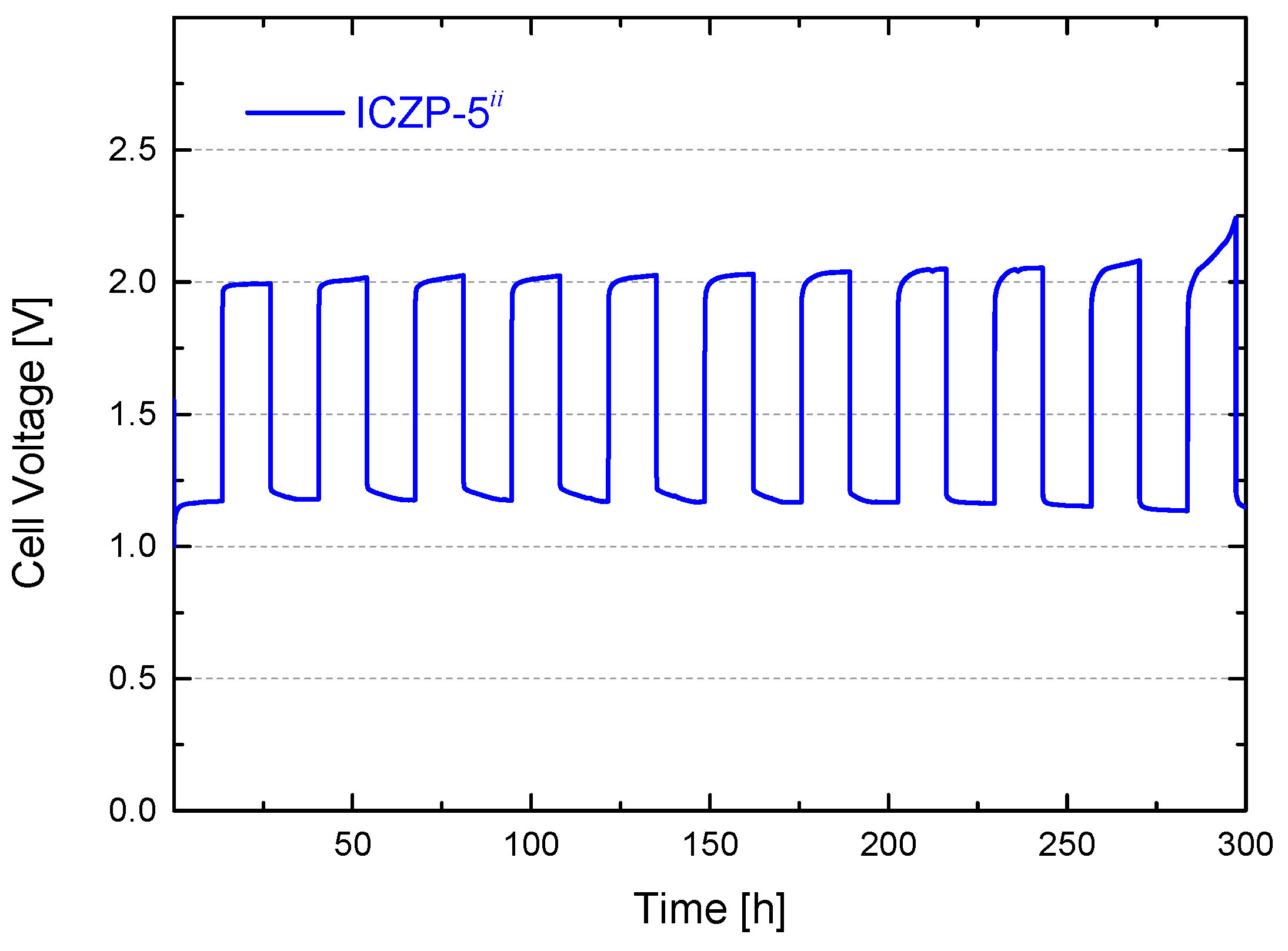
3.4. Full Cell Cycle Life Assessment under Closer Battery Operating Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc–air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, H.; Li, W.; Liu, H. New modification procedure of zinc powder in neodymium nitrate solution for improving the electrochemical properties of alkaline zinc electrodes. J. Phys. Chem. Solids 2009, 70, 45–54. [Google Scholar] [CrossRef]
- Vatsalarani, J.; Trivedi, D.C.; Ragavendran, K.; Warrier, P.C. Effect of Polyaniline Coating on “Shape Change” Phenomenon of Porous Zinc Electrode. J. Electrochem. Soc. 2005, 152, A1974–A1978. [Google Scholar] [CrossRef]
- Devyatkina, T.I.; Gunko, Y.L.; Mikhalenko, M.G. Development of Ways to Diminish Corrosion of Zinc Electrode. Russ. J. Appl. Chem. 2001, 74, 1122–1125. [Google Scholar] [CrossRef]
- Banik, S.J.; Akolkar, R. Suppressing Dendritic Growth during Alkaline Zinc Electrodeposition using Polyethylenimine Additive. Electrochim. Acta 2014, 179, 475–781. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, H.; Ai, X.; Zhu, X. A study of calcium zincate as negative electrode materials for secondary batteries. J. Power Sources 2001, 103, 93–97. [Google Scholar] [CrossRef]
- Jo, Y.N.; Kang, S.H.; Prasanna, K.; Eom, S.W.; Lee, C.W. Shield effect of polyaniline between zinc active material and aqueous electrolyte in zinc-air batteries. Appl. Surf. Sci. 2017, 422, 406–412. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Blázquez, J.A.; Grande, H.-J. Systematic cycle life assessment of a secondary zinc–air battery as a function of the alkaline electrolyte composition. Energy Sci. Eng. 2018, 6, 174–186. [Google Scholar] [CrossRef]
- Mainar, A.R.; Colmenares, L.C.; Blázquez, J.A.; Urdampilleta, I. A brief overview of secondary zinc anode development: The key of improving zinc-based energy storage systems. Int. J. Energy Res. 2017, 42, 903–918. [Google Scholar] [CrossRef]
- Mainar, A.R.; Leonet, O.; Bengoechea, M.; Boyano, I.; de Meatza, I.; Kvasha, A.; Guerfi, A.; Blázquez, J.A. Alkaline aqueous electrolytes for secondary zinc–air batteries: An overview. Int. J. Energy Res. 2016, 40, 1032–1049. [Google Scholar] [CrossRef]
- Bass, K.; Wicox, P.J.; Smith, J. Methods for the reduction of shape change and dendritic growth in zinc-based secondary cells. J. Power Sources 1991, 35, 333–351. [Google Scholar] [CrossRef]
- Adler, T.C.; McLarnon, F.R.; Cairns, E.J. Investigations of a New Family of Alkaline-Fluoride-Carbonate Electrolytes for Zinc/Nickel Oxide Cells. Ind. Eng. Chem. Res. 1998, 37, 3237–3241. [Google Scholar] [CrossRef]
- Sato, Y.; Niki, H.; Takamura, T. Effects of Carbonate on the Anodic Dissolution and the Passivation of Zinc Electrode in Concentrated Solution of Potassium Hydroxide. J. Electrochem. Soc. 1971, 118, 1269–1272. [Google Scholar] [CrossRef]
- Adler, T.C.; McLarnon, F.R.; Cairns, E.J. Low-Zinc-Solubility Electrolytes for Use in Zinc/Nickel Oxide Cells. J. Electrochem. Soc. 1993, 140, 289–294. [Google Scholar] [CrossRef]
- Parker, J.F.; Pala, I.R.; Chervin, C.N.; Long, J.W.; Rolison, D.R. Minimizing Shape Change at Zn Sponge Anodes in Rechargeable Ni–Zn Cells: Impact of Electrolyte Formulation. J. Electrochem. Soc. 2016, 163, A351–A355. [Google Scholar] [CrossRef]
- Thornton, R.F.; Carlson, E.J. Properties of Alternate Electrolytes for Secondary Zinc Batteries. J. Electrochem. Soc. 1980, 127, 1448–1452. [Google Scholar] [CrossRef]
- Jorné, J.; Adler, T.C.; Cairns, E.J. Visual Observations of Early Shape Changes in a Zinc/Nickel Oxide Cell. J. Electrochem. Soc. 1995, 142, 771–774. [Google Scholar] [CrossRef]
- Shivkumar, R.; Kalaignan, G.P.; Vasudevan, T. Effect of additives on zinc electrodes in alkaline battery systems. J. Power Sources 1995, 55, 53–62. [Google Scholar] [CrossRef]
- Hilder, M.; Winther-Jensen, B.; Clark, N.B. The effect of binder and electrolyte on the performance of thin zinc-air battery. Electrochim. Acta 2012, 69, 308–314. [Google Scholar] [CrossRef]
- Jain, R.; Adler, T.C.; McLarnon, F.R.; Cairns, E.J. Development of long-lived high-performance zinc-calcium/nickel oxide cells. J. Appl. Electrochem. 1992, 22, 1039–1048. [Google Scholar] [CrossRef]
- Luo, Z.; Sang, S.; Wu, Q.; Liu, S. A Conductive Additive for Zn Electrodes in Secondary Ni/Zn Batteries: The Magneli Phase Titanium Sub-Oxides Conductive Ceramic TinO2n−1. ECS Electrochem. Lett. 2013, 2, A21–A24. [Google Scholar] [CrossRef]
- Vatsalarani, J.; Geetha, S.; Trivedi, D.C.; Warrier, P.C. Stabilization of zinc electrodes with a conducting polymer. J. Power Sources 2006, 158, 1484–1489. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, Y. Effects of ionomer films on secondary alkaline zinc electrodes. J. Power Sources 1998, 73, 266–270. [Google Scholar] [CrossRef]
- Miyazaki, K.; Lee, Y.S.; Fukutsuka, T.; Abe, T. Suppression of Dendrite Formation of Zinc Electrodes by the Modification of Anion-Exchange Ionomer. Electrochemistry 2012, 80, 725–727. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, Q.; Liang, M.; Lv, D.; Xu, M.; Li, H.; Li, W. Investigation on synergism of composite additives for zinc corrosion inhibition in alkaline solution. Mater. Chem. Phys. 2011, 128, 214–219. [Google Scholar] [CrossRef]
- Zhu, J.-L.; Zhou, Y.-H.; Yang, H. Effects of lanthanum and neodymium hydroxides on secondary alkaline zinc electrode. J. Power Sources 1997, 69, 169–173. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Walther, F.; Sann, J.; Janek, J.; Schröder, D. Homogeneous Coating with an Anion-Exchange Ionomer Improves the Cycling Stability of Secondary Batteries with Zinc Anodes. ACS Appl. Mater. Interfaces 2018, 10, 8640–8648. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yang, Z.; Huang, J.; Xie, X.; Zhang, Z. The superior cycling performance of the hydrothermal synthesized carbon-coated ZnO as anode material for zinc/nickel secondary cells. J. Power Sources 2015, 276, 162–169. [Google Scholar] [CrossRef]
- Gan, W.; Zhou, D.; Zhao, J.; Zhou, L. Stable zinc anodes by in situ polymerization of conducting polymer to conformally coat zinc oxide particles. J. Appl. Electrochem. 2015, 45, 913–919. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z. Synthesis of ZnO/polypyrrole composites and an application in Zn/Ni rechargeable batteries. RSC Adv. 2014, 4, 19205–19209. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yi, C.-W.; Kim, K. Characteristics and Electrochemical Performance of the TiO2-Coated ZnO Anode for Ni-Zn Secondary Batteries. J. Phys. Chem. C 2011, 115, 2572–2577. [Google Scholar] [CrossRef]
- Bonnick, P.; Dahn, J.R. A Simple Coin Cell Design for Testing Rechargeable Zinc-Air or Alkaline Battery Systems. J. Electrochem. Soc. 2012, 159, A981–A989. [Google Scholar] [CrossRef]
- Hwang, B.; Oh, E.-S.; Kim, K. Observation of electrochemical reactions at Zn electrodes in Zn-air secondary batteries. Electrochim. Acta 2016, 216, 484–489. [Google Scholar] [CrossRef]
- Malone, E.; Berry, M.; Lipson, H. Freeform fabrication and characterization of Zn-air batteries. Rapid Prototyp. J. 2008, 14, 128–140. [Google Scholar] [CrossRef]
- Kainthla, R.; David, C.; Manko, J. Anodic Zinc Electrode for Use in an Alkaline Based Electrochemical Cell. U.S. Patent 2003/0113630 A1, 19 June 2003. [Google Scholar]
- Stamm, J.; Varzi, A.; Latz, A.; Horstmann, B. Modeling nucleation and growth of zinc oxide during discharge of primary zinc-air batteries. J. Power Sources 2017, 360, 136–149. [Google Scholar] [CrossRef]
- Mainar, A.R.; Colmenares, L.C.; Leonet, O.; Alcaide, F.; Iruin, J.J.; Weinberger, S.; Hacker, V.; Iruin, E.; Urdanpilleta, I.; Blazquez, J.A. Manganese oxide catalysts for secondary zinc air batteries: From electrocatalytic activity to bifunctional air electrode performance. Electrochim. Acta 2016, 217, 80–91. [Google Scholar] [CrossRef]
- Kim, H.-W.; Lim, J.-M.; Lee, H.-J.; Eom, S.-W.; Hong, Y.T.; Lee, S.-Y. Artificially engineered, bicontinuous anion-conducting/-repelling polymeric phases as a selective ion transport channel for rechargeable zinc–air battery separator membranes. J. Mater. Chem. A 2016, 4, 3711–3720. [Google Scholar] [CrossRef]
- Krejčí, I.; Vanýsek, P.; Trojánek, A. Transport of Zn(OH)42− ions through modified microporous polypropylene membranes. J. Electrochem. Soc. 1993, 140, 2279–2283. [Google Scholar] [CrossRef]
- Zawodzinski, T.A., Jr.; Derouin, C.; Radzinski, S.; Sherman, R.J.; Smith, V.T.; Springer, T.E.; Gottesfeld, S. Water Uptake by and Transport Through Nafion® 117 Membranes. J. Electrochem. Soc. 1993, 140, 1041–1047. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.; Jin, W.; Jiang, Q.; Sun, L.; Jiang, L.; Sun, G. KOH modified Nafion112 membrane for high performance alkaline direct ethanol fuel cell. Int. J. Hydrogen Energy 2011, 36, 5104–5109. [Google Scholar] [CrossRef]
- Majsztrik, P.; Bocarsly, A.; Benziger, J. Water permeation through nafion membranes: The role of water activity. J. Phys. Chem. B 2008, 112, 16280–16289. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Maeda, T.; Nakano, A.; Takenaka, H. Properties of Nafion membranes under PEM water electrolysis conditions. Int. J. Hydrogen Energy 2011, 36, 10527–10540. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, H.; Benziger, J. Transport of liquid water through Nafion membranes. J. Memb. Sci. 2012, 392–393, 88–94. [Google Scholar] [CrossRef]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Spring Street: New York, NY, USA, 1996; ISBN 0306453347. [Google Scholar]
- Ko, Y.; Park, S.-M. Zinc Oxidation in Dilute Alkaline Solutions Studied by Real-Time Electrochemical Impedance Spectroscopy. J. Phys. Chem. C 2012, 116, 7260–7268. [Google Scholar] [CrossRef]
- Shivkumar, R.; Kalaignan, G.P.; Vasudevan, T. Studies with porous zinc electrodes with additives for secondary alkaline batteries. J. Power Sources 1998, 75, 90–100. [Google Scholar] [CrossRef]
- Renuka, R.; Srinivasan, L.; Ramamurthy, S.; Veluchamy, A.; Venkatakrishnan, N. Cyclic voltammetric study of zinc and zinc oxide electrodes in 5.3 M. KOH. J. Appl. Electrochem. 2001, 31, 655–661. [Google Scholar] [CrossRef]
- Mele, C.; Bozzini, B. Spectroelectrochemical investigation of the anodic and cathodic behaviour of zinc in 5.3 M. KOH. J. Appl. Electrochem. 2015, 45, 43–50. [Google Scholar] [CrossRef]
- Ghaemi, M.; Amrollahi, R.; Ataherian, F.; Kassaee, M.Z. New advances on bipolar rechargeable alkaline manganese dioxide–zinc batteries. J. Power Sources 2003, 117, 233–241. [Google Scholar] [CrossRef]
- Ghavami, R.K.; Rafiei, Z. Performance improvements of alkaline batteries by studying the effects of different kinds of surfactant and different derivatives of benzene on the electrochemical properties of electrolytic zinc. J. Power Sources 2006, 162, 893–899. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry. Principle, Methods and Applications; Oxford University Press Inc.: New York, NY, USA, 1993; pp. 176–185. ISBN 0198553889. [Google Scholar]
- Thomas, S.; Cole, I.S.; Sridhar, M.; Birbilis, N. Revisiting zinc passivation in alkaline solutions. Electrochim. Acta 2013, 97, 192–201. [Google Scholar] [CrossRef]
- Liu, M.B.; Cook, G.M.; Yao, N.P. Passivation of zinc anodes in KOH electrolytes. J. Electrochem. Soc. 1981, 128, 1663–1668. [Google Scholar] [CrossRef]
- Horn, Q.C.; Shao-Horn, Y. Morphology and Spatial Distribution of ZnO Formed in Discharged Alkaline Zn/MnO2 AA Cells. J. Electrochem. Soc. 2003, 150, A652–A658. [Google Scholar] [CrossRef]
- Schmid, M.; Willert-Porada, M. Electrochemical behavior of zinc particles with silica based coatings as anode material for zinc air batteries with improved discharge capacity. J. Power Sources 2017, 351, 115–122. [Google Scholar] [CrossRef]
- Bockelmann, M.; Reining, L.; Kunz, U.; Turek, T. Electrochemical characterization and mathematical modeling of zinc passivation in alkaline solutions: A review. Electrochim. Acta 2017, 237, 276–298. [Google Scholar] [CrossRef]




| Coating Material | Coating Method | Active Material | Ref. |
|---|---|---|---|
| Carbon | Hydrothermal synthesis | ZnO | [29] |
| Hydroxide coatings | Applying a negative current | Zinc foil | [27] |
| Ionomer solution | Spin coater | ZnO | [25] |
| Polyaniline | Applying thin layer | ZnO | [3] |
| Polypyrrole | In situ polymerization | ZnO | [30] |
| Polypyrrole | Ultrasound-assisted chemical polymerization | ZnO | [31] |
| Polypyrrole | Electropolymerization | Zinc foil | [23] |
| TiO2 | Sol-gel | ZnO | [32] |
| Sample | BET (m2 g−1) | Ecorr (VHg/HgO) | Icorr (mA) | CR * (mgZn cm−1·s−1) | Eff** (%) | ∆Ep (VHg/HgO) | |iC/iA| | ||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 20th | 1st | 20th | ||||||
| ICZP-0i | 0.199 | −1.427 | 3.087 | 10.4 | — | — | — | — | — |
| ICZP-5i | 0.052 | −1.440 | 2.231 | 2.0 | 81 | — | — | — | — |
| ICZP-0ii | 0.199 | −1.476 | 1.348 | 4.5 | 56 | 0.631 | 0.780 | 0.94 | 0.86 |
| ICZP-1ii | 0.201 | −1.460 | 1.842 | 6.3 | 40 | 0.613 | 0.717 | 1.03 | 0.96 |
| ICZP-5ii | 0.052 | −1.484 | 1.489 | 1.3 | 87 | 0.731 | 0.663 | 0.91 | 1.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mainar, A.R.; Colmenares, L.C.; Grande, H.-J.; Blázquez, J.A. Enhancing the Cycle Life of a Zinc–Air Battery by Means of Electrolyte Additives and Zinc Surface Protection. Batteries 2018, 4, 46. https://doi.org/10.3390/batteries4030046
Mainar AR, Colmenares LC, Grande H-J, Blázquez JA. Enhancing the Cycle Life of a Zinc–Air Battery by Means of Electrolyte Additives and Zinc Surface Protection. Batteries. 2018; 4(3):46. https://doi.org/10.3390/batteries4030046
Chicago/Turabian StyleMainar, Aroa R., Luis C. Colmenares, Hans-Jürgen Grande, and J. Alberto Blázquez. 2018. "Enhancing the Cycle Life of a Zinc–Air Battery by Means of Electrolyte Additives and Zinc Surface Protection" Batteries 4, no. 3: 46. https://doi.org/10.3390/batteries4030046
APA StyleMainar, A. R., Colmenares, L. C., Grande, H.-J., & Blázquez, J. A. (2018). Enhancing the Cycle Life of a Zinc–Air Battery by Means of Electrolyte Additives and Zinc Surface Protection. Batteries, 4(3), 46. https://doi.org/10.3390/batteries4030046