Consumer-Based Evaluation of Commercially Available Protected 18650 Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results
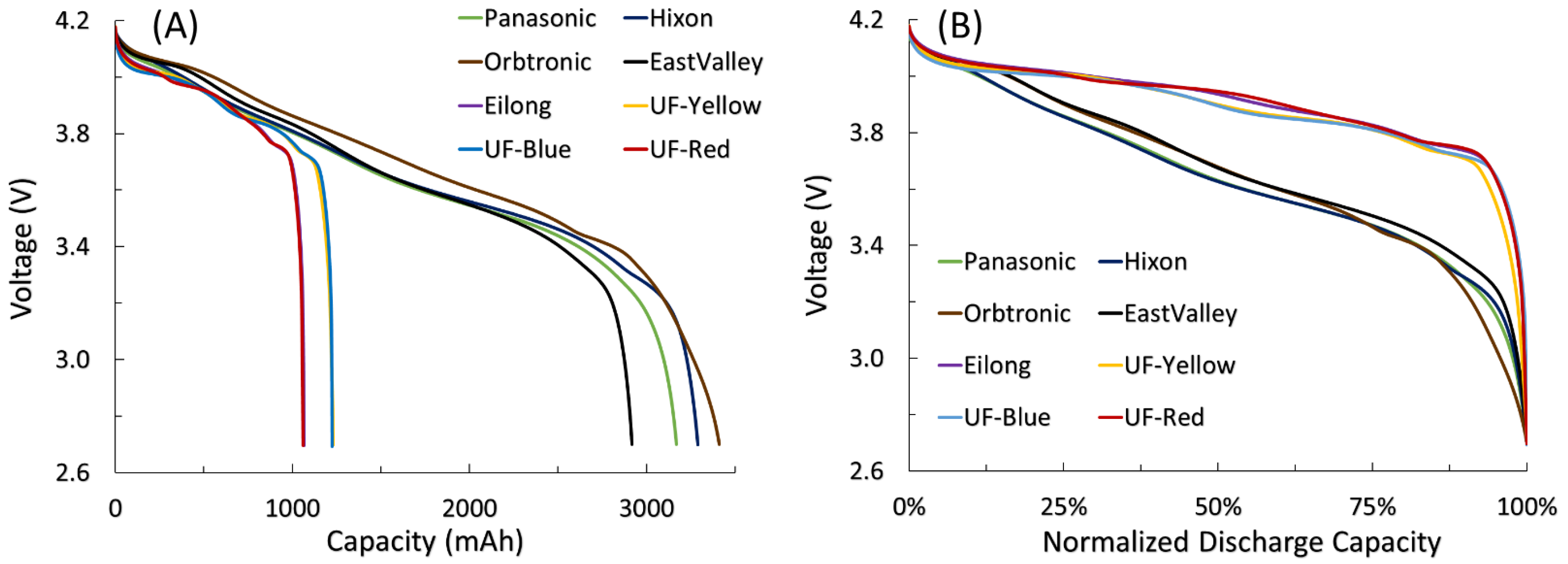
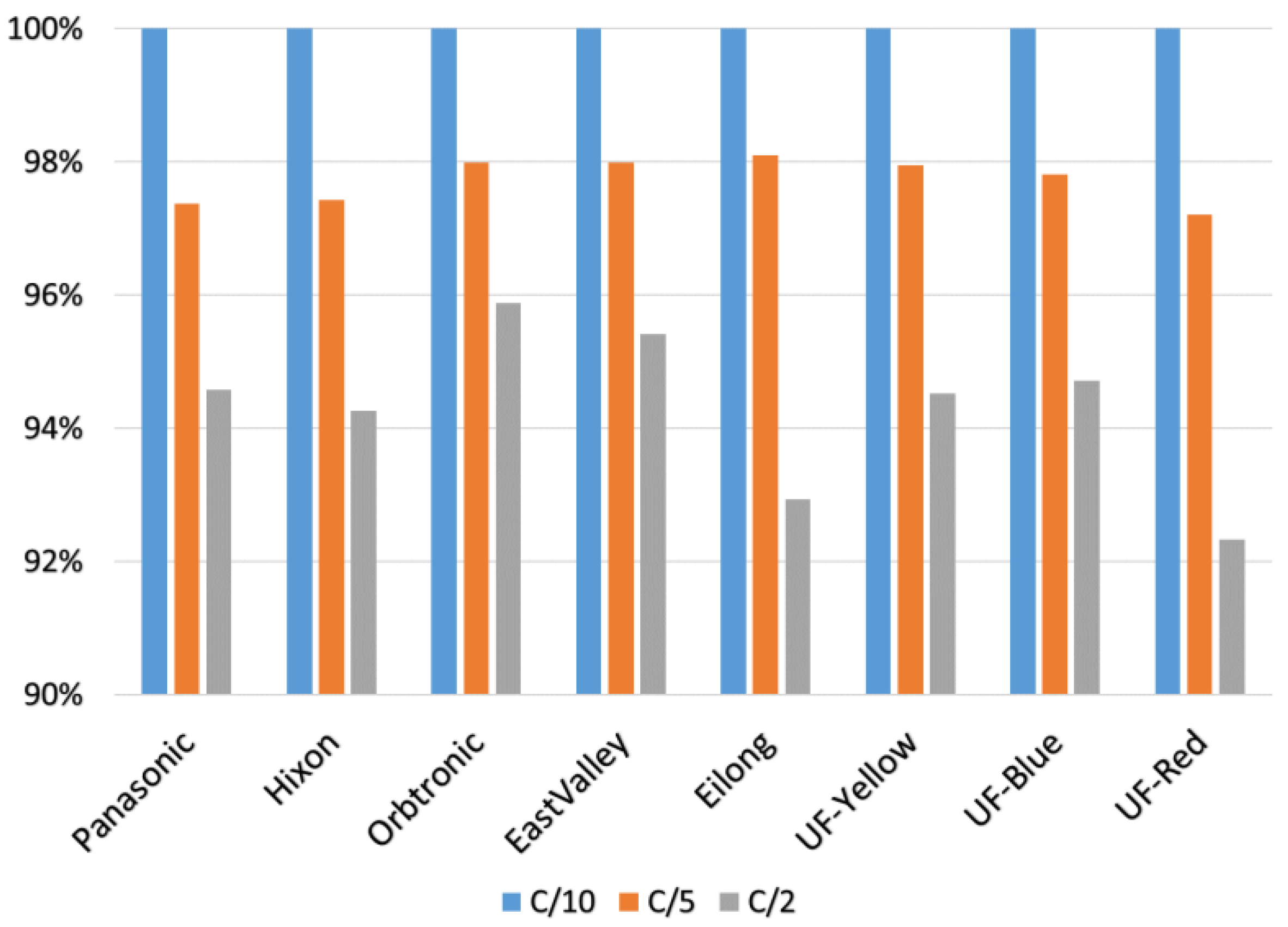
3.1. Capacity
3.2. Rate Capability
3.3. Cost/Energy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muenzel, V.; Hollenkamp, A.F.; Bhatt, A.I.; de Hoog, J.; Brazil, M.; Thomas, D.A.; Mareels, I. A Comparative Testing Study of Commercial 18650-Format Lithium-Ion Battery Cells. J. Electrochem. Soc. 2015, 162, A1592–A1600. [Google Scholar] [CrossRef] [Green Version]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Hossain, S.; Tipton, A.; Mayer, S.; Anderman, M.; Surumpudi, S. Lithium-ion cells for aerospace applications. In Proceedings of the IECEC-97: Thirty-Second Intersociety Energy Conversion Engineering Conference (Cat. No. 97CH6203), Honolulu, HI, USA, 27 July–1 August 1997; Volume 1, pp. 35–38. [Google Scholar]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Uitz, M.; Sternad, M.; Breuer, S.; Täubert, C.; Traußnig, T.; Hennige, V.; Hanzu, I.; Wilkening, M. Aging of Tesla’s 18650 Lithium-Ion Cells: Correlating Solid-Electrolyte-Interphase Evolution with Fading in Capacity and Power. J. Electrochem. Soc. 2017, 164, A3503–A3510. [Google Scholar] [CrossRef]
- Hesse, H.C.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-Ion Battery Storage for the Grid—A Review of Stationary Battery Storage System Design Tailored for Applications in Modern Power Grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef]
- Augeard, A.; Singo, T.; Desprez, P.; Perisse, F.; Menecier, S.; Abbaoui, M. Arc analysis to the CID of li-ion battery cells in high-current applications. In Proceedings of the 2014 IEEE 60th Holm Conference on Electrical Contacts (Holm), New Orleans, LA, USA, 12–15 October 2014; pp. 1–7. [Google Scholar]
- Reddy, T. Linden’s Handbook of Batteries, Fourth Edition, 4th ed.; McGraw-Hill: New York, NY, USA, 2002; ISBN 978-0-07-162421-3. [Google Scholar]
- Morley, R. Lithium-Ion 18650 Battery Failures. Hartwood Consulting 3. Available online: http://hartwood.com.au/project_item/lithium-ion-18650-battery-failures/ (accessed on 31 August 2018).
- Burke, A.; Miller, M. Performance Characteristics of Lithium-ion Batteries of Various Chemistries for Plug-in Hybrid Vehicles. In Proceedings of the EVS24 International Battery, Hybrid and Fuel Cell Electric Vehicle Symposium, Stavanger, Norway, 13–16 May 2009; pp. 1–13. [Google Scholar]
- Barkholtz, H.M.; Fresquez, A.; Chalamala, B.R.; Ferreira, S.R. A Database for Comparative Electrochemical Performance of Commercial 18650-Format Lithium-Ion Cells. J. Electrochem. Soc. 2017, 164, A2697–A2706. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-Y.; Xu, T.; Ge, S.; Zhang, G.; Yang, X.-G.; Ji, Y. A Fast Rechargeable Lithium-Ion Battery at Subfreezing Temperatures. J. Electrochem. Soc. 2016, 163, A1944–A1950. [Google Scholar] [CrossRef] [Green Version]
- Krieger, E.M.; Cannarella, J.; Arnold, C.B. A comparison of lead-acid and lithium-based battery behavior and capacity fade in off-grid renewable charging applications. Energy 2013, 60, 492–500. [Google Scholar] [CrossRef]
- Tröltzsch, U.; Kanoun, O.; Tränkler, H.-R. Characterizing aging effects of lithium ion batteries by impedance spectroscopy. Electrochim. Acta 2006, 51, 1664–1672. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Bugga, R.V.; Smart, M.C. Lithium Plating Behavior in Lithium-Ion Cells. ECS Trans. 2010, 25, 241–252. [Google Scholar] [CrossRef]
- Kim, C.S.; Jeong, K.M.; Kim, K.; Yi, C.W. Effects of capacity ratios between anode and cathode on electrochemical properties for lithium polymer batteries. Electrochim. Acta 2015, 155, 431–436. [Google Scholar] [CrossRef]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Van Mierlo, J. Cost Projection of State of the Art Lithium-Ion Batteries for Electric Vehicles Up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]


| Cell/Model | Nominal Voltage (V) * | Nameplate Capacity (mAh) * | Height (mm) | Diameter (mm) | Weight (g) |
|---|---|---|---|---|---|
| Panasonic NCR18650B | 3.7 | 3400 | 69.53 ± 0.00 | 18.54 ± 0.13 | 47.62 ± 0.03 |
| Hixon NCR18650 | 3.7 | 3400 | 69.27 ± 0.17 | 18.52 ± 0.02 | 47.88 ± 0.07 |
| Orbtronic FBA | 3.7 | 3500 | 68.96 ± 0.09 | 18.56 ± 0.15 | 48.78 ± 0.05 |
| EastValley B35-18650 | 3.7 | 3500 | 69.85 ± 0.08 | 18.52 ± 0.05 | 46.48 ± 0.02 |
| Eilong TR18650 | 3.7 | 9800 | 66.34 ± 0.11 | 18.27 ± 0.07 | 35.52 ± 0.20 |
| UF-Yellow BRC18650 | 3.7 | 5000 | 67.16 ± 0.00 | 18.13 ± 0.02 | 36.25 ± 0.76 |
| UF-Blue TR18650 | 3.7 | 5000 | 66.66 ± 0.14 | 18.08 ± 0.03 | 34.59 ± 0.20 |
| UF-Red BRC18650 | 3.7 | 4000 | 66.38 ± 0.12 | 18.05 ± 0.00 | 33.99 ± 1.38 |
| Step | Description |
|---|---|
| 1 | Discharge to 2.7 V @ C/10 |
| 2 | Charge to 4.2 V @ C/5 rate with C/50 taper |
| 3 | Discharge to 2.7 V @ C/10 |
| 4 | Repeat Steps 2–3 three times |
| 5 | Repeat Steps 2–4 @ C/5 discharge rate |
| 6 | Repeat Steps 2–4 @ C/2 discharge rate |
| 7 | Charge cell to 3.7 V |
| Cell Type | Nameplate Capacity (mAh) | C/10 (mAh) | C/5 (mAh) | C/2 (mAh) | C/10 (%) | C/5 (%) | C/2 (%) |
|---|---|---|---|---|---|---|---|
| Panasonic | 3400 | 3177 | 3094 | 3005 | 93.4 | 91.0 | 88.4 |
| Hixon | 3400 | 3264 | 3180 | 3076 | 96.0 | 93.5 | 90.5 |
| Orbtronic | 3500 | 3399 | 3330 | 3259 | 97.1 | 95.2 | 93.1 |
| EastValley | 3500 | 2924 | 2865 | 2789 | 83.5 | 81.9 | 79.7 |
| Eilong * | 9800 | 944 | 927 | 882 | 9.6 | 9.5 | 9.0 |
| UF-Yellow * | 5000 | 1178 | 1154 | 1113 | 23.6 | 23.1 | 22.3 |
| UF-Blue * | 5000 | 1203 | 1177 | 1140 | 24.1 | 23.5 | 22.8 |
| UF-Red * | 4000 | 1013 | 986 | 938 | 25.3 | 24.6 | 23.5 |
| Type | Nominal Voltage (V) | Nameplate Capacity (mAh) | Cost/Cell ($) | Cost/Capacity ($/Ah) | Cost/Energy ($/Wh) |
|---|---|---|---|---|---|
| Panasonic | 3.7 | 3400 | $10.44 | $3.07 | $0.83 |
| Hixon | 3.7 | 3400 | $10.75 | $3.16 | $0.85 |
| Orbtronic | 3.7 | 3500 | $15.00 | $4.28 | $1.16 |
| EastValley | 3.7 | 3500 | $7.45 | $2.13 | $0.58 |
| Eilong | 3.7 | 9800 | $1.80 | $0.18 | $0.05 |
| UF-Yellow | 3.7 | 5000 | $2.31 | $0.46 | $0.13 |
| UF-Blue | 3.7 | 5000 | $1.50 | $0.30 | $0.08 |
| UF-Red | 3.7 | 4000 | $1.50 | $0.37 | $0.10 |
| Type | Nominal Voltage (V) | C/10 (mAh) | Cost/Cell ($) | Cost/Capacity ($/Ah) | Cost/Energy ($/Wh) | Gravimetric Energy Density (Wh/kg) | Vol. Energy Density (Wh/L) |
|---|---|---|---|---|---|---|---|
| Panasonic | 3.6 | 3177 | $10.44 | $3.29 | $0.91 | 240.2 | 609.64 |
| Hixon | 3.6 | 3264 | $10.75 | $3.29 | $0.91 | 245.4 | 630.06 |
| Orbtronic | 3.6 | 3399 | $15.00 | $4.41 | $1.23 | 250.9 | 655.83 |
| EastValley | 3.6 | 2924 | $7.45 | $2.55 | $0.71 | 226.5 | 559.70 |
| Eilong | 3.7 | 944 | $1.80 | $1.91 | $0.52 | 98.3 | 200.83 |
| UF-Yellow | 3.7 | 1178 | $2.31 | $1.96 | $0.53 | 120.2 | 251.43 |
| UF-Blue | 3.7 | 1203 | $1.50 | $1.25 | $0.34 | 128.7 | 260.28 |
| UF-Red | 3.7 | 1013 | $1.50 | $1.48 | $0.40 | 110.5 | 220.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baksa, S.; Yourey, W. Consumer-Based Evaluation of Commercially Available Protected 18650 Cells. Batteries 2018, 4, 45. https://doi.org/10.3390/batteries4030045
Baksa S, Yourey W. Consumer-Based Evaluation of Commercially Available Protected 18650 Cells. Batteries. 2018; 4(3):45. https://doi.org/10.3390/batteries4030045
Chicago/Turabian StyleBaksa, Steven, and William Yourey. 2018. "Consumer-Based Evaluation of Commercially Available Protected 18650 Cells" Batteries 4, no. 3: 45. https://doi.org/10.3390/batteries4030045





