Increase in the Surface Catalytic Ability by Addition of Palladium in C14 Metal Hydride Alloy
Abstract
:1. Introduction
2. Experimental Setup
3. Results and Discussions
3.1. Properties of Pd
3.2. Chemical Composition
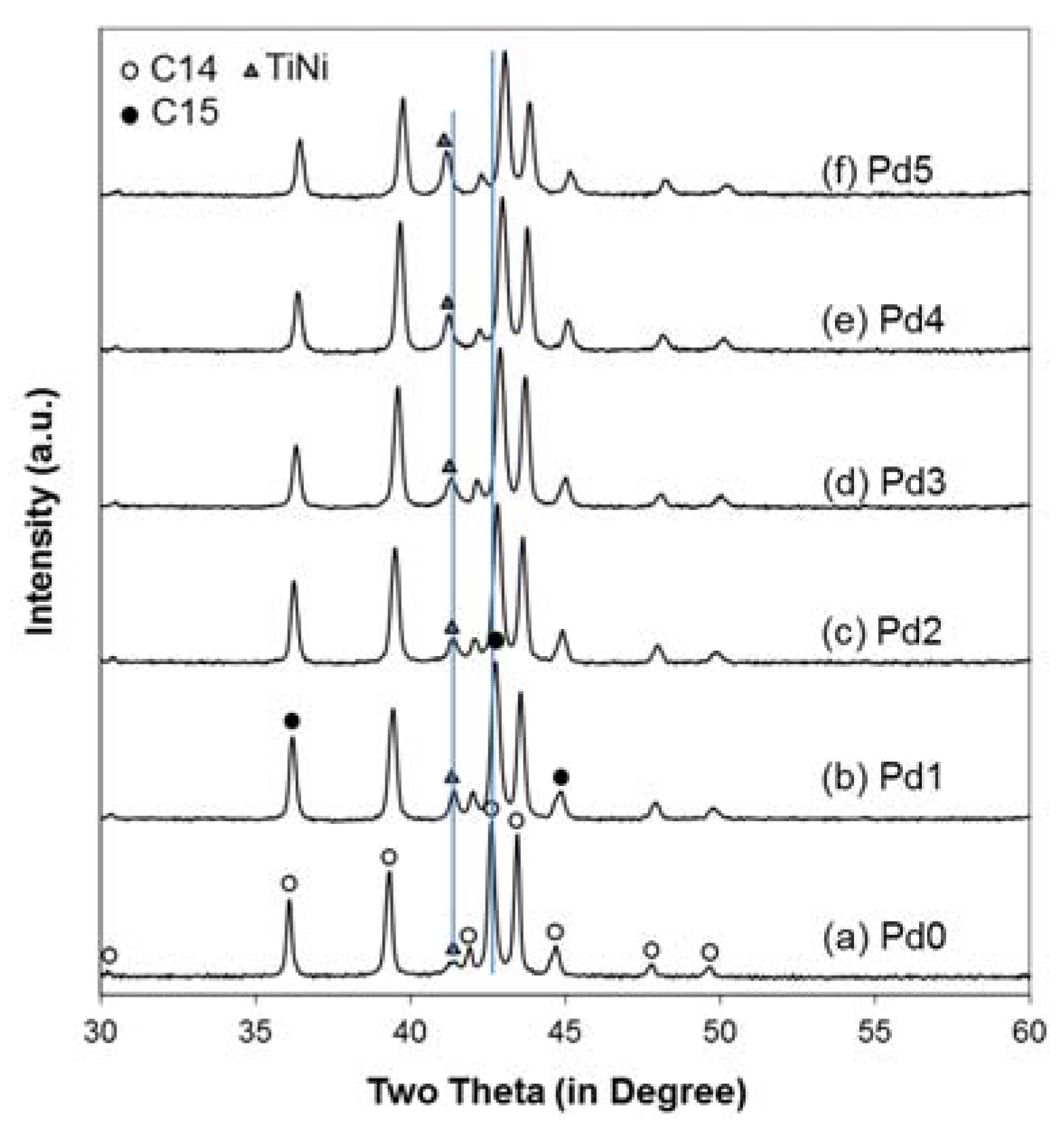
3.3. XRD Analysis
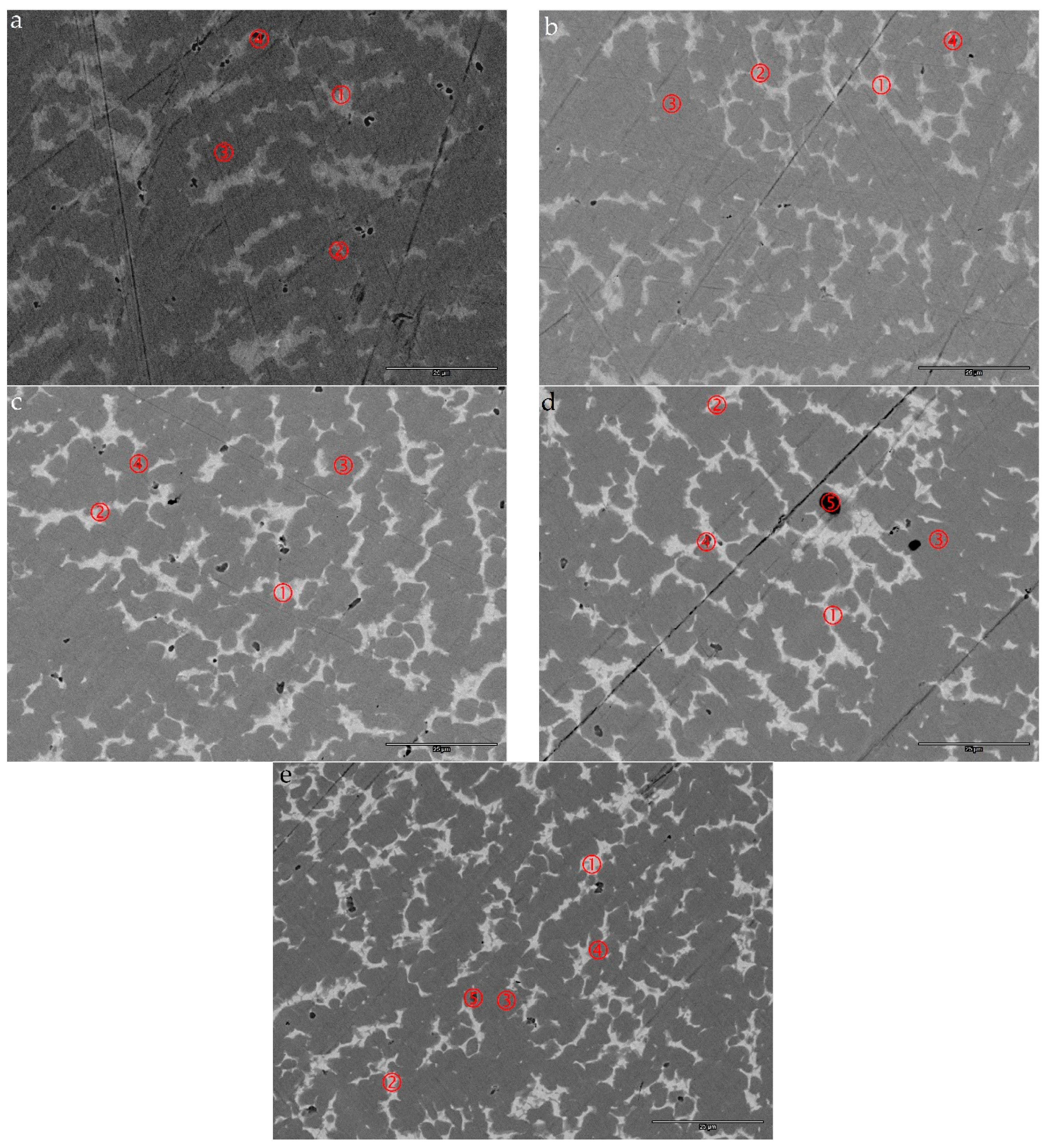
3.4. SEM/EDS Analysis
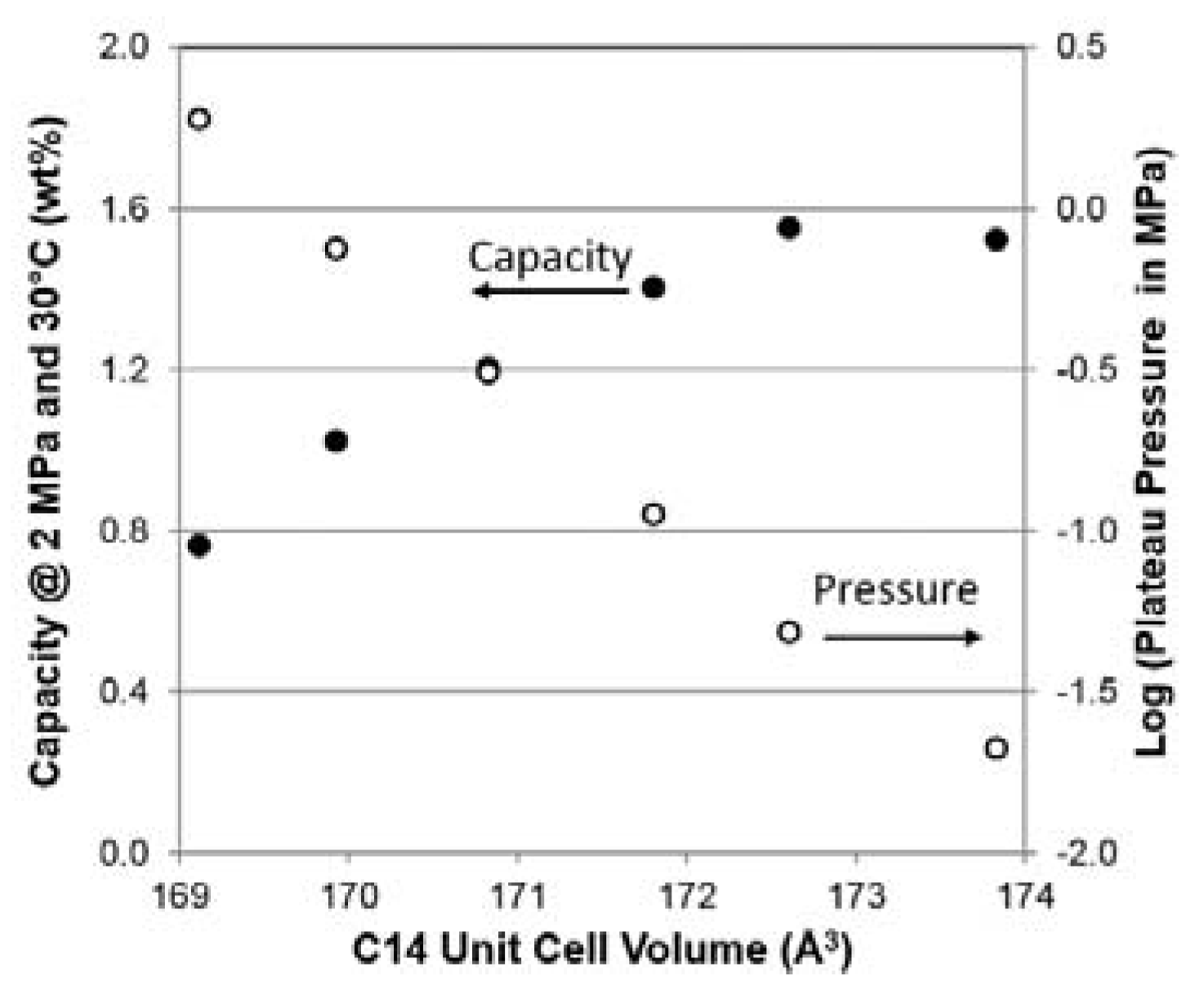
3.5. PCT Analysis
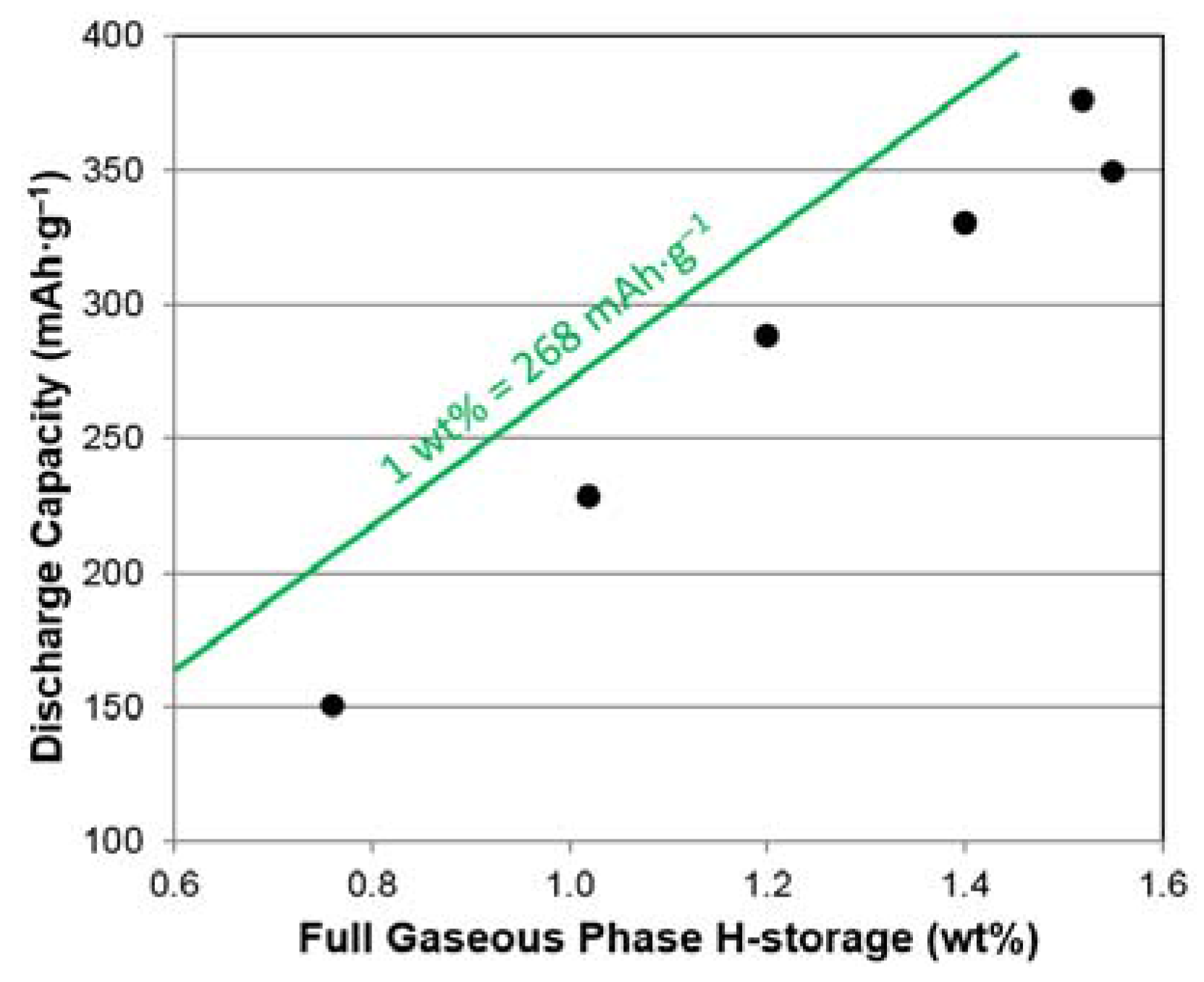
3.6. Electrochemical Analysis
3.7. Magnetic Susceptibility
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MH | Metal hydride |
| H-storage | Hydrogen storage |
| HRD | High-rate dischargeability |
| AM | Arc melting |
| RD | Replacement-diffusion |
| MA | Mechanical alloying |
| TA | Thermal annealing |
| IM | Induction melting |
| MS | Melt spinning |
| LM | Levitation melting |
| WI | Wet impregnation |
| GP | Gaseous phase |
| EC | Electrochemical |
| Io | Surface exchange current |
| XRD | X-ray diffractometer |
| SEM | Scanning electron microscope |
| PCT | Pressure concentration temperature |
| ICP-OES | Inductively coupled plasma optical emission spectrometer |
| EDS | Energy dispersive spectroscopy |
| M.S. | Magnetic susceptibility |
| ΔHh | Heat of hydride formation |
| hcp | Hexagonal close-packed |
| fcc | Face-centered-cubic |
| bcc | Body-centered-cubic |
| IMC | Intermetallic compound |
| e/a | Average electron density |
| VC14 | Unit cell volume of the C14 phase |
| FWHM | Full width at half maximum |
| BEI | Back-scattering electron image |
| ΔSh | Change in entropy |
| T | Absolute temperature |
| R | Ideal gas constant |
| D | Bulk diffusion coefficient |
| R | Surface charge-transfer resistance |
| C | Surface double-layer capacitance |
| MS | Saturated magnetic susceptibility |
| H1/2 | Applied magnetic field strength corresponding to half of saturated magnetic susceptibility |
References
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Young, K.; Nei, J.; Fierro, C. Reviews on the U.S. Patents regarding nickel/metal hydride batteries. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Shaltiel, D.; Jacob, I.; Davidov, D. Hydrogen absorption and desorption properties of AB2 Laves-phase pseudobinary compounds. J. Less Common Met. 1977, 53, 117–131. [Google Scholar] [CrossRef]
- Jacob, I.; Shaltiel, D.; Davidov, D.; Miloslavski, I. A phenomenological model for the hydrogen absorption capacity in pseudobinary Laves phase compounds. Solid State Commun. 1977, 23, 669–671. [Google Scholar] [CrossRef]
- Shaltiel, D. Hydride properties of AB2 Laves-phase compounds. J. Less Common Met. 1978, 62, 407–416. [Google Scholar] [CrossRef]
- Mendelsohn, M.H.; Gruen, D.M. The pseudo-binary system Zr(V1−xCrx)2: Hydrogen absorption and stability considerations. J. Less Common Met. 1981, 78, 275–280. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.; Ouchi, T.; Meng, T.; Nei, J.; Wu, X. Studies on incorporation of Mg in Zr-based AB2 metal hydride alloys. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Ouchi, T.; Huang, B.; Reichman, B. Effects of La-addition to the structure, hydrogen storage, and electrochemical properties of C14 metal hydride alloys. Electrochim. Acta 2015, 174, 815–825. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Moghe, D. The importance of rare-earth additions in Zr-based AB2 metal hydride alloys. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Wong, D.F.; Young, K.; Nei, J.; Wang, L.; Ng, K.Y.S. Effects of Nd-addition on the structural, hydrogen storage, and electrochemical properties of C14 metal hydride alloys. J. Alloys Compd. 2015, 647, 507–518. [Google Scholar] [CrossRef]
- Griessen, R.; Riesterer, T. Heat of Formation Models. In Hydrogen in Intermetallic Compounds I; Schlapbach, L., Ed.; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Osumi, Y. Suiso Kyuzou Goukin; Agune Co. Ltd.: Tokyo, Japan, 1993. (In Japanese) [Google Scholar]
- Graham, T. On the absorption and dislytic separation of gases by colloid septa. Philos. Trans. R. Soc. Lond. 1866, 156, 399–439. [Google Scholar] [CrossRef]
- Smith, D.P. Hydrogen in Metals; The University of Chicago Press: Chicago, IL, USA, 1947. [Google Scholar]
- Mackay, K.M. Hydrogen Compounds of the Metallic Elements; E.&F.N. Spon Ltd.: London, UK, 1966. [Google Scholar]
- Muetterties, E.L. The transition metal-hydrogen interaction. In Transition Metal Hydrides; Muetterties, E.L., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1971. [Google Scholar]
- Sakamoto, Y.; Yuwasa, K.; Hirayama, H. X-ray investigation of the absorption of hydrogen by several palladium and nickel solid solution alloys. J. Less Common Met. 1982, 88, 115–124. [Google Scholar] [CrossRef]
- Żurowski, A.; Łukaszewski, M.; Czerwiński, A. Electrosorption of hydrogen into palladium-rhodium alloys Part 2. Pd-rich electrodes of various thickness. Electrochim. Acta 2008, 53, 7812–7816. [Google Scholar] [CrossRef]
- Rousselot, S.; Bichat, M.-P.; Guay, D.; Roué, L. Structure and electrochemical behavior of metastable Mg50Ti50 alloy prepared by ball milling. J. Power Sources 2008, 175, 621–624. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Hubkowska, K.; Koss, U.; Czerwiński, A. On the nature of voltammetric signals originating from hydrogen electrosorption into palladium-noble metal alloys. Materials 2013, 6, 4817–4835. [Google Scholar] [CrossRef] [PubMed]
- Badri, V.; Hermann, A.M. Metal hydride batteries: Pd nanotube incorporation into the negative electrode. Int. J. Hydrogen Energy 2000, 25, 249–253. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Kohno, T.; Yamamoto, M.; Kanda, M. Electrochemical properties of mechanically ground Mg2Ni alloy. J. Alloys Compd. 1999, 293–295, 643–647. [Google Scholar] [CrossRef]
- Janot, R.; Rougier, A.; Aymard, L.; Lenain, C.; Herrera-Urbina, R.; Narzi, G.A.; Tarascon, J.M. Enhancement of hydrogen storage in MgNi by Pd-coating. J. Alloys Compd. 2003, 356–357, 438–441. [Google Scholar] [CrossRef]
- Rivera, M.A.; Pal, U.; Wang, X.; Gonzalez-Rodriguez, J.G.; Gamboa, S.A. Rapid activation of MmNi5−xMx based MH alloy through Pd Nanoparticle impregnation. J. Power Sources 2006, 155, 470–474. [Google Scholar] [CrossRef]
- Visintin, A.; Castro, E.B.; Real, S.G.; Trica, W.E.; Wang, C.; Soriaga, M.P. Electrochemical activation and electrocatalytic enhancement of a hydride-forming metal alloy modified with palladium, platinum and nickel. Electrochim. Acta 2006, 51, 3658–3667. [Google Scholar] [CrossRef]
- Shan, X.; Payer, J.H.; Jennings, W.D. Mechanism of increased performance and durability of Pd-treated metal hydriding alloys. Int. J. Hydrogen Energy 2009, 34, 363–369. [Google Scholar] [CrossRef]
- Uchida, H.H.; Wulz, H.-G.; Fromm, E. Catalytic effect of nickel, iron and palladium on hydriding titanium and storage materials. J. Less Common Met. 1991, 172–174, 1076–1083. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kohno, T.; Iwakura, C. Electrochemical properties of hydrogen storage alloys modified with foreign metals. Electrochim. Acta 1993, 38, 789–791. [Google Scholar] [CrossRef]
- Hjort, P.; Krozer, A.; Kasemo, B. Hydrogen sorption kinetics in partly oxidized Mg films. J. Alloys Compd. 1996, 237, 74–80. [Google Scholar] [CrossRef]
- Visintin, A.; Tori, C.A.; Garaventta, G.; Triaca, W.E. The electrochemical performance of Pd-coated metal hydride electrodes with different binding additives in alkaline solution. J. Electrochem. Soc. 1998, 145, 4169–4172. [Google Scholar] [CrossRef]
- Cuevas, F.; Hirscher, M. The hydrogen desorption kinetics of Pd-coated LaNi5-type films. J. Alloys Compd. 2000, 313, 269–275. [Google Scholar] [CrossRef]
- Hara, M.; Hatano, Y.; Abe, T.; Watanabe, K.; Naitoh, T.; Ikeno, S.; Honda, Y. Hydrogen absorption by Pd-coated ZrNi prepared by using Barrel-sputtering system. J. Nucl. Mat. 2003, 320, 265–271. [Google Scholar] [CrossRef]
- Park, H.J.; Goo, N.H.; Lee, K.S. In situ Pd deposition on Mg2Ni electrode for Ni/MH secondary batteries during charge cycles. J. Electrochem. Soc. 2003, 150, A1328–A1332. [Google Scholar] [CrossRef]
- Barsellini, D.B.; Visintin, A.; Triaca, W.E.; Soriaga, M.P. Electrochemical characterization of a hydride-forming metal alloy surface-modified with palladium. J. Power Sources 2003, 124, 309–313. [Google Scholar] [CrossRef]
- Parimala, R.; Ananth, M.V.; Ramaprabhu, S.; Raju, M. Effect of electroless coating of Cu, Ni and Pd on ZrMn0.2V0.2Fe0.8Ni0.8 alloy used as anodes in Ni-MH batteries. Int. J. Hydrogen Energy 2004, 29, 509–513. [Google Scholar] [CrossRef]
- Yoshimura, K.; Yamada, Y.; Okada, M. Hydrogenation of Pd capped Mg thin films at room temperature. Surf. Sci. 2004, 566–568, 751–754. [Google Scholar] [CrossRef]
- Souza, E.C.; Ticianelli, E.A. Structural and electrochemical properties of MgNi-based alloys with Ti, Pt and Pd additives. Int. J. Hydrogen Energy 2000, 25, 249–253. [Google Scholar] [CrossRef]
- Xin, G.; Yang, J.; Fu, H.; Zheng, J.; Li, X. Pd capped MgxTi1−x films: Promising anode materials for alkaline secondary batteries with superior discharge capacities and cyclic stabilities. Int. J. Hydrogen Energy 2013, 38, 10625–10629. [Google Scholar] [CrossRef]
- Jung, H.; Cho, S.; Lee, W. A catalytic effect on hydrogen absorption kinetics in Pd/Ti/Mg/Ti multilayer thin films. J. Alloys Compd. 2015, 635, 203–206. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, Y.; Ouyang, L.; Wang, H. Thermodynamic tuning of Mg-based hydrogen storage alloys: A review. Materials 2013, 6, 4654–4674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, R.; Zhang, T.; Kou, H.; Li, J.; Xue, X. Hydrogenation properties of Pd-coated Zr-based Laves phase compounds. Vacuum 2014, 109, 191–196. [Google Scholar] [CrossRef]
- Geng, M. Electrochemical characteristics of Ni-Pd-coated MmNi5-based alloy powder for nickel-metal hydride batteries. J. Alloys Compd. 1995, 217, 90–93. [Google Scholar] [CrossRef]
- Geng, M. Electrochemical characterization of MmNi5-based alloy powder coated with palladium and nickel-palladium. J. Alloys Compd. 1994, 215, 151–153. [Google Scholar] [CrossRef]
- Williams, M.; Lototsky, M.V.; Davids, M.W.; Linkov, V.; Yartyes, V.A.; Solberg, J.K. Chemical surface modification for the improvement of the hydrogenation kinetics and poisoning resistance of TiFe. J. Alloys Compd. 2011, 509, S770–S774. [Google Scholar] [CrossRef]
- Williams, M.; Lototsky, M.; Nechaev, A.; Linkov, V.; Vartys, V.; Li, Q. Surface-modified AB5 alloys with enhanced hydrogen absorption kinetics. In Carbon Nanomaterials in Clean Energy Hydrogen Systems; Barabowski, B., Zaginaichenko, S.Y., Schur, D.V., Skorokhod, V.V., Veziroglu, A., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 625–636. [Google Scholar]
- Van Mal, H.H.; Buschow, K.H.J.; Miedema, A.R. Hydrogen absorption in LaNi5 and related compounds: Experimental observations and their explanation. J. Less Common Met. 1974, 35, 65–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, J.; Yuan, H.; Chen, S.; Wang, D.; Zang, T. Synthesis of hydrogen storage compound Mg2Ni0.75Pd0.25 and studies on hydriding-dehydriding properties. Acta Sci. Nat. Univ. Nan Kaiensis 1991, 1, 93–98. (In Chinese) [Google Scholar]
- Zaluski, L.; Zaluska, A.; Ström-Olsen, J.O. Hydrogen absorption in nanacrystalline Mg2Ni formed by mechanical alloying. J. Alloys Compd. 1995, 217, 245–249. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Tessier, P.; Ström-Olsen, J.O.; Schulz, R. Effects of relaxation on hydrogen absorption in Fe-Yi produced by ball-milling. J. Alloys Compd. 1995, 227, 53–57. [Google Scholar] [CrossRef]
- Tsukahara, M.; Takahashi, K.; Mishima, T.; Isomura, A.; Sakai, T. Influence of various additives in vanadium-based alloys V3TiNi0.56 on secondary phase formation, hydrogen storage properties and electrode properties. J. Alloys Compd. 1996, 245, 59–65. [Google Scholar] [CrossRef]
- Yamashita, I.; Tanaka, H.; Takeshita, H.; Kuriyama, N.; Sakai, T.; Uehara, I. Hydrogenation characteristics of TiFe1−xPdx (0.05 ≤ x≤ 0.30) alloys. J. Alloys Compd. 1997, 253–254, 238–240. [Google Scholar] [CrossRef]
- Wang, C.S.; Lei, Y.Q.; Wang, Q.D. Effects of Nb and Pd on the electrochemical properties of a Ti-Ni hydrogen-storage electrode. J. Power Sources 1998, 70, 222–227. [Google Scholar] [CrossRef]
- Yang, X.G.; Zhang, W.K.; Lei, Y.Q.; Wang, Q.D. Electrochemical properties of Zr-V-Ni system hydrogen storage alloys. J. Electrochem. Soc. 1999, 146, 1245–1250. [Google Scholar] [CrossRef]
- Zeppelin, F.; Reule, H.; Hirscher, M. Hydrogen desorption kinetics of nanostructured MgH2 composite materials. J. Alloys Compd. 2002, 330–332, 723–726. [Google Scholar] [CrossRef]
- Ovshinsky, S.R.; Young, R. High Power Nickel-Metal Hydride Batteries and High Power Alloys/Electrodes for Use Therein. U.S. Patent 6,413,670 B1, 2 July 2002. [Google Scholar]
- Yamaura, S.; Kim, H.; Kimura, H.; Inoue, A.; Arata, Y. Thermal stabilities and discharge capacities of melt-spun Mg-Ni-based amorphous alloys. J. Alloys Compd. 2002, 339, 230–235. [Google Scholar] [CrossRef]
- Yamaura, S.; Kim, H.; Kimura, H.; Inoue, A.; Arata, Y. Electrode properties of rapidly solidified Mg67Ni23Pd10 amorphous alloy. J. Alloys Compd. 2002, 347, 239–243. [Google Scholar] [CrossRef]
- Yamaura, S.; Kimura, H.; Inoue, A. Discharge capacities of melt-spun Mg-Ni-Pd amorphous alloys. J. Alloys Compd. 2003, 358, 173–176. [Google Scholar] [CrossRef]
- Ma, J.; Hatano, Y.; Abe, T.; Watanabe, K. Effects of Pd addition on electrochemical properties of MgNi. J. Alloys Compd. 2004, 372, 251–258. [Google Scholar] [CrossRef]
- Ma, T.; Hatano, Y.; Abe, T.; Watanabe, K. Effects of bulk modification by Pd on electrochemical properties of MgNi. J. Alloys Compd. 2005, 391, 313–317. [Google Scholar] [CrossRef]
- Miyamura, H.; Takada, M.; Kikuchi, S. Characteristics of hydride electrode using Ti-Fe-Pd-X alloys. J. Alloys Compd. 2005, 404–406, 675–678. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Y.; Sun, L.; Xu, F.; Tan, Z.; Yuan, H.; Zhang, T. Effects of Pd substitution on the electrochemical properties of Mg0.9−xTi0.1PdxNi (x = 0.04–0.1) hydrogen storage electrode alloys. J. Power Sources 2006, 158, 1463–1471. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Y.; Chu, H.; Sun, L.; Xu, F.; Tan, Z.; Yuan, H.; Zhang, T. The electrochemical performances of Mg0.9Ti0.1Ni1−xPdx (x = 0–0.15) hydrogen storage electrode alloys. J. Power Sources 2006, 159, 155–158. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Y.; Tan, Z.; Xu, F.; Sun, L.; Zhang, T.; Yuan, H. Effects of Pd substitution for Ni on the corrosion performances of Mg0.9Ti0.1Ni1–xPdx hydrogen storage electrode alloys. Trans. Nonferrous Met. Soc. China 2006, 16, 497–501. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Y.; Sun, L.; Xu, F.; Yuan, H. The hydrogen desorption kinetics of Mg0.9−xTi0.1PdxNi (x = 0.04, 0.06, 0.08, 0.1) electrode alloys. J. Alloys Compd. 2007, 446–447, 121–123. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Balogh, M.P.; Meyer, M.S.; Meisner, G.P. Hydrogen Generation Material. U.S. Patent Application 2006/0,057,049 A1, 16 March 2006. [Google Scholar]
- Yermakov, A.Y.; Mushnikov, N.V.; Uimin, M.A.; Gaviko, V.S.; Tankeev, A.P.; Skripov, A.V.; Soloninin, A.V.; Buzlukov, A.L. Hydrogen reaction kinetics of Mg-based alloys synthesized by mechanical milling. J. Alloys Compd. 2006, 425, 367–372. [Google Scholar] [CrossRef]
- Berlouis, L.E.A.; Honnor, P.; Hall, P.J.; Morris, S.; Dodd, S.B. An investigation of the effect of Ti, Pd, and Zr on the dehydriding kinetics of MgH2. J. Mater. Sci. 2006, 41, 6403–6408. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Wondergem, H.J.; Bakker, F.; Notten, P.H.L. Mg-Ti based materials for electrochemical hydrogen storage. J. Mater. Res. 2007, 22, 1640–1649. [Google Scholar] [CrossRef]
- Spassov, T.; Todorova, S.; Jung, W.; Borissova, A. Hydrogen sorption properties of ternary intermetallic Mg-(Ir,Rh,Pd)-Si compounds. J. Alloys Compd. 2007, 429, 306–310. [Google Scholar] [CrossRef]
- Yvon, K.; Rapin, J.-Ph.; Penin, N.; Ma, Z.; Chou, M.Y. LaMg2PdH7, a new complex metal hydride containing tetrahedral [PdH4]4− anion. J. Alloys Compd. 2007, 446–447, 34–38. [Google Scholar] [CrossRef]
- Jeng, R.; Lee, S.; Hsu, C.; Wu, Y.; Lin, J. Effects of the addition of Pd on the hydrogen absorption-desorption characteristics of Ti33V33Cr34 alloys. J. Alloys Compd. 2008, 464, 467–471. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Li, R.; Gao, M.; Zhong, K.; Miao, H.; Pan, H. Electrochemical performances of the Pd-added Ti-V-based hydrogen storage alloys. Int. J. Hydrogen Energy 2008, 33, 728–734. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Mi, G.; Zhang, Z.; Wang, L. Crystallographic and electrochemical characteristics of Ti-Zr-Ni-Pd quasicrystalline alloys. Int. J. Hydrog. Energy 1008, 34, 6925–6929. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Y.; Chu, H.; Ding, Y.; Wu, Y. Electrochemical impedance study of discharge characteristics of Pd substituted MgNi-based hydrogen storage electrode alloys. J. Alloys Compd. 2009, 481, 826–829. [Google Scholar] [CrossRef]
- Gao, L.; Yao, E.; Nakamura, J.; Zhang, W.; Chua, H. Hydrogen storage in Pd-Ni doped defective carbon nanotubes through the formation of CHx (x = 1, 2). Carbon 2010, 48, 3250–3255. [Google Scholar] [CrossRef]
- Rousselot, S.; Gazeau, A.; Guay, D.; Roué, L. Influence of Pd on the structure and electrochemical hydrogen storage properties of Mg50Ti50 alloy prepared by ball milling. Electrochim. Acta 2010, 55, 611–619. [Google Scholar] [CrossRef]
- Ruiz, F.C.; Peretti, H.A.; Visintin, A. Electrochemical hydrogen storage in ZrCrNiPdx alloys. Int. J. Hydrogen Energy 2010, 35, 5963–5967. [Google Scholar] [CrossRef]
- Okonska, I.; Jurczyk, M. Hydriding properties of Mg-3d/M-type nanocomposites (3d = Cu, Ni; M = Ni, Cu, Pd). Phys. Status Solidi A 2010, 207, 1139–1143. [Google Scholar] [CrossRef]
- Emami, H.; Cuevas, F. Hydrogenation properties of shape memory Ti(Ni,Pd) compounds. Intermetallics 2011, 19, 876–886. [Google Scholar] [CrossRef]
- Anik, M.; Özdemir, G.; Küҫükdeveci, N. Electrochemical hydrogen storage characteristics of Mg-Pd-Ni ternary alloys. Int. J. Hydrogen Energy 2011, 36, 6744–6750. [Google Scholar] [CrossRef]
- Prigent, J.; Joubert, J.-M.; Gupta, M. Investigation of modification of hydrogenation and structural properties of LaNi5 intermetallic compound induced by substitution of Ni by Pd. J. Solid State Chem. 2011, 184, 123–133. [Google Scholar] [CrossRef]
- Anik, M. Improvement of the electrochemical hydrogen storage performance of magnesium based alloys by various additive elements. Int. J. Hydrogen Energy 2012, 37, 1905–1911. [Google Scholar] [CrossRef]
- Lin, K.; Mai, Y.; Chiu, W.; Yang, J.; Chan, S.L.I. Synthesis and characterization of metal hydride/carbon aerogel composites for hydrogen storage. J. Nanomater. 2012, 20154. [Google Scholar] [CrossRef]
- Etiemble, A.; Rousselot, S.; Guo, W.; Idrissi, H.; Roué, L. Influence of Pd addition on the electrochemical performance of Mg-Ni-Ti-Al-based metal hydride for Ni-MH batteries. Int. J. Hydrogen Energy 2013, 38, 10625–10629. [Google Scholar] [CrossRef]
- Santos, S.F.; Castro, J.F.R.; Ticianelli, E.A. Microstructures and electrode performances of Mg50Ni(50−x)Pdx alloys. Cent. Eur. Chem. 2013, 11, 485–491. [Google Scholar] [CrossRef]
- Williams, M.; Sibanyoni, J.M.; Lototskyy, M.; Pollet, B.G. Hydrogen absorption study of high-energy reactive ball milled Mg composites with palladium additives. J. Alloys Compd. 2013, 580, S144–S148. [Google Scholar] [CrossRef]
- Nikkuni, F.R.; Santos, S.F.; Ticianelli, E.A. Microstructures and electrochemical properties of Mg49Ti6Ni(45−x)Mx (M = Pd and Pt) alloy electrodes. Int. J. Energy Res. 2013, 37, 706–712. [Google Scholar] [CrossRef]
- Teresiak, A.; Uhlemann, M.; Thomas, J.; Eckert, J.; Gebert, A. Influence of Co and Pd on the formation of nanostructured LaMg2Ni and its hydrogen reactivity. J. Alloys Compd. 2014, 582, 647–658. [Google Scholar] [CrossRef]
- Balcerzak, M.; Nowak, M.; Jakubowicz, J.; Jurczyk, M. Electrochemical behavior of Nanocrystalline TiNi doped by MWCNTs and Pd. Renew. Energy 2014, 62, 432–438. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, X.; Zhu, Y.; Zhan, L.; Pu, Z.; Wan, N.; Li, L. Effects of additive Pd on the structures and electrochemical hydrogen storage properties of Mg67Co33-based composited or alloys with BCC phase. J. Alloys Compd. 2015, 622, 580–586. [Google Scholar] [CrossRef]
- Zhan, L.; Zhang, Y.; Zhu, Y.; Zhuang, X.; Dong, J.; Guo, X.; Chen, J.; Wang, Z.; Li, L. The electrochemical hydrogen storage properties of Mg67−xPdxCo33 (x = 1, 3, 5, 7) electrodes with BCC phase. J. Alloys Compd. 2016, 662, 396–403. [Google Scholar] [CrossRef]
- Banerjee, S.; Dasgupta, K.; Kumar, A.; Ruz, P.; Vishwanadh, B.; Joshi, J.B.; Sudarsan, V. Comparative evaluation of hydrogen storage behavior of Pd dopes carbon nanotubes prepared by wet impregnation and polyol methods. Int. J. Hydrogen Energy 2015, 40, 3268–3276. [Google Scholar] [CrossRef]
- Dündar-Tekkaya, E.; Yürüm, Y. Effect of loading bimetallic mixture of Ni and Pd on hydrogen storage capacity of MCM-41. Int. J. Hydrog. Energy 2015, 40, 7636–7643. [Google Scholar] [CrossRef]
- Ismail, N.; Madian, M.; El-Shall, M.S. Reduced graphene oxide doped with Ni/Pd nanoparticles for hydrogen storage application. J. Ind. Eng. Chem. 2015, 30, 328–335. [Google Scholar] [CrossRef]
- Balcerzak, M. Electrochemical and structural studies on Ti-Zr-Ni and Ti-Zr-Ni-Pd alloys and composites. J. Alloys Compd. 2016, 658, 576–587. [Google Scholar] [CrossRef]
- Giasafaki, D.; Charalambopoulou, G.; Tampaxis, Ch.; Dimos, K.; Gournis, D.; Stubos, A. Comparing hydrogen sorption in different Pd-doped pristine and surface-modified nanoporous carbons. Carbon 2016, 98, 1–14. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 85. [Google Scholar] [CrossRef]
- Abdul, J.M. Development of titanium alloys for hydrogen storage. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, December 2015. Available online: http://wiredspace.wits.ac.za/handle/10539/21151 (accessed on 11 August 2017).
- Zhan, L.; Zhang, Y.; Zhu, Y.; Zhuang, X.; Wan, N.; Qu, Y.; Guo, X.; Chen, J.; Wang, Z.; Li, L. Electrochemical performances of Mg45M5Co50 (M = Pd, Zr) ternary hydrogen storage electrodes. Trans. Nonferrous Met. Soc. China 2016, 26, 1388–1395. [Google Scholar] [CrossRef]
- Tosques, J.; Guerreiro, B.H.; Martin, M.H.; Roué, L.; Guay, D. Hydrogen solubility of bcc PdCu and PdCuAg alloys prepared by mechanical alloys. J. Alloys Compd. 2017, 698, 725–730. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Li, F.; Ouchi, T. Structural, thermodynamics, and electrochemical properties of TixZr1−x(VNiCrMnCoAl)2 C14 Laves phase alloys. J. Alloys Compd. 2008, 464, 238–247. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Koch, J.; Morii, K.; Shimizu, T. Studies of Sn, Co, Al, and Fe additives in C14/C15 Laves alloys for NiMH battery application by orthogonal arrays. J. Alloys Compd. 2009, 486, 559–569. [Google Scholar] [CrossRef]
- Price of Palladium. Available online: http://www.apmex.com/spotprices/palladium-price (accessed on 18 October 2016).
- Price of Nickel. Available online: www.infomine.com/investment/metal-prices/nickel/ (accessed on 18 October 2016).
- Rennert, P.; Radwan, A.M. Structural investigation of the Laves phase MgZn2 with model potential calculations. Phys. Status Solidi B 1977, 79, 167–173. [Google Scholar] [CrossRef]
- Douglas, B.E.; Ho, S.M. Structure and Chemistry of Crystalline Solids; Springer Science + Business, Inc.: New York, NY, USA, 2006. [Google Scholar]
- Yakoubi, A.; Baraka, O.; Bouhafs, B. Structural and electronic properties of the Laves phase based on rare earth type BaM2 (M = Rh, Pd, Pt). Results Phys. 2012, 2, 58–65. [Google Scholar] [CrossRef]
- Palladium Hydride. Available online: https://en.wikipedia.org/wiki/Palladium_hydride (accessed on 18 October 2016).
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Nihon Kinzoku Gakkai. Hi Kagaku Ryouronteki Kinzoku Kagobutsu; Maruzen: Tokyo, Japan, 1975; p. 296. [Google Scholar]
- Crystal Structure of the Elements. Available online: http://www.periodictable.com/Properties/A/CrystalStructure.html (accessed on 13 October 2016).
- Lide, D.R. CRC Handbook of Chemistry and Physics, 74th ed.; CRC Press Inc.: Boca Raton, FL, USA, 1993. [Google Scholar]
- Murray, J.L. Ti-Zr binary phase diagram. In ASM Handbook, Vol. 3 Alloy Phase Diagram; Baker, H., Ed.; ASM International: Materials Park, OH, USA, 1992. [Google Scholar]
- Zhu, J.H.; Liu, C.T.; Pike, L.M.; Liaw, P.K. Enthalpies of formation of binary Laves phases. Intermetallics 2002, 10, 579–595. [Google Scholar] [CrossRef]
- Liu, C.T.; Zhu, J.H.; Brady, M.P.; McKamey, C.G.; Pike, L.M. Physical metallurgy and mechanical properties of transition-metal Laves phase alloys. Intermetallics 2000, 8, 1119–1129. [Google Scholar] [CrossRef]
- Johnston, R.L.; Hoffmann, R. Structure-bonding relationships in the Laves phases. Z. Anorg. Allg. Chem. 1992, 616, 105–120. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Fetcenko, M.A. Pressure-composition-temperature hysteresis in C14 Laves phase alloys: Part 1. Simple ternary alloys. J. Alloys Compd. 2009, 480, 428–433. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K.Y.S. Determination of C14/C15 phase abundance in Laves phase alloys. Mater. Chem. Phys. 2012, 135, 520–527. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Li, F.; Ouchi, T.; Koch, J. Effect of vanadium substitution in C14 Laves phase alloys for NiMH battery application. J. Alloys Compd. 2009, 468, 482–492. [Google Scholar] [CrossRef]
- Young, K.; Regmi, R.; Lawes, G.; Ouchi, T.; Fetcenko, M.A.; Wu, A. Effect of aluminum substitution in C14-rich multi-component alloys for NiMH battery application. J. Alloys Compd. 2010, 490, 282–292. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Ouchi, T.; Li, F.; Koch, J. Effect of Sn-substitution in C14 Laves phase alloys for NiMH battery application. J. Alloys Compd. 2009, 469, 406–416. [Google Scholar] [CrossRef]
- Boriskina, N.G.; Kenina, E.M. Phase equilibria in the Ti-TiPd-TiNi system alloys. In Proceedings of the 4th International Conference on Titanium 80, Science & Technology, Kyoto, Japan, 19–22 May 1980; Kimura, H., Izumi, O., Eds.; The Metallurgical Society of AIME: Warrendale, PA, USA, 1980; pp. 2917–2927. [Google Scholar]
- Ghost, G. Nickel-Palladium-Titanium. In Light Metal System. Part 4 Selected Systems from Al-Si-Ti to Ni-Si-Ti; Effenberg, D., Ilyenko, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 425–434. [Google Scholar]
- Thoma, D.J.; Perepezko, J.H. A geometric analysis of solubility ranges in Laves phases. J. Alloys Compd. 1995, 224, 330–341. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Wang, L. Annealing effects on Laves phase-related body-centered-cubic solid solution metal hydride alloys. J. Alloys Compd. 2016, 654, 216–225. [Google Scholar] [CrossRef]
- Bououdina, M.; Soubeyroux, J.L.; De Rango, P.; Fruchart, D. Phase stability and neutron diffraction studies of the laves phase compounds Zr(Cr1−xMox)2 with 0.0 ≤ x ≤ 0.5 and their hydrides. Int. J. Hydrogen Energy 2000, 25, 1059–1068. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Reichman, B.; Fetcenko, M.A. Effect of molybdenum content on structural, gaseous storage, and electrochemical properties of C14-predominated AB2 metal hydride alloys. J. Power Sources 2011, 196, 8815–8821. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Lin, X.; Reichman, B. Effects of Zn-addition to C14 metal hydride alloys and comparisons to Si, Fe, Cu, Y, and Mo-additives. J. Alloys Compd. 2016, 655, 50–59. [Google Scholar] [CrossRef]
- Boettinger, W.J.; Newbury, D.E.; Wang, K.; Bendersky, L.A.; Chiu, C.; Kattner, U.R.; Young, K.; Chao, B. Examination of multiphase (Zr, Ti) (V, Cr, Mn, Ni)2 Ni-MH electrode alloys: Part I. Dendritic solidification structure. Metall. Mater. Trans. 2010, 41A, 2033–2047. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Wang, K.; Boettinger, W.J.; Newbury, D.E.; Young, K.; Chao, B. Examination of multiphase (Zr, Ti) (V, Cr, Mn, Ni)2 Ni-MH electrode alloys: Part II. Solid-state transformation of the interdendric B2 phase. Metall. Mater. Trans. 2010, 41A, 1891–1906. [Google Scholar] [CrossRef]
- Liu, Y.; Young, K. Microstructure investigation on metal hydride alloys by electron backscatter diffraction technique. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Wong, D.F.; Young, K.; Ouchi, T.; Ng, K.Y.S. First-principles point defect models for Zr7Ni10 and Zr2Ni7 phases. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Koch, J.; Fetcenko, M.A. Compositional optimization of vanadium-free hypo-stoichiometric AB2 metal hydride for Ni/MH battery application. J. Alloys Compd. 2012, 510, 97–106. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Reichman, B.; Blankenship, R. Improvement in −40 °C electrochemical properties of AB2 metal hydride alloy by silicon incorporation. J. Alloys Compd. 2013, 575, 65–72. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Meng, T.; Wong, D.F. Studies on the synergetic effects in multi-phase metal hydride alloys. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Nei, J. Effects of vanadium/nickel contents in Laves phase-related body-centered-cubic solid solution metal hydride alloys. Batteries 2015, 1, 34–53. [Google Scholar] [CrossRef]
- Sinha, V.K.; Pourarian, F.; Wallace, W.E. Hydrogenation characteristics of Zr1−xTixMnFe alloys. J. Less Common Met. 1982, 87, 283–296. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Lee, J. The hydrogenation characteristics of Ti-Zr-V-Mn-Ni C14 type Laves phase alloys for metal hydride electrodes. J. Alloys Compd. 1997, 253–254, 601–604. [Google Scholar] [CrossRef]
- Pourbaix, M.J.N.; Van Muylder, J.; De Zoubov, N. Electrochemical properties of the platinum metals. Platinum Met. Rev. 1959, 3, 100–106. [Google Scholar]
- Young, K.; Reichman, B.; Fetcenko, M.A. Electrochemical performance of AB2 metal hydride alloys measured at −40 °C. J. Alloys Compd. 2013, 580, S349–S352. [Google Scholar] [CrossRef]
- Young, K.; Huang, B.; Regmi, R.K.; Lawes, G.; Liu, Y. Comparisons of metallic clusters imbedded in the surface of AB2, AB5, and A2B7 alloys. J. Alloys Compd. 2010, 506, 831–840. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Liu, Y.; Nei, J. Microstructures of the oxides on the activated AB2 and AB5 metal hydride alloys surface. J. Alloys Compd. 2014, 606, 97–104. [Google Scholar] [CrossRef]
- Stucki, F.; Schlapbach, L. Magnetic properties of LaNi5, FeTi, Mg2Ni and their hydrides. J. Less Common Met. 1980, 74, 143–151. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Pawlik, D.; Shen, H.T. Transmission electron microscope studies in the surface oxide on the La-containing AB2 metal hydride alloy. J. Alloys Compd. 2016, 672, 356–365. [Google Scholar] [CrossRef]
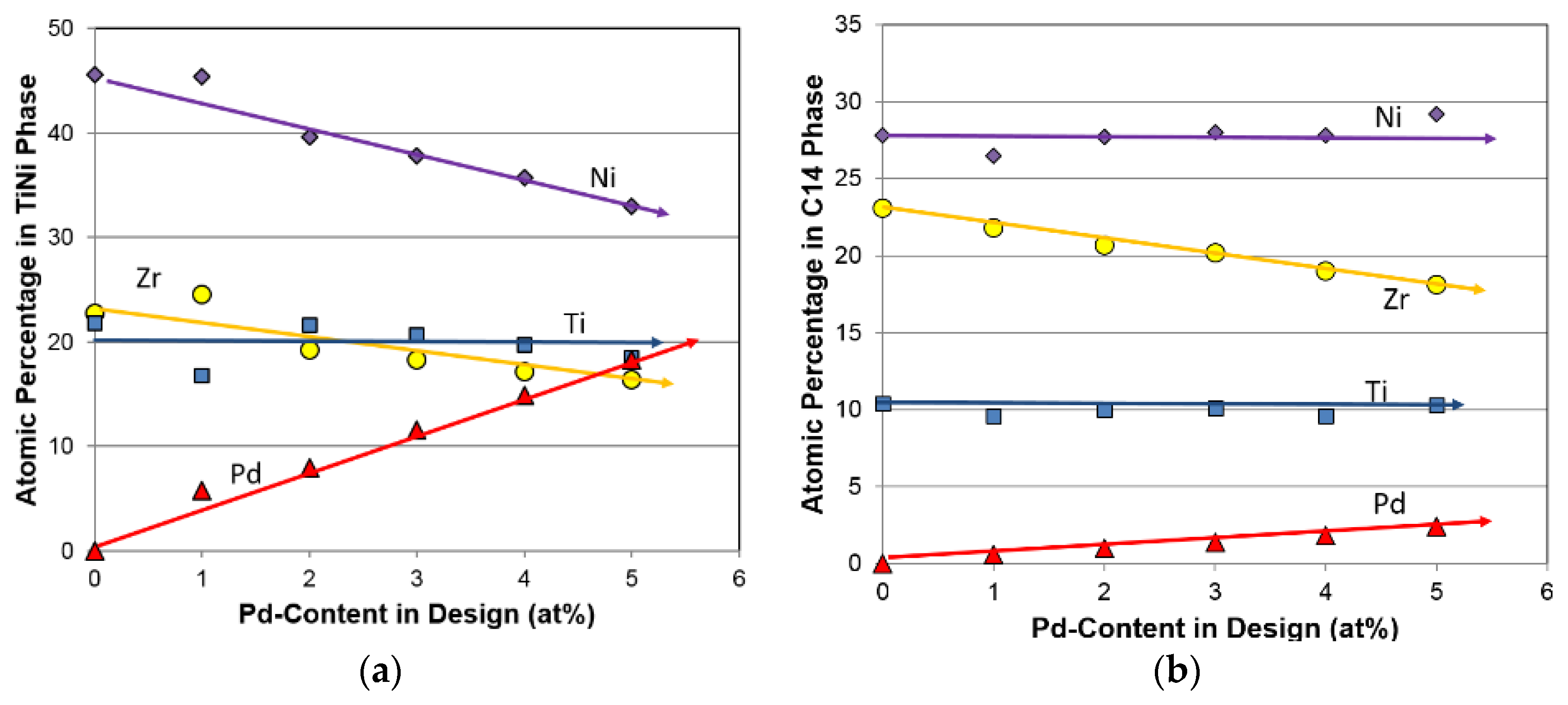
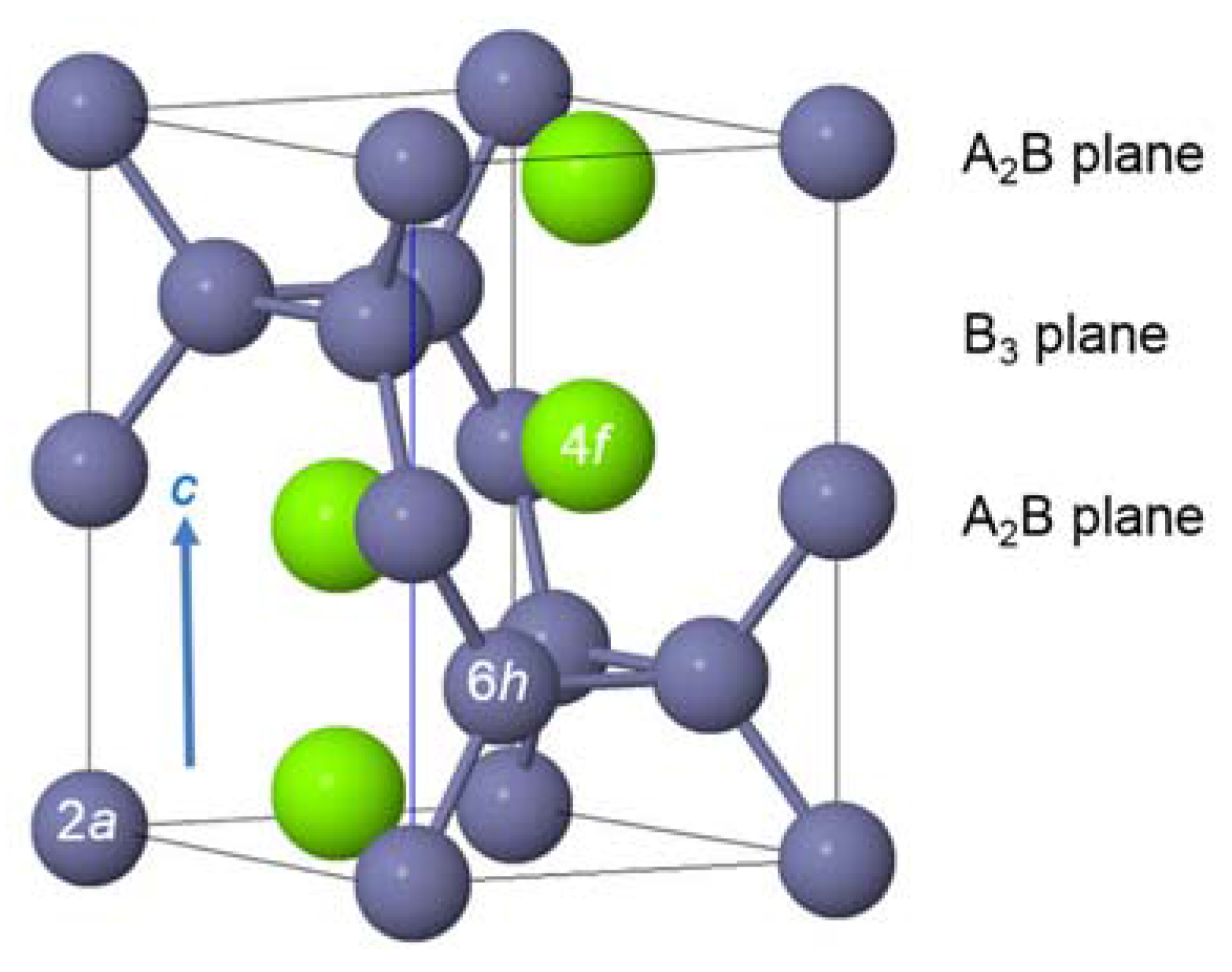
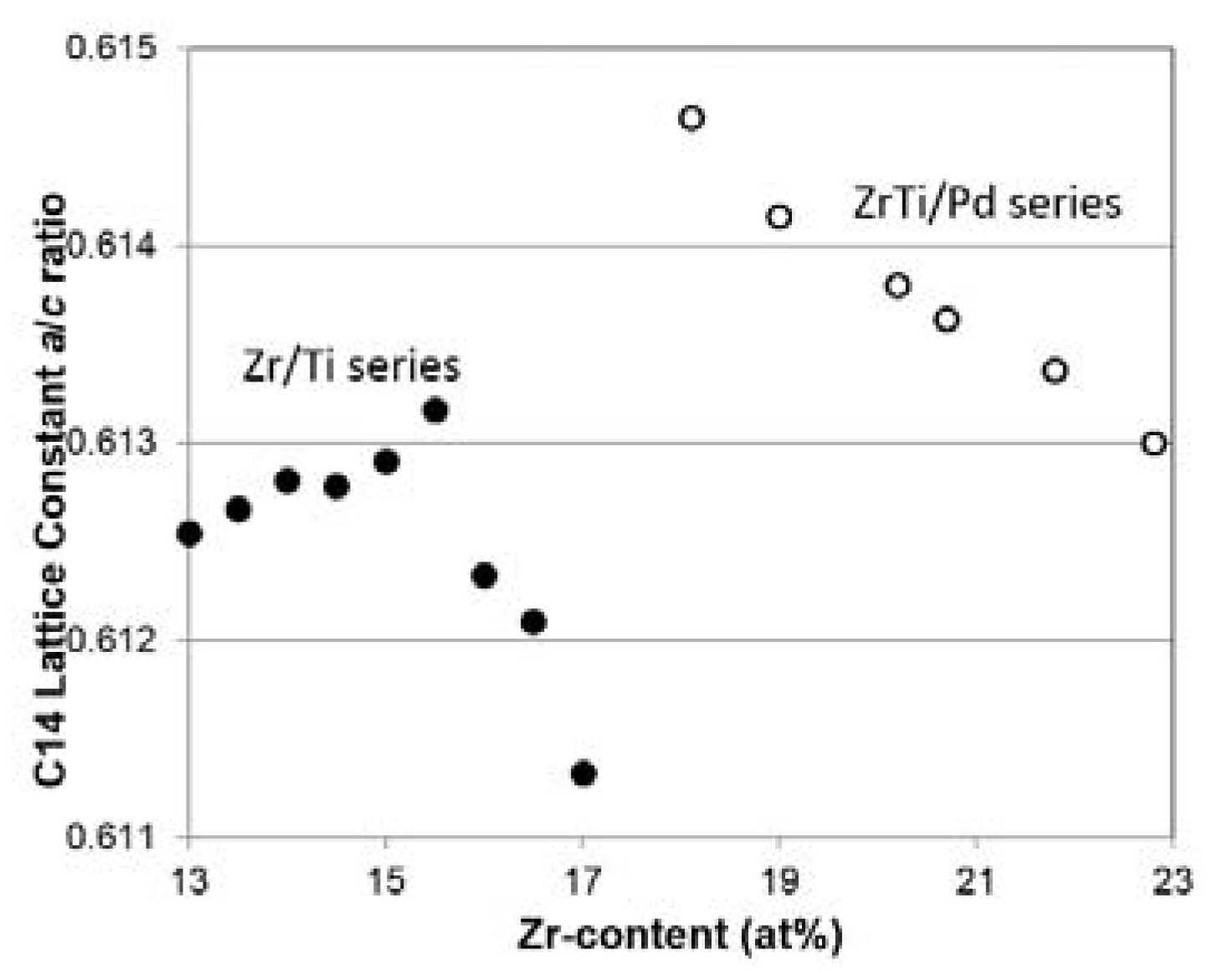

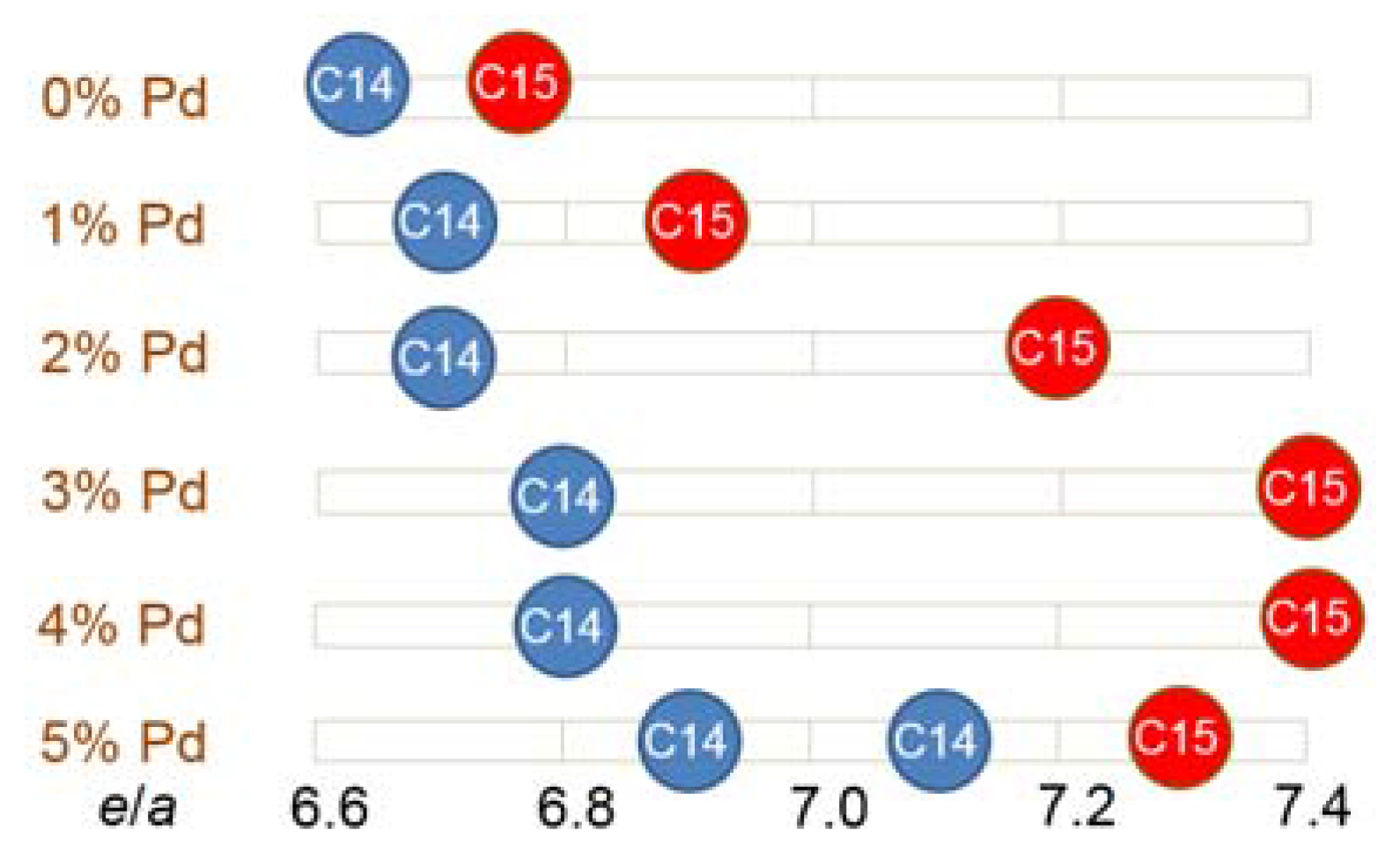




| Host | Preparation | Application | Amount | Major Effect(s) of Pd | Reference |
|---|---|---|---|---|---|
| LaNi5 | AM | GP | 16 at % | Increased plateau pressure | [47] |
| Mg2Ni | RD | GP | 8.3 at % | Increased absorption kinetics | [48] |
| Mg2Ni | MA | GP | <1 wt. % | Increased absorption kinetics | [49] |
| TiFe | MA | GP | <1 wt. % | Increased activation | [50] |
| V3TiNi0.56 | AM | EC | 1 & 5 at % | Increased capacity | [51] |
| TiFe | AM + TA | GP | 2.5 to 15 at % | Increased activation | [52] |
| Ti2Ni | AM | EC | 9.6 at % | Increased HRD and cycle life | [53] |
| AB2 | AM | EC | 3.3 at % | Increased cycle life | [54] |
| Mg | MA | GP | 14 wt. % | Increased desorption kinetics | [55] |
| AB2 | IM | EC | 1 to 4 at % | Increased HRD | [56] |
| Mg2Ni | MS | EC | 5 to 20 at % | Increased capacity and cycle life | [57,58] |
| MgNix | MS | EC | 10 at % | Easy amorphization | [59] |
| MgNi | MA | EC | 1 to 10 at % | Increased cycle life | [60] |
| MgNi | MA | EC | 10 at % | Increased cycle life | [61] |
| TiFe | AM | EC | 5 to 10% | Increased EC activity | [62] |
| Mg0.9Ti0.1Ni | MA | EC | 0 to 7.5 at % | Increased cycle life and Io | [63,64,65,66] |
| Li3BN2 | MA | GP | 5 to 10 wt. % | Increased desorption kinetics | [67] |
| Mg | MA | GP | 5 wt. % | Decreased absorption kinetics | [68] |
| Mg | MA | GP | 10 wt. % | Increased desorption kinetics | [69] |
| MgTix | MA | EC | 5 at % | Increased activation | [70] |
| Mg6Pd7Si3 | TA | GP | 44 at % | Increased cycle life | [71] |
| LaMg2Pd | TA | GP | 25 at % | Novel MH alloy | [72] |
| TiVCr | AM | GP | 0 to 0.5 at % | Increased capacity and activation | [73] |
| TiVCr | LM | EC | 0 to 3 at % | Increased capacity, cycle life, and activation | [74] |
| TiZrNi | AM | EC | 0 to 7 at % | Increased capacity, HRD, and cycle life | [75] |
| MgNi | MA | EC | 0 to 5 at % | Increased HRD and cycle life | [76] |
| C | WI | GP | 0 to 6 at % | Increased capacity | [77] |
| MgTi | MA | EC | 3.3 at % | Increased capacity and Io | [78] |
| AB2 | AM + TA | EC | 5 to 10 wt. % | Increased HRD | [79] |
| Mg2Ni | MA | EC | 10 wt. % | Increased capacity | [80] |
| TiNi | IM | GP | 0 to 2.5 at % | Decreased Capacity | [81] |
| MgNi | MA | EC | 3.5 at % | Increased capacity, HRD, and cycle life | [82] |
| LaNi5 | AM + TA | GP | 4 to 25 at % | Increase in plateau pressure | [83] |
| Mg2Ni | MA | EC | 3.3 at % | Increased capacity and cycle life | [84] |
| C | WI | GP | 5 wt. % | Decreased absorption kinetics | [85] |
| MgNi | MA | EC | 5 at % | Increased HRD and cycle life | [86] |
| MgNi | MA | EC | 0 to 5 at % | Increased capacity and cycle life | [87] |
| Mg | MA | GP | 0.1 to 5 wt. % | Increased absorption and desorption | [88] |
| MgNi | MA | EC | 0 to 4 at % | Increased capacity | [89] |
| LaMg2Ni | IM | GP | 5 at % | Increased absorption and desorption | [90] |
| TiNi | MA | EC | 5 wt. % | Increased capacity and cycle life | [91] |
| Mg2Co | MA | EC | 5 at % | Increased capacity, Io, and cycle life | [92,93] |
| WMCNT | WI | GP | 5 wt. % | Increased capacity | [94] |
| Na2SiO3 | TA | GP | 2.5 to 5 wt. % | Increased capacity | [95] |
| Graphene | WI | GP | 5 to 10 wt. % | Increased capacity | [96] |
| TiNi, Ti2Ni | MA + TA | EC | 5 wt. % | Increased capacity and cycle life | [97] |
| C | WI | GP | 0 to 13 wt. % | Increased capacity | [98] |
| Mg6Pd | TA | GP | 14 at % | Novel MH alloy | [99] |
| TiVCr | AM | GP | 0.05 to 0.1 at % | Increased capacity | [100] |
| MgCo | MA | EC | 5 at % | Increased HRD | [101] |
| PdCu, PdCuAg | MA | GP | 15 to 100 at % | Increased capacity | [102] |
| Property | Zr | Ti | Pd | V | Cr | Mn | Co | Ni | Al |
|---|---|---|---|---|---|---|---|---|---|
| Atomic Number | 40 | 22 | 46 | 23 | 24 | 25 | 27 | 28 | 13 |
| Number of Outer-layer e− | 4 | 4 | 10 | 5 | 6 | 7 | 9 | 10 | 3 |
| Earth Crust Abundance (%) | 0.013 | 0.66 | 6 × 10−7 | 0.019 | 0.014 | 0.11 | 0.003 | 0.009 | 8.1 |
| Radius (Å) [112] | 1.771 | 1.614 | 1.521 | 1.491 | 1.423 | 1.428 | 1.385 | 1.377 | 1.582 |
| Electronegativity | 1.33 | 1.54 | 2.20 | 1.63 | 1.66 | 1.55 | 1.88 | 1.91 | 1.61 |
| Crystal Structure [113] | hcp | hcp | fcc | bcc | bcc | bcc | hcp | fcc | fcc |
| Melting Point (°C) [114] | 1855 | 1668 | 1555 | 1910 | 1907 | 1246 | 1495 | 1455 | 660 |
| ΔHh (kJ·mol−1) [11] | −94 | −67 | −20 | −35 | −8 | −8 | 15 | −3 | 3 |
| Number of IMC with Ni [115] | 8 | 3 | 0 | 3 | 0 | 0 | 0 | - | 4 |
| Alloy | Source | Ti | Zr | V | Cr | Mn | Co | Ni | Pd | Al | e/a | B/A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pd0 | Design | 12.0 | 22.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 0.0 | 0.4 | 6.771 | 1.87 |
| ICP | 11.9 | 22.9 | 10.0 | 7.5 | 8.0 | 7.1 | 32.2 | 0.0 | 0.4 | 6.773 | 1.87 | |
| Pd1 | Design | 12.0 | 21.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 1.0 | 0.4 | 6.831 | 1.87 |
| ICP | 12.0 | 21.3 | 10.3 | 7.5 | 8.5 | 7.0 | 31.9 | 1.1 | 0.4 | 6.834 | 1.91 | |
| Pd2 | Design | 12.0 | 20.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 2.0 | 0.4 | 6.891 | 1.87 |
| ICP | 12.0 | 20.5 | 9.9 | 7.5 | 8.6 | 6.9 | 32.2 | 2.0 | 0.4 | 6.900 | 1.90 | |
| Pd3 | Design | 12.0 | 19.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 3.0 | 0.4 | 6.951 | 1.87 |
| ICP | 12.0 | 19.5 | 10.1 | 7.5 | 8.6 | 7.0 | 32.1 | 2.8 | 0.4 | 6.949 | 1.92 | |
| Pd4 | Design | 12.0 | 18.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 4.0 | 0.4 | 7.011 | 1.87 |
| ICP | 11.9 | 18.7 | 10.1 | 7.6 | 8.7 | 7.0 | 31.9 | 3.7 | 0.4 | 6.996 | 1.92 | |
| Pd5 | Design | 12.0 | 17.8 | 10.0 | 7.5 | 8.1 | 7.0 | 32.2 | 5.0 | 0.4 | 7.071 | 1.87 |
| ICP | 11.9 | 17.8 | 10.2 | 7.4 | 8.6 | 7.1 | 32.0 | 4.6 | 0.4 | 7.055 | 1.92 |
| Structural Property | Pd0 | Pd1 | Pd2 | Pd3 | Pd4 | Pd5 |
|---|---|---|---|---|---|---|
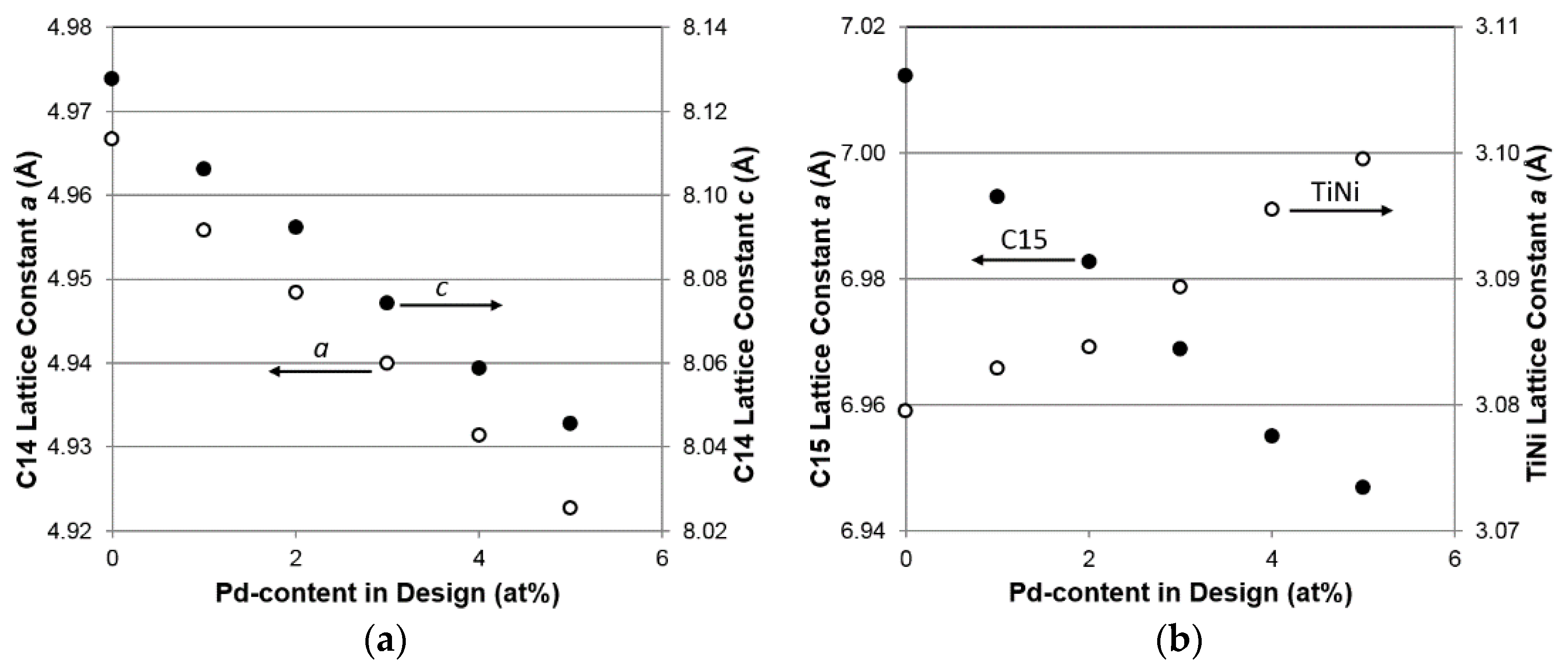
| a, C14 (Å) | 4.9739 | 4.9631 | 4.9561 | 4.9471 | 4.9394 | 4.9328 |
| c, C14 (Å) | 8.1134 | 8.0915 | 8.0767 | 8.0598 | 8.0427 | 8.0254 |
| a/c, C14 (Å) | 0.61305 | 0.61337 | 0.61363 | 0.61380 | 0.61415 | 0.61465 |
| VC14 (Å3) | 173.83 | 172.61 | 171.81 | 170.83 | 169.93 | 169.12 |
| a, C15 (Å) | 7.0121 | 6.9929 | 6.9827 | 6.9689 | 6.9550 | 6.9468 |
| a, TiNi (Å) | 3.0795 | 3.0829 | 3.0846 | 3.0893 | 3.0955 | 3.0995 |
| FWHM, C14 (103) | 0.237 | 0.25 | 0.249 | 0.241 | 0.234 | 0.243 |
| C14 Crystallite Size (Å) | 482 | 446 | 448 | 469 | 491 | 465 |
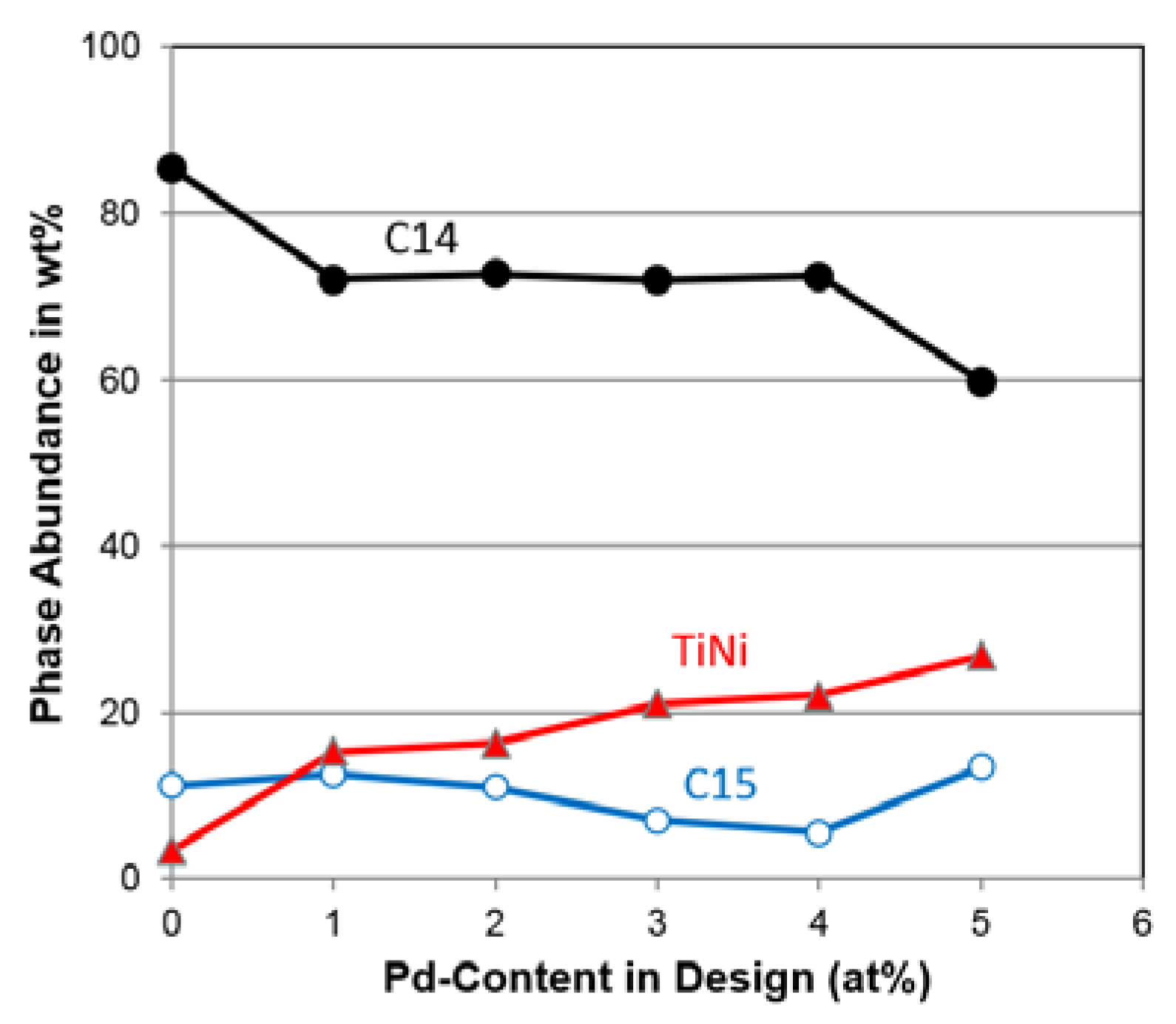
| C14 Abundance (%) | 85.4 | 72.1 | 72.7 | 72.0 | 72.4 | 59.8 |
| C15 Abundance (%) | 11.2 | 12.6 | 11.0 | 7.0 | 5.5 | 13.4 |
| TiNi Abundance (%) | 3.4 | 15.3 | 16.3 | 21.0 | 22.1 | 26.8 |
| Alloy | Location | Ti | Zr | V | Cr | Mn | Co | Ni | Pd | Al | e/a | B/A | Phase |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pd1 | Pd1-1 | 16.8 | 24.5 | 1.4 | 0.5 | 2.3 | 2.7 | 45.4 | 5.8 | 0.5 | 7.3 | 1.1 | TiNi |
| Pd1-2 | 13.3 | 20.8 | 9.2 | 5.1 | 8.2 | 6.7 | 35.2 | 1.0 | 0.5 | 6.9 | 1.8 | C15 | |
| Pd1-3 | 9.6 | 21.8 | 12.4 | 10.6 | 10.2 | 7.8 | 26.5 | 0.6 | 0.5 | 6.7 | 2.1 | C14 | |
| Pd1-4 | 7.8 | 58.9 | 5.0 | 2.6 | 4.2 | 3.0 | 17.4 | 0.9 | 0.3 | - | - | ZrO2 | |
| Pd2 | Pd2-1 | 21.6 | 19.2 | 2.1 | 0.9 | 3.6 | 4.3 | 39.6 | 8.0 | 0.7 | 7.2 | 1.0 | TiNi |
| Pd2-2 | 11.6 | 20.8 | 7.2 | 4.0 | 7.1 | 5.1 | 40.9 | 2.8 | 0.5 | 7.2 | 1.8 | C15 | |
| Pd2-3 | 10.0 | 20.7 | 12.4 | 9.6 | 10.1 | 8.0 | 27.7 | 1.0 | 0.5 | 6.7 | 2.2 | C14 | |
| Pd2-4 | 5.7 | 69.1 | 3.3 | 2.3 | 3.0 | 2.3 | 12.5 | 1.6 | 0.3 | - | - | ZrO2 | |
| Pd3 | Pd3-1 | 20.7 | 18.3 | 2.0 | 0.8 | 4.3 | 3.8 | 37.8 | 11.6 | 0.7 | 7.3 | 1.0 | TiNi |
| Pd3-2 | 12.7 | 19.7 | 6.0 | 3.0 | 6.4 | 4.5 | 41.9 | 5.3 | 0.5 | 7.4 | 1.7 | C15 | |
| Pd3-3 | 10.1 | 20.2 | 11.9 | 10.1 | 9.7 | 8.1 | 28.0 | 1.4 | 0.4 | 6.8 | 2.2 | C14 | |
| Pd3-4 | 19.5 | 51.8 | 3.3 | 1.5 | 3.2 | 2.2 | 15.3 | 3.0 | 0.2 | - | - | ZrO2 | |
| Pd4 | Pd4-1 | 19.7 | 17.2 | 2.0 | 0.8 | 5.4 | 3.4 | 35.7 | 14.9 | 0.8 | 7.4 | 0.9 | TiNi |
| Pd4-2 | 10.7 | 18.9 | 7.5 | 4.2 | 6.9 | 4.8 | 40.8 | 5.7 | 0.6 | 7.4 | 1.8 | C15 | |
| Pd4-3 | 9.6 | 19.0 | 12.1 | 10.9 | 10.2 | 8.1 | 27.8 | 1.8 | 0.5 | 6.8 | 2.3 | C14 | |
| Pd4-4 | 3.6 | 80.1 | 1.8 | 1.0 | 1.3 | 1.3 | 7.6 | 3.2 | 0.1 | - | - | ZrO2 | |
| Pd4-5 | 1.7 | 0.2 | 42.8 | 41.1 | 8.8 | 2.4 | 2.8 | 0.1 | 0.1 | - | - | BCC | |
| Pd5 | Pd5-1 | 18.5 | 16.4 | 2.3 | 1.0 | 6.6 | 3.1 | 33.0 | 18.3 | 0.8 | 7.5 | 0.9 | TiNi |
| Pd5-2 | 12.4 | 18.0 | 7.7 | 4.1 | 6.7 | 4.7 | 38.7 | 7.2 | 0.5 | 7.3 | 1.7 | C15 | |
| Pd5-3 | 12.2 | 16.6 | 10.7 | 6.8 | 8.8 | 7.4 | 33.6 | 3.5 | 0.4 | 7.1 | 2.1 | C14 | |
| Pd5-4 | 10.3 | 18.1 | 11.5 | 10.4 | 9.4 | 8.3 | 29.2 | 2.4 | 0.4 | 6.9 | 2.2 | C14 | |
| Pd5-5 | 7.3 | 51.4 | 7.3 | 5.0 | 5.2 | 4.2 | 17.4 | 2.0 | 0.2 | - | - | ZrO2 |
| Gaseous Phase Property | Unit | Pd0 | Pd1 | Pd2 | Pd3 | Pd4 | Pd5 |
|---|---|---|---|---|---|---|---|
| Maximum capacity @ 2 MPa and 30 °C | wt. % | 1.52 | 1.55 | 1.40 | 1.20 | 1.02 | 0.76 |
| Reversible Capacity @ 30 °C | wt. % | 1.25 | 1.32 | 1.23 | 1.11 | 0.92 | 0.69 |
| Capacity @ 0.002 MPa and 30 °C | wt. % | 0.30 | 0.23 | 0.17 | 0.10 | 0.09 | 0.06 |
| Desorption Pressure @ 0.75 wt. % and 30 °C | MPa | 0.021 | 0.048 | 0.112 | 0.306 | 0.745 | 1.882 |
| Slope Factor @ 30 °C | % | 78 | 81 | 78 | 66 | 53 | 44 |
| Hysteresis @ 30 °C | 0.21 | 0.05 | 0.03 | 0.05 | 0.03 | 0.00 | |
| −ΔHh | kJ·mol H2−1 | 41.6 | 40.5 | 35.6 | 30.9 | 28.0 | - |
| −ΔSh | J·mol H2−1·K−1 | 127 | 125 | 118 | 111 | 109 | - |
| Electrochemical and Magnetics Properties | Unit | Pd0 | Pd1 | Pd2 | Pd3 | Pd4 | Pd5 |
|---|---|---|---|---|---|---|---|
| 3rd Cycle High-rate Discharge Capacity | mAh·g−1 | 300 | 335 | 327 | 285 | 226 | 143 |
| 3rd Cycle Full Discharge Capacity | mAh·g−1 | 376 | 349 | 330 | 288 | 228 | 150 |
| 3rd Cycle HRD | % | 80 | 96 | 99 | 99 | 99 | 98 |
| Activation Cycle # to Achieve 92% HRD | 6 | 1 | 1 | 1 | 1 | 1 | |
| Diffusion Coefficient, D | 10−10 cm2·s−1 | 2.1 | 4.4 | 6.2 | 2.0 | 4.1 | 4.5 |
| Surface Reaction Current, Io | mA·g−1 | 12.8 | 24.7 | 28.8 | 25.2 | 22.1 | 17.1 |
| Charge-transfer Resistance @ −40 °C | Ω·g | 158.6 | 29.1 | 28.3 | 22.9 | 15.7 | 39.9 |
| Double-layer Capacitance @ −40 °C | F·g−1 | 0.18 | 0.16 | 0.15 | 0.16 | 0.13 | 0.10 |
| RC Product @ −40 °C | s | 28.4 | 4.8 | 4.2 | 3.6 | 2.0 | 4.0 |
| Total Saturated Magnetic Susceptibility, MS | emu·g−1 | 0.035 | 0.015 | 0.008 | 0.018 | 0.011 | 0.013 |
| Applied Field @ M.S. = ½ MS, H1/2 | kOe | 0.50 | 0.47 | 0.34 | 0.61 | 0.77 | 0.36 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.-H.; Ouchi, T.; Nei, J.; Chang, S. Increase in the Surface Catalytic Ability by Addition of Palladium in C14 Metal Hydride Alloy. Batteries 2017, 3, 26. https://doi.org/10.3390/batteries3030026
Young K-H, Ouchi T, Nei J, Chang S. Increase in the Surface Catalytic Ability by Addition of Palladium in C14 Metal Hydride Alloy. Batteries. 2017; 3(3):26. https://doi.org/10.3390/batteries3030026
Chicago/Turabian StyleYoung, Kwo-Hsiung, Taihei Ouchi, Jean Nei, and Shiuan Chang. 2017. "Increase in the Surface Catalytic Ability by Addition of Palladium in C14 Metal Hydride Alloy" Batteries 3, no. 3: 26. https://doi.org/10.3390/batteries3030026





