1. Introduction
Lithium-ion (Li-ion) battery technology can enable a broad introduction of electrified vehicles, mainly due to its high energy capacity. Li-ion batteries also have other important properties, e.g., long lifetime and the possibility of fast charging. However, Li-ion batteries have a drawback compared to most other battery technologies in that the electrolyte is flammable and the battery may go into a thermal runaway, that is, the battery may self-heat, resulting in a rapid pressure and temperature increase in the cell; this will release flammable and toxic gases but can also cause projectiles and fire [
1,
2,
3,
4,
5,
6,
7]. Thermal runaway may happen when the battery moves out of the stable operating window of the Li-ion cell and can be caused by, e.g., short circuiting, overheating, overcharging or mechanical damage.
Li-ion batteries are used in very large numbers for consumer products like cell phones, laptop computers, etc. Incidents have occurred with these batteries, but the consequences are in most cases not that serious due to the limited size of the batteries. With the increased number of electric vehicles (EVs) on the roads, the safety issues surrounding Li-ion technology have become more important, taking into consideration the large size of the batteries in automotive applications. Incidents involving EVs have indeed happened, some of them resulting in fires. But these fires have not yet resulted in any more serious consequences.
Notable EV fires include three car fires involving the battery EV (BEV) Tesla Model S that occurred in 2013. In two of them, the driver hit road debris at highway speed, while one was caused by a crash into a concrete barrier and a tree resulting in significant deformations. The first fire was a result of penetration from beneath of the battery pack. Mass media attention was high regarding these incidents and the fires caused a drop in Tesla Motors stock prices. Up to the present time, the authors are aware of three additional incidents involving the Tesla Model S, however, possibly caused by electrical faults outside of the vehicle. In any case, compared to the annual average number of automobile fires in the USA, of the order of 1/1000 automobiles [
8], the number of car fires in the Tesla Model S is significantly lower. Larsson
et al. [
9] estimated in 2014 the number of fires in the Tesla Model S as 1/10,000 cars. With the increased statistics now available, although with still limited amounts of data, and with the sales numbers of more than 100,000 Model S vehicles, the estimate deceases somewhat to about 1/20,000 cars. This comparison does not take into account the age of the cars involved, as older cars may be more prone to fires, but it still shows that the risks involving EVs should not be overstated. In 2014, the National Highway Traffic Safety Administration (NHTSA) investigated the fires and did not find any defect trends [
10], but Tesla did voluntarily chose to reinforce the underbody of their cars with arming plates [
11] in order to lower the frequency and the effect of hitting road debris.
Other incidents include the Fisker Karma plug-in hybrid EV (PHEV). In October 2012, Hurricane Sandy caused the flooding of a harbor in Newark, New Jersey. The flooding lasted several hours and, thereafter, 16 brand-new Fisker Karma were destroyed by fire. The cars were completely covered with salt water during the flooding, an extreme situation where electrical short circuits are likely to occur. Mass media attention was high on the Fisker Karma fires even though other vehicles including other PHEVs/hybrid EVs (HEVs) also burnt. Prior to Hurricane Sandy, some other fire incidents occurred involving Fisker Karma, one of them outside a supermarket shortly after the driver left the car. These incidents are examples where EV fires have been the focus of the mass media. Other fires have happened, during charging or as spontaneous fires, but have not gained as much media interest.
Besides the few incidents in electrified vehicles, incidents have occurred in other situations. The Boeing 787 Dreamliner Li-ion battery fire incidents in 2013–2014 [
12], as well as serious accidents on cargo airplanes involving Li-ion batteries in the cargo hold, have increased the awareness of the safety risks associated with this type of battery [
13]. In 2016, the Federal Aviation Administration (FAA) warned that there is a risk of catastrophic aircraft loss if a Li-ion battery fire or explosion occurs in the cargo hold since existing fire suppression systems cannot control such a fire [
14]. As a consequence, the ICAO Air Navigation Commission (ANC) has issued strict regulations, effective 1 April 2016, for the transportation of Li-ion batteries as cargo on passenger aircraft [
15].
These incidents and their consequences clearly demonstrate the necessity of putting safe vehicles on the market, not only for the safety of humans in or near the vehicles, but also for economic and environmental reasons. The EV has the potential to be safer than conventional combustion engine cars, simply because the main fire source, gasoline/diesel, is removed [
16]. In any case, the safety of a battery system depends on several things, e.g., cell chemistry, cell design and system design, including thermal management system and control strategies. Common cathode chemistries contain cobalt, e.g., lithium cobalt oxide (LCO), LiCoO
2, lithium nickel manganese cobalt (NMC), LiNi
xMn
yCo
zO
2, and lithium nickel cobalt aluminum (NCA), LiNi
xCo
yAl
zO
2. Lithium phosphates [
17] are also used, e.g., lithium iron phosphate (LFP), LiFePO
4. For the anode, various forms of carbon are dominant, while lithium titanate oxide (LTO), Li
4Ti
5O
12, is used in lower volumes. This paper focuses mainly on carbon-LFP cells, which are currently seen as state of the art on the market when it comes to safety, although many battery systems for automotive applications use less stable chemistries in order to obtain, e.g., higher energy density. Abuse test results from cell level are presented and their impact is discussed on battery system and vehicle level.
4. Fire Characteristics on Cell Level
The measurement and gas collection system of the single burning item (SBI) apparatus were used for the fire tests. The SBI apparatus is normally used for the classification of building materials according to the European Classification scheme EN13823 [
18]. The experimental setup is shown in
Figure 4. The battery cells were placed on a wire grating. A 15 kW propane burner was placed underneath the cells and was ignited 2 min after the start of the test.
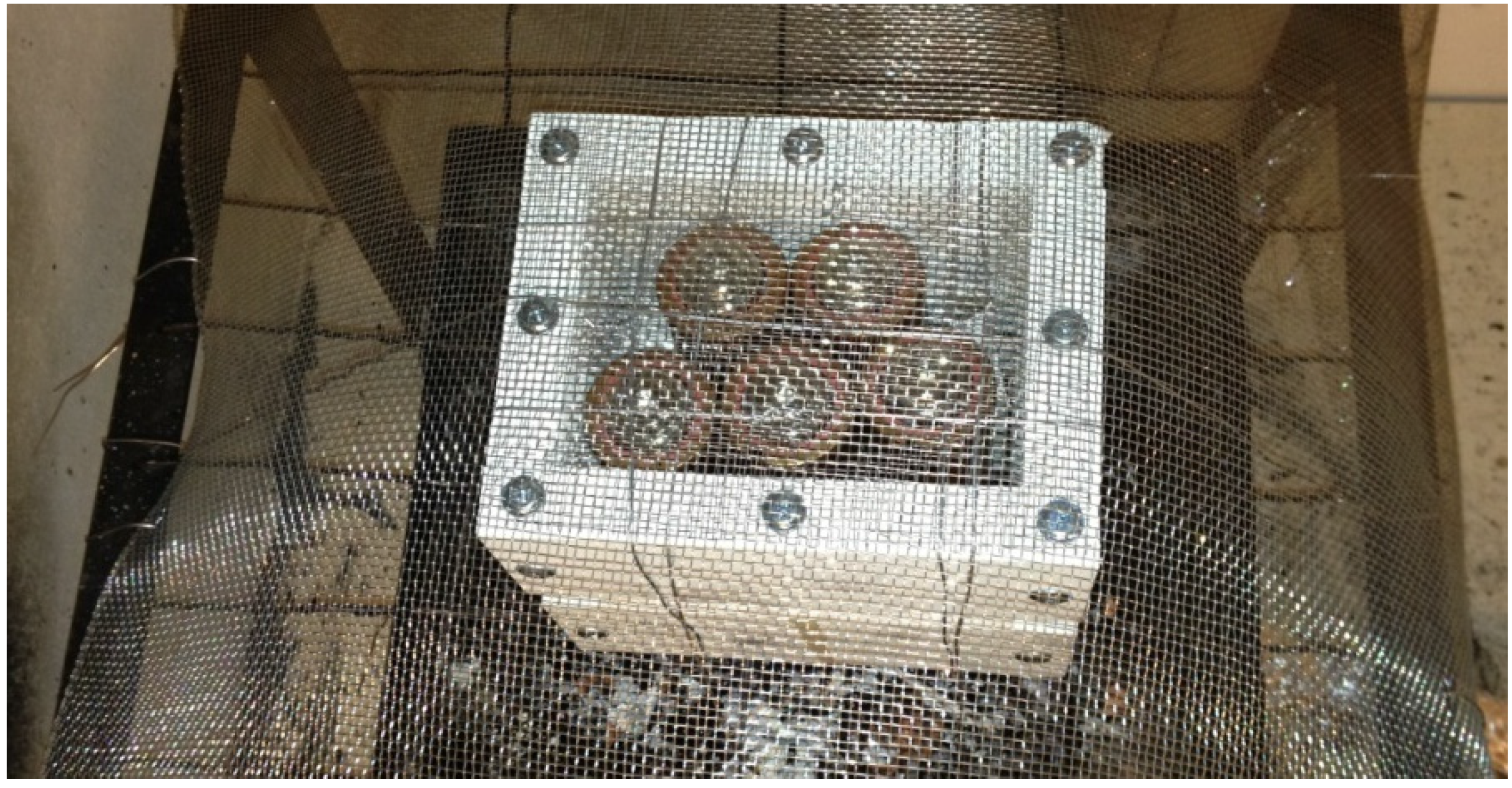
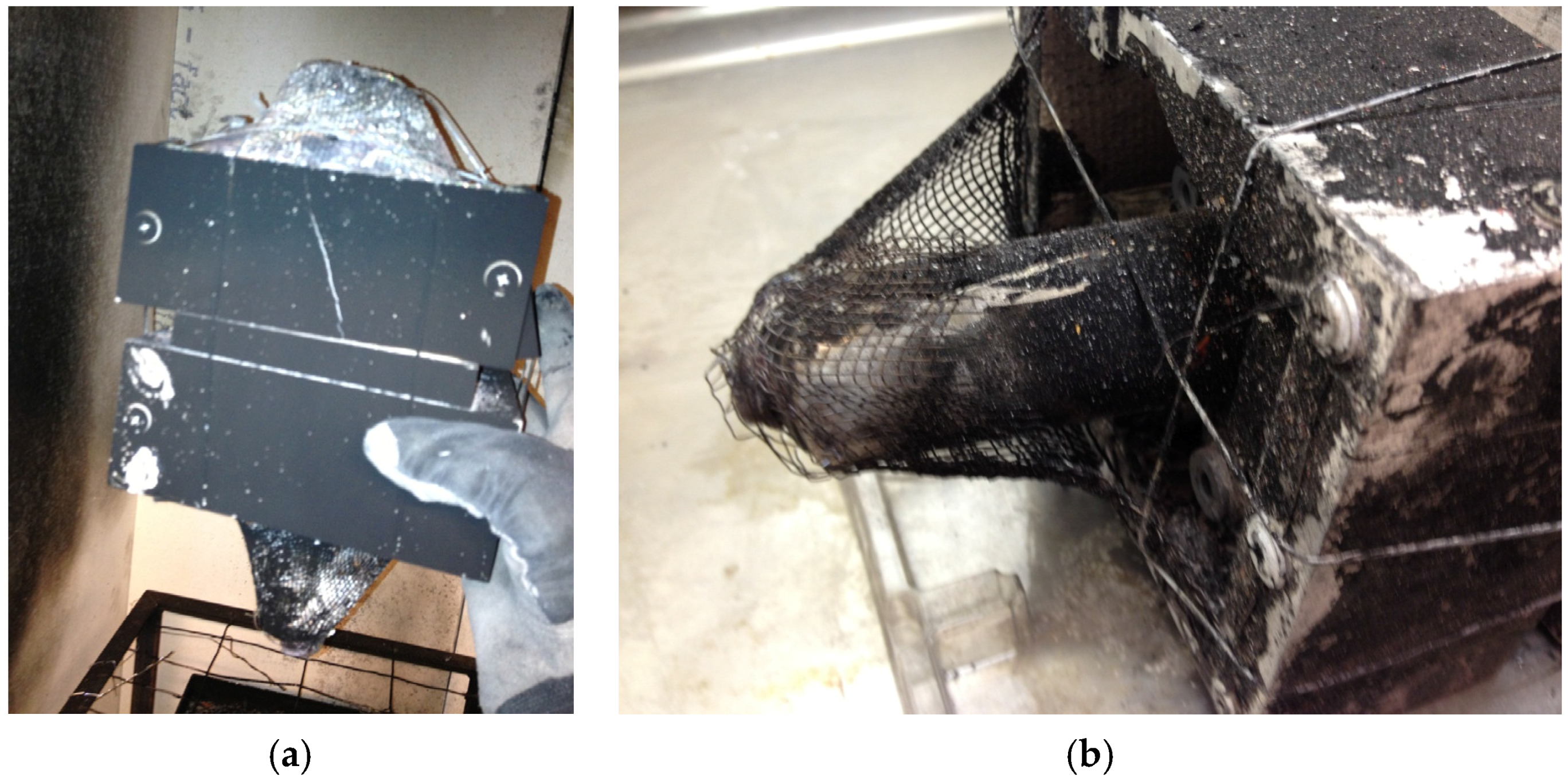
Tests were performed on EiG and Lifetech cells. Five cells were tested at the same time. The EiG cells were fastened together with steel wire, while the Lifetech cells were placed inside a protection box made of walls of non-combustible silica board and steel net at the bottom and top. Additionally, a secondary layer of steel net was used at the top, nailed to the wire grating to protect from hazardous projectiles (
Figure 5). A blank test was conducted at the beginning of each test day in order to make a blank for the gas analysis and to measure the burner influence on the heat release rate (HRR). HRR values were calculated by the oxygen consumption method and corrected for CO
2 [
18]. The gases from the fire were collected in the duct flow as seen in
Figure 4. In the tests of EiG cells with 100% SOC a duct flow of 0.6 m
3/s was used, while for the other tests of EiG cells and for the Lifetech cells, the flow was decreased to 0.4 m
3/s in order to increase emission concentrations. All tests were video recorded. A heated (180 °C) sub-flow was taken out to the Fourier transform infrared spectroscopy (FTIR, Thermo Fisher Scientific, Waltham, MA, USA) with an Antaris IGS analyzer (Nicolet), with a gas cell (heated to 180 °C), that measured gases, e.g., hydrogen fluoride (HF). Each test used a fresh primary filter (heated to 180 °C) which was analyzed for fluoride content after the test. All fluoride found was assumed to be in the form of HF. For the measured HRR the combined expanded uncertainty is +/−5 kW. The detection limit was 2 ppm for HF. For a detailed description of the experiment, see Larsson
et al. [
4] and Andersson
et al. [
19].
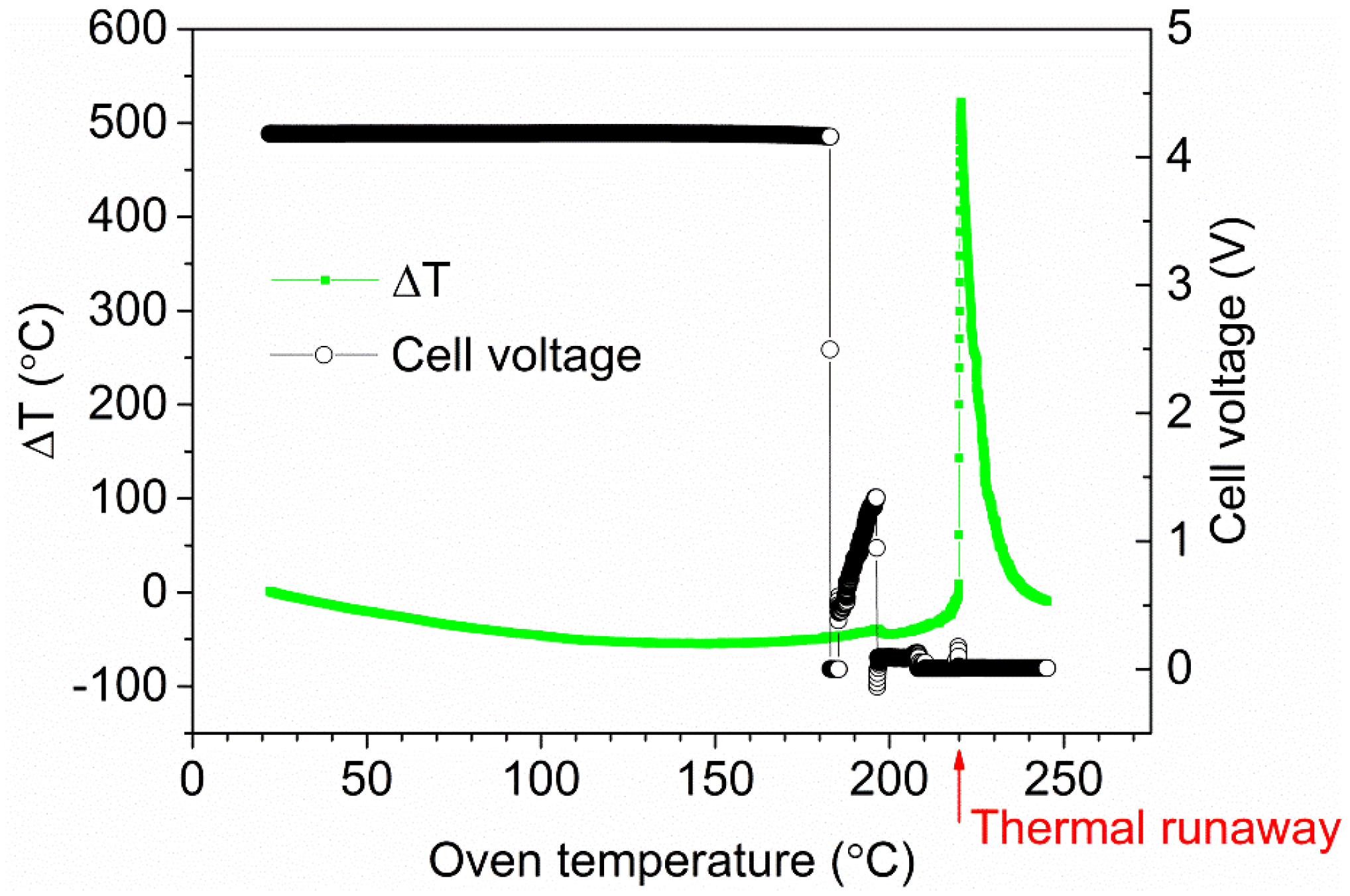
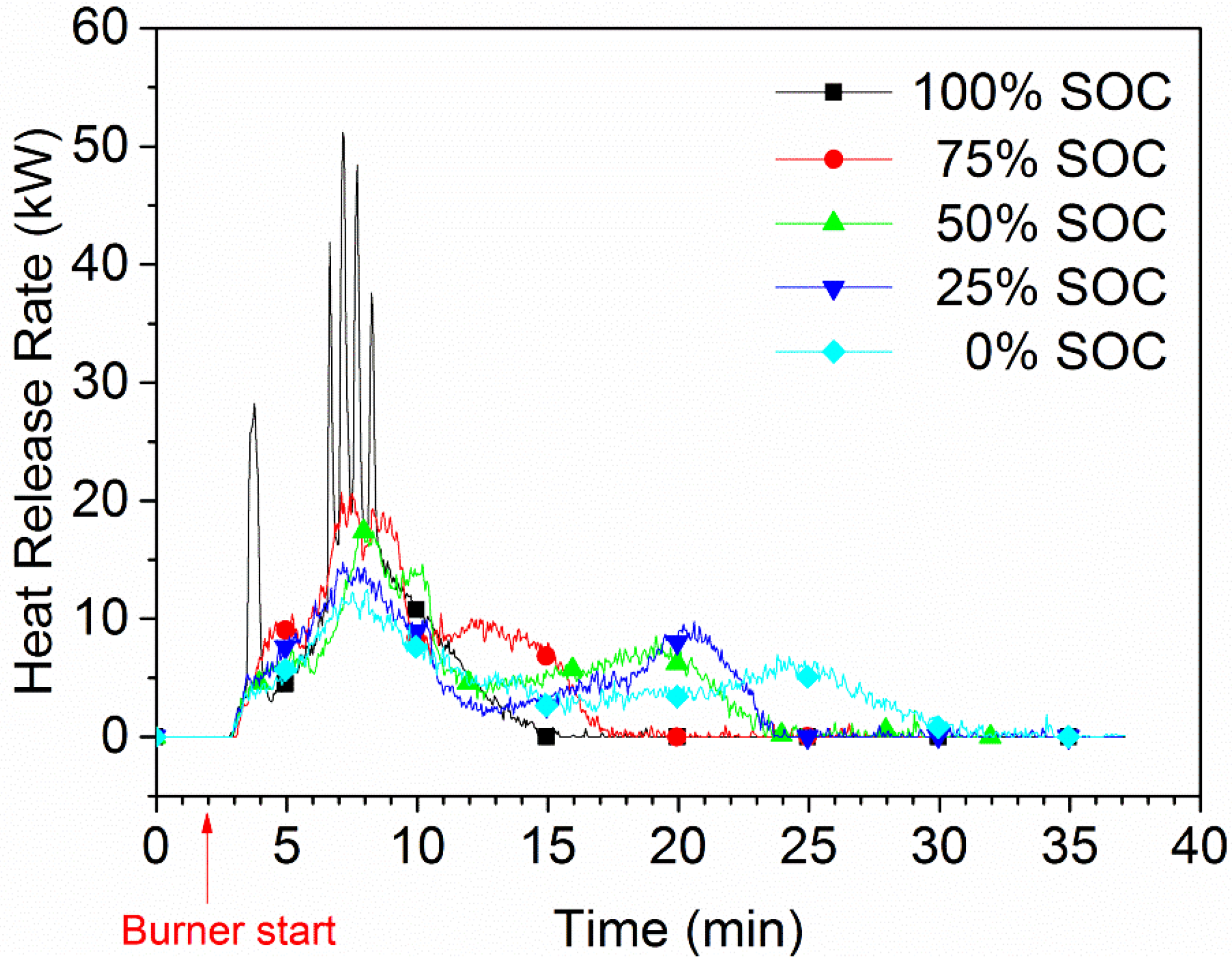
The HRR for various SOC levels for a five-cell-pack of EiG cells is shown in
Figure 6. A strong dependence between SOC and HRR can be observed, and lower SOC values result in lower HRR peaks. For a 100% SOC, there are rapid heat releases and outbursts, one per cell, while no outburst or HRR peak can be seen for cells with a lower SOC.
For an example of an outburst see
Figure 7. The total heat release (THR) has a relatively low dependence on SOC and was roughly 8 MJ for the five-cell-pack, corresponding to 6.5 MJ/kg battery cell. Ribière
et al. [
5] found, based on an 11 Wh pouch cell with LiMn
2O
4 (LMO) cathode, a heat of combustion of 4 MJ/kg, which is in the same order as that measured in our study.
The nominal energy content of the five-cell-pack is 112 Wh. Electrified vehicles typically have 10–30 kWh of batteries, and an extrapolation of our values to the energy released for this size of battery pack gives a THR of 700–2100 MJ, which corresponds to a fire of about 20–50 L of gasoline.
6. Cell Venting and Toxic Gases
The gases released from a Li-ion battery cell can be toxic, e.g., CO, but the fluoride emissions are of most concern. Hydrogen fluoride (HF) is one of them, but there are also others, e.g., phosphorous oxyfluoride (POF
3). They are formed from the fluorine content used in the Li-ion cell; the binder (e.g., PVdF) and the commonly used Li-salt, hexafluorophosphate (LiPF
6). The reaction formulas for the salt decomposition can be seen in the following equations [
20]:
HF has a relatively well-known toxicity [
21], while the toxicity of POF
3 is unknown. However, POF
3 might be more toxic than HF as in the case of the chlorine analogue POCl
3/HCl [
22]. POF
3 could not be observed in the fire tests on Li-ion cells reported here, but a fire study on electrolytes in a Cone calorimeter by Andersson
et al. [
19] indicated that the POF
3 production might be approximately 1:20 of the HF production, which indicates that POF
3 may also have been released in the present tests, but that the concentration was below the detection limit (6 ppm). In the previous study [
4], the real time HF production rate for EiG cells was determined.
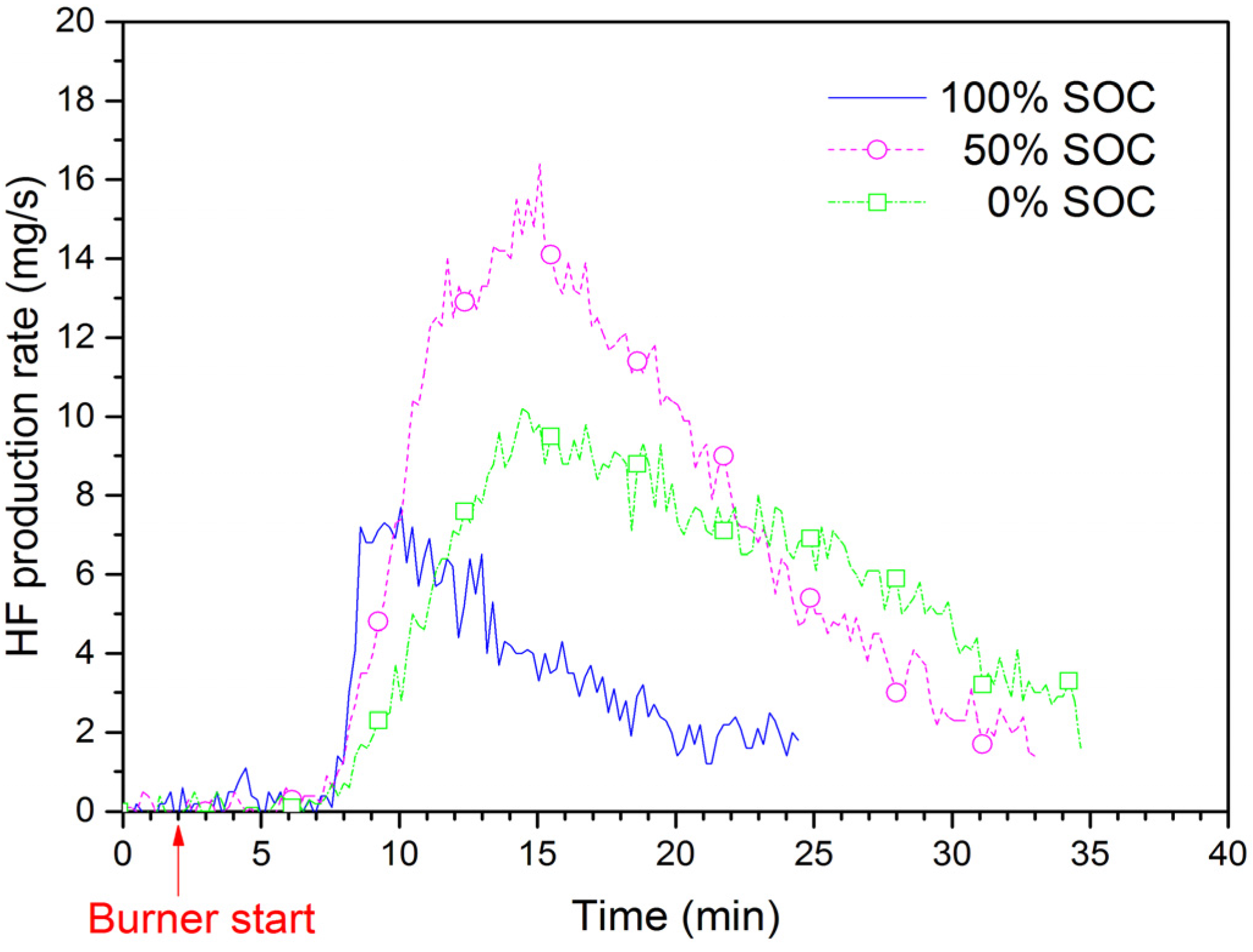
Figure 10 shows the HF production rate for EiG cells with different SOC during the fire tests. The highest rate is for 50% SOC, while 100% SOC has the lowest rate. The total amount of HF from both FTIR and the sampling filter is shown in
Table 2, and values are between 5.6 g and 14 g HF for a five-cell-pack. Ribière
et al. [
5] measured HF in their studies of another type of pouch cell and, if we normalize their values against the cell electrical energy, a value of 37–69 mg/Wh is obtained, with the higher HF amounts for lower SOC, as seen in
Table 2. These amounts are in the same order as our results, 50–120 mg/Wh; however, in contrast to this study, Ribière
et al. [
5] found the highest HF production rate for the fully charged (100% SOC) cells.
The extrapolation of the Larsson
et al. [
4] data to a larger battery pack size typically used in EVs gives an indication of the potential amount of released HF. A battery pack for an EV, based on the tested EiG cell, could, for example, have 432 cells. This corresponds to 108 cells in series and four cells in parallel, which results in a battery pack with 9.7 kWh and a 346 V nominal voltage. The extrapolation factor is then 432/5 = 86.4, resulting in about 400–1200 g HF, depending on the SOC level. These values are in the same order of magnitude as those reported by Lecocq
et al. [
23] for fire tests on a complete EV.
7. Cell Safety Mechanisms
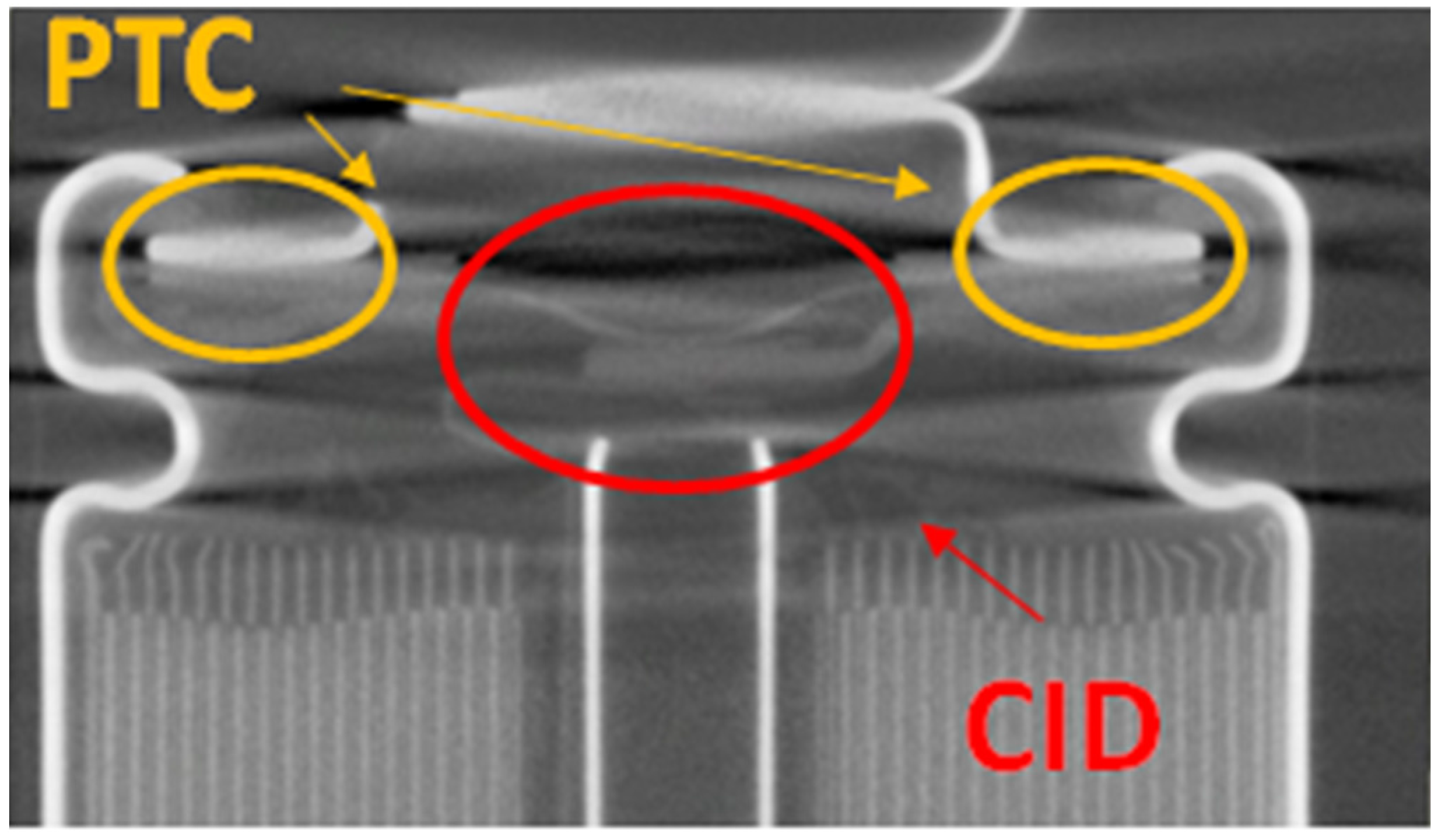
Cylindrical 18650 cells for consumer products typically have a cobalt or cobalt mixture-based cathodes (e.g., NMC, NCA), which are not as thermally stable as LFP [
24]. A number of safety mechanisms [
25] are often included in 18650 cells used in consumer products for low voltage systems. An example of such a safety mechanism is the current interrupter device (CID). The CID is a disc which is part of the current pathway. In case of overpressure in the cell, the CID is mechanically released due to the pressure, letting the cell go into open circuit mode. The CID is typically activated at a predesigned stage, before the cell can go into thermal runaway, by using shutdown additives [
26]. Positive temperature coefficient (PTC) is another safety mechanism, which protects the cell by rapidly increasing the resistance in the current pathway when trigged by an overtemperature, significantly lowering the current passing through the cell. In any case, the CID and PTC do not work that well in battery systems with multiple cells that are electrically connected in a series and thereby at a higher voltage [
27], e.g., in batteries used in electrified vehicles.
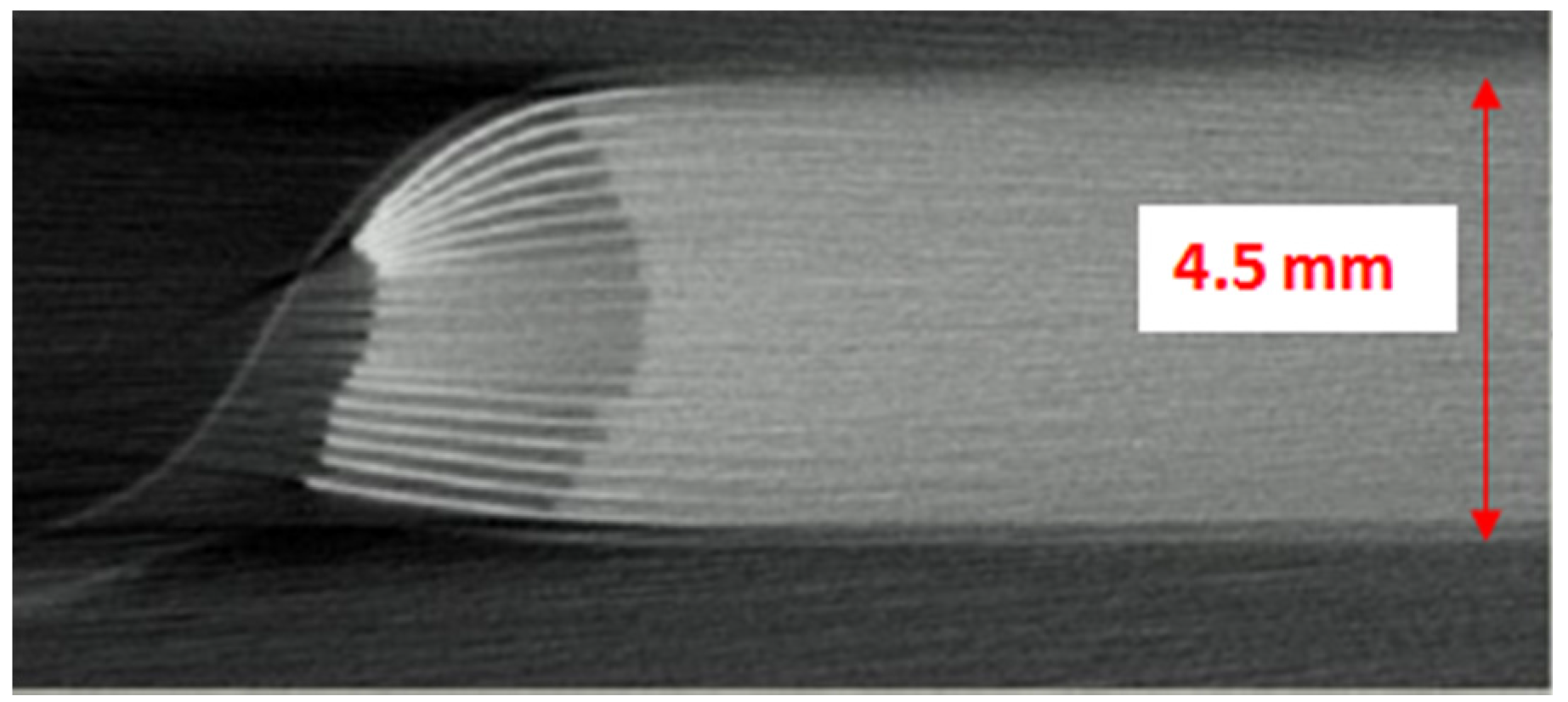
Figure 11 shows a cross section X-ray photo of an 18650 cell where PTC and CID are shown.
Shutdown separators are widely used in commercial Li-ion batteries as a safety protection for some abuse situations, e.g., overcharge and short circuit. The pores in the separator are closed at overtemperatures, which lead to a hindered ion transport between cathode and anode and thus an open circuit. The shutdown separator usually consists of a layered structure where one layer has a lower melting temperature than the other layer. When the first layer melts the pores in the separator are closed, while the second layer sustains the cell integrity, thereby prohibiting internal short circuit.
Figure 12 shows differential scanning calorimetry (DSC) measurements of a polypropylene (PP) separator and of a shutdown separator with polyethylene (PE) and PP; the latter exhibits two melting temperatures, corresponding to the two materials. In case of, e.g., an overcharge leading to an increased cell temperature, the PE will melt at around 130 °C, lowering the current and thereby the heating process. It may work less well in some situations, e.g., when the current is interrupted too late or when the cooling is poor due to the battery system design. In those cases, the melting temperature of the second layer of PP, around 160 °C, can be reached, leading to the total disintegration of the separator, followed by an internal cell short circuit. The use of shutdown separators in large battery systems has shown not to have the same safety benefits as in small batteries, since the higher battery voltage in cases where many cells are electrically connected in a series, as with EV batteries, for example, can lead to separator breakdown [
28].
In order to account for the drawback that some of the typical safety devices used in cells for consumer products cannot be used in Li-ion cells for EVs, other safety mechanisms such as special additives in the electrolyte are used. Li-ion cells for EV typically use cells which have higher quality manufacturing, more pure raw materials and safer chemistry such as the LFP, which can withstand abuse better [
3].
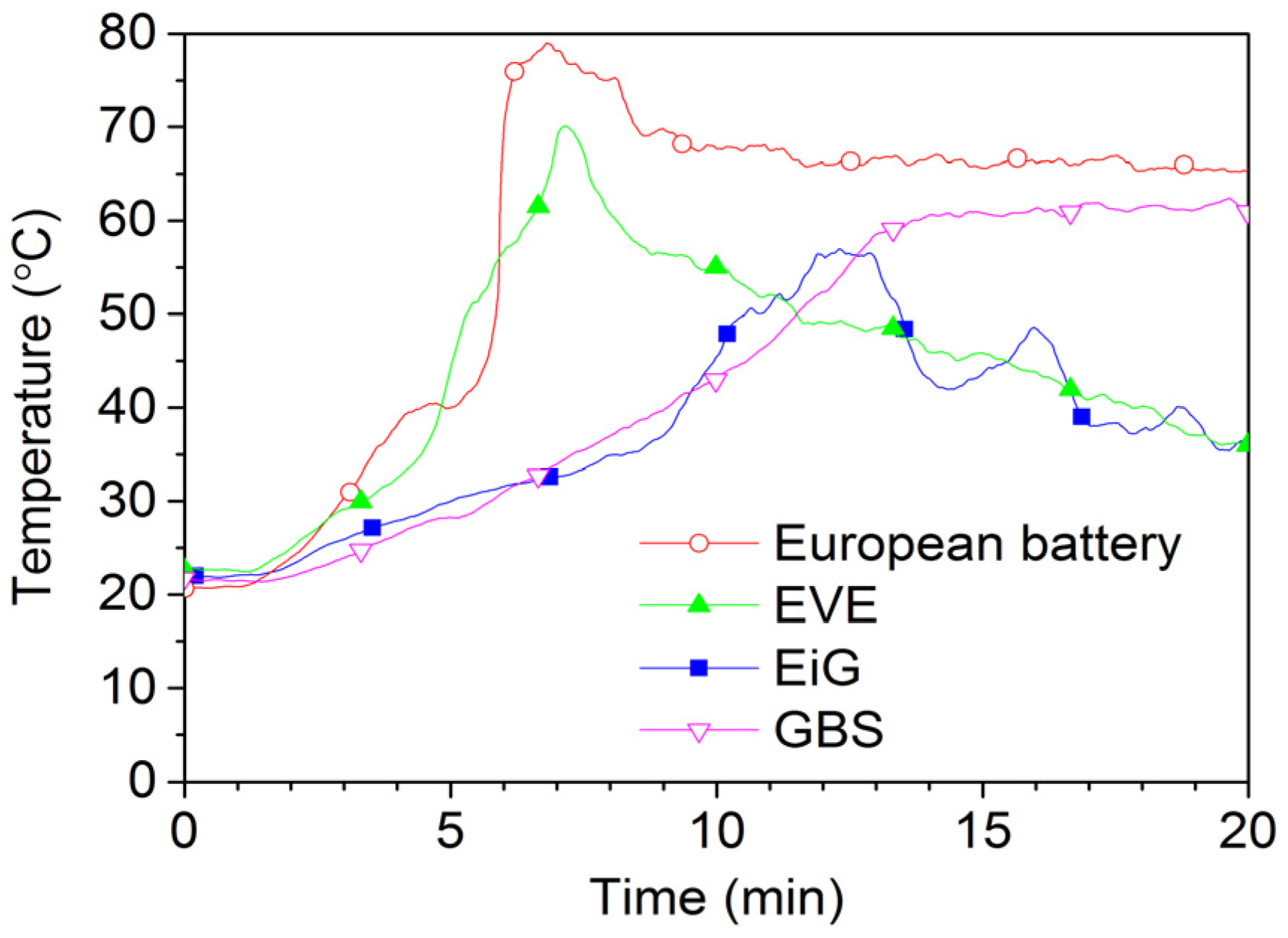
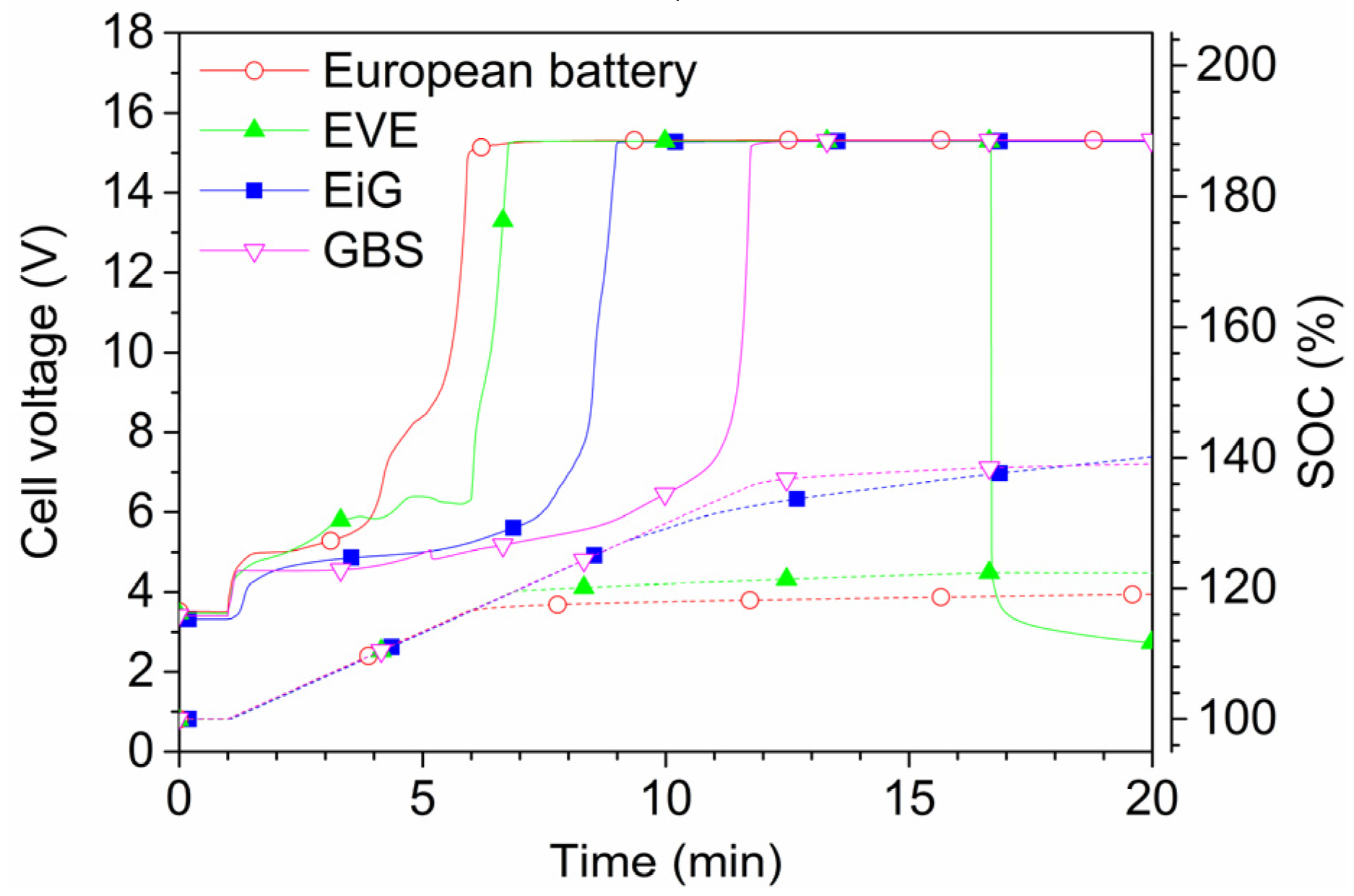
Figure 13 and
Figure 14 show 2C-rate overcharging of four LFP-based cells with a capacity between 7 Ah and 45 Ah. The GBS cell has a cathode of LFMP,
i.e., LFP with manganese. The charger voltage was max 15.3 V and the charger was started after 1 min and was active during the complete test; however, for the overcharge of EVE, the charger was switched off at around 17 min, as seen by the voltage drop in
Figure 14. The temperatures reached less than 80 °C, well below the onset temperature of the thermal runaway. However, the cells swell and gases are emitted. Four European battery cells were tested and the result from one of them is shown in
Figure 13 and
Figure 14. In fact, one of the European Battery cell unexpectedly caught fire. A situation of an overcharge abuse in the field might occur in case of a failure in the battery management system (BMS). High charge currents can occur, e.g., during fast charging or during breaking (recuperation) of an EV, which makes those cases especially sensitive to errors in the overcharge protection. In principle, the consequences for overcharging of LFP cells are less dramatic than for other Li-ion chemistries, but the temperature increase starts at a lower state of overcharge [
24].
In the case of a short circuit of a Li-ion battery, the current can be very high [
3]. A measurement of a low-ohmic short circuit on a single pouch cell from European Battery is shown in
Figure 15. The voltage and current were measured with 1 kHz by an oscilloscope and cell surface temperatures (by 18 type K thermocouples on both sides of the cell) by a data logger at 1 Hz. The short circuit peak current is close to 1100 A and then lowered to a plateau of about 700 A. High currents generate a lot of heat, but for this cell the average temperature increase is only about 5 °C since the short circuit is stopped when the positive terminal burns off from the cell. In the case of a large battery pack with cell terminals that do not burn off, the current and the generated heat can be substantial, and in the case of burnt off terminal tabs the flames might ignite vented flammable battery gases or plastic parts inside a battery system.
9. Conclusions
There is relatively good knowledge about the safety risks and safety devices used in consumer cells. Using Li-ion in the automotive sector puts higher demands on the battery since the batteries are significantly larger and have harsher environmental conditions, e.g., vibrations, humidity, larger temperature variations. The different Li-ion chemistries show diverse hazards where the LFP is less reactive but safety measures are still needed for all Li-ion batteries. A high level of safety is achieved by adding several safety layers from the cell to vehicle level; however, the risk for a cascading fire in a complete battery pack starting from a single cell is not yet well studied, and the knowledge about possible counteractions is thus also limited. Sometimes things go wrong even though smart safety strategies are used. The exploded cylindrical cell due to a cell vent malfunction showed this and underlines the importance of using many safety layers.
The toxic gas emissions from Li-ion batteries, e.g., HF and POF3, can pose a serious risk for a person. A replacement of the Li-salt LiPF6 to a non-fluorine salt and change of fluorine binder could resolve this risk. Intense research is ongoing in this field, but the required properties for a Li-ion battery in EVs are complex and demanding.