1. Introduction
The synergetic effects in multi-phase metal hydride (MH) alloys refer to the presence of microsegregated secondary phases occurring in the melted alloys that effectively provide beneficial effects [
1]. Some examples of synergetic effects in the gaseous phase and electrochemical environment are summarized in
Table 1. In general, the synergetic effects in the gaseous phase hydrogen storage can improve the storage capacity and reversibility, and are characterized by a continuous transition in the pressure-concentration-temperature (PCT) isotherm, from the plateau pressure corresponding to the phase with a stronger metal–hydrogen (M–H) bond strength to the phase with a weaker M–H bond strength (Figure 7b in [
2]). The interface region between two phases is considered to be critical for the synergy to take place. Transmission electron microscopy (TEM) studies have demonstrated the interface between the main C14 phase and other secondary phases (C15, Zr
7Ni
10,
etc.) are clean [
3,
4], and strong crystallographic orientation alignment can be established by electron beam back-scattering diffraction pattern studies [
5,
6]. Some synergetic effects in the electrochemical environment are similar to those in the gaseous phase,
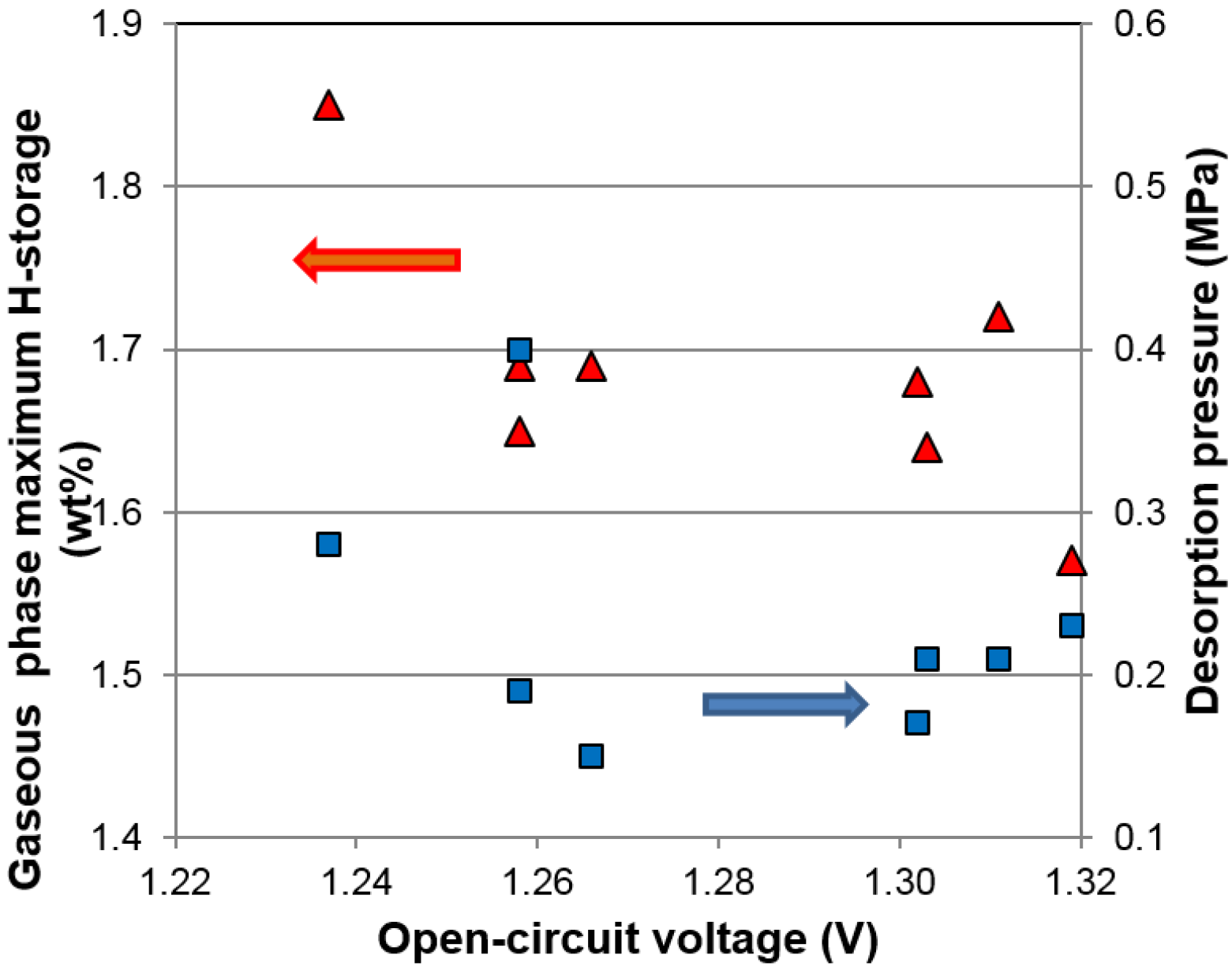
i.e., improvement in capacity and hydrogen absorption/desorption rate. Interestingly, the properties established by the scope of the gaseous phase can be further enhanced by synergetic effects in the electrochemical environment. For example, it has been found that the electrochemical discharge capacities of certain multi-phase MH alloys can be increased substantially by lowering the equivalent plateau pressure (observed through open-circuit voltage (
OCV) changes during charge/discharge [
7]) through synergetic effects [
8,
9]. Therefore, a systematic study in the difference between the synergetic effects in the two environments is of significant interest.
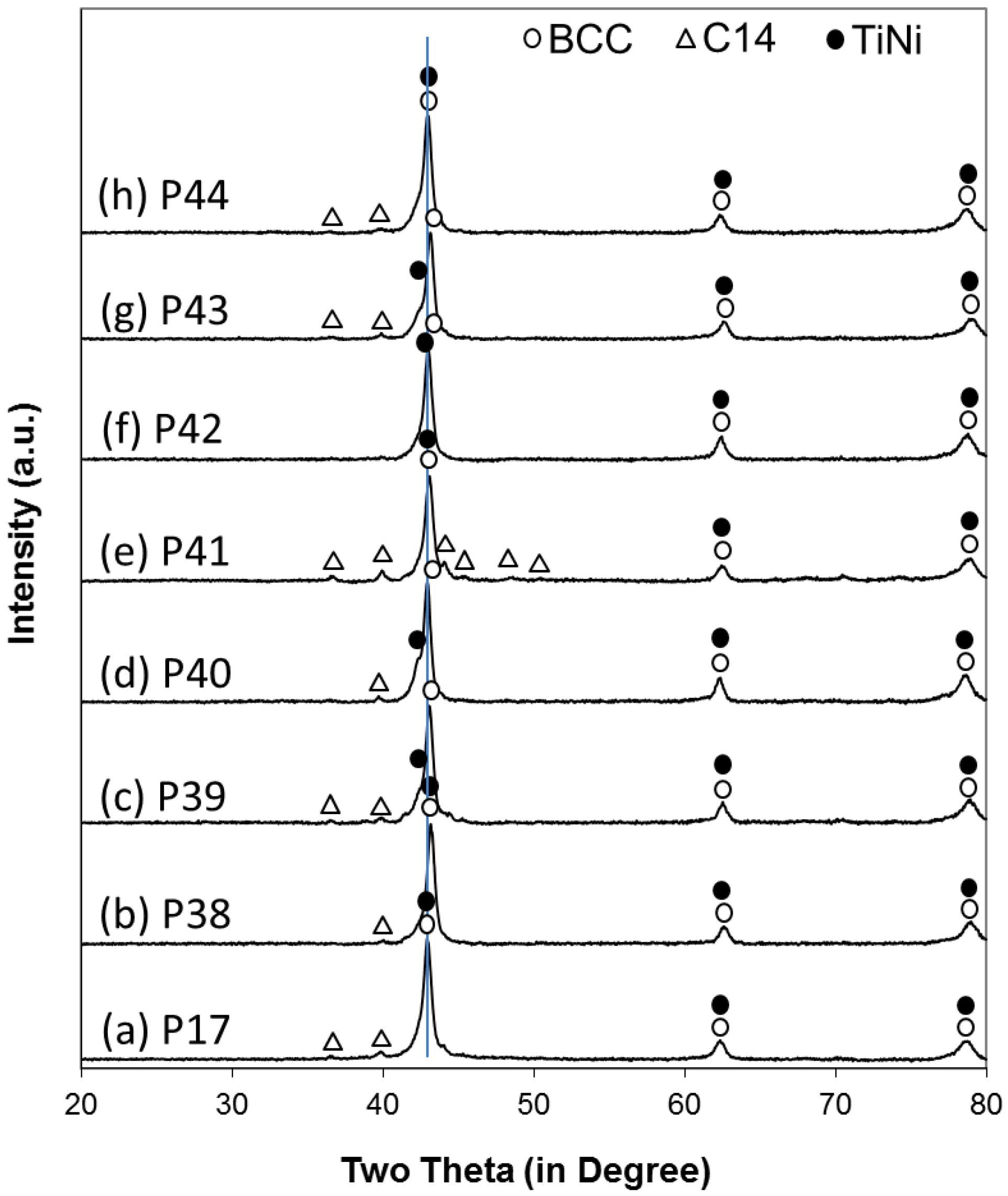
Laves phase-related body-centered-cubic (BCC) solid solution alloys were chosen for this study. By combining the high-capacity main storage BCC phase with catalytic phases, such as C14, TiNi, and Ti
2Ni, one alloy in the family demonstrated a 30% increase in capacity when discharged at a current density of 100 mA·g
‒1, which is adequate for electric vehicle applications [
31]. The development of this family of alloys proceeded in stages and the X-ray diffraction (XRD) and PCT analyses demonstrated strong evidence of synergetic effects between the main storage phase and the catalytic phase [
32,
33,
34,
35]. With a base alloy of P17 (Ti
15.6Zr
2.1V
44Cr
11.2Mn
6.9Co
1.4Ni
18.5Al
0.3), the V/Ni content was adjusted to increase the high-rate dischargeability (
HRD) [
36]. The synergetic effects in an electrochemical environment were studied in detail using this same base alloy, P17, with substitutions of other A-site atoms (B, Mg, Y, Zr, Nb, Mo, La, and Nd) and presented here.
4. Discussion
It is generally believed that in a multi-phase MH system the phase with a lower plateau pressure and more negative ∆
H is a main storage phase, which has a larger hydrogen storage capacity (Zr
7Ni
10, for example [
57]), and the secondary phase with a higher plateau pressure and less negative ∆
H is considered to be the catalytic phase (Zr
2Ni
7, for example [
57]). In the case of funneling phenomenon, the gaseous phase hydrogen storage is accomplished with the catalytic phase as the necessary funnel to move hydrogen in and out of the alloy [
2]. In the case of Laves phase-related BCC MH alloy, the BCC phase is considered to be the main storage phase; although it has a large hydrogen storage capacity, it has limited absorption/desorption kinetics that require an additional catalytic phase to facilitate the hydrogen storage process [
58]. Therefore, both C14 and TiNi were considered to be catalytic phases in this family of alloys [
32,
33,
34,
35,
36]. However, the ∆
H values estimated from Equation (3) for the C14 and TiNi phases are much lower than those of the BCC phase, and the visible plateau in the pressure between 0.1 MPa and 1 MPa was assigned to the BCC phase. It is necessary to verify the correctness of such an assignment.
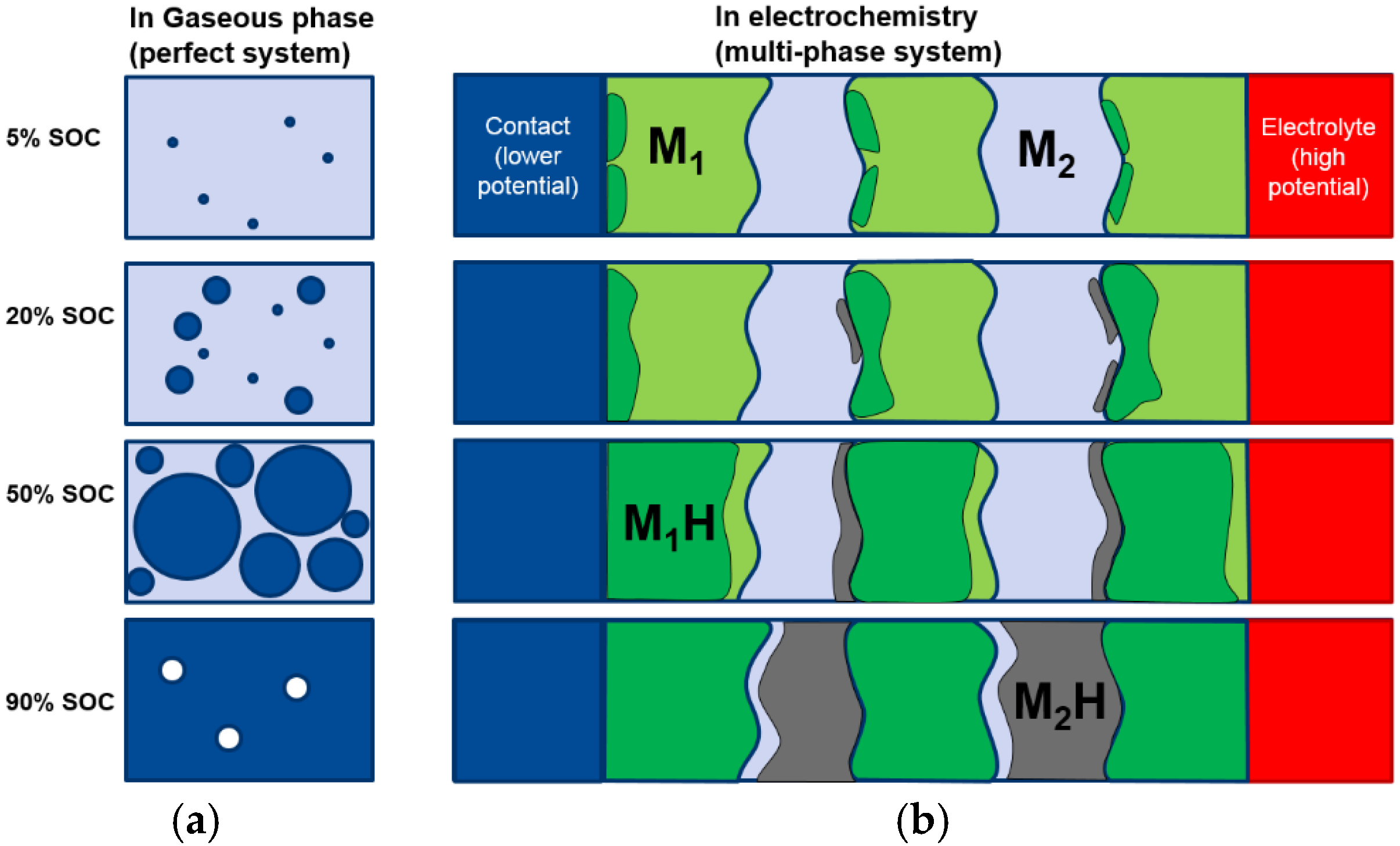
In order to investigate the phase abundance evolution during both gaseous phase and electrochemical hydrogen absorption/desorption, an alloy with a target composition of P17 (
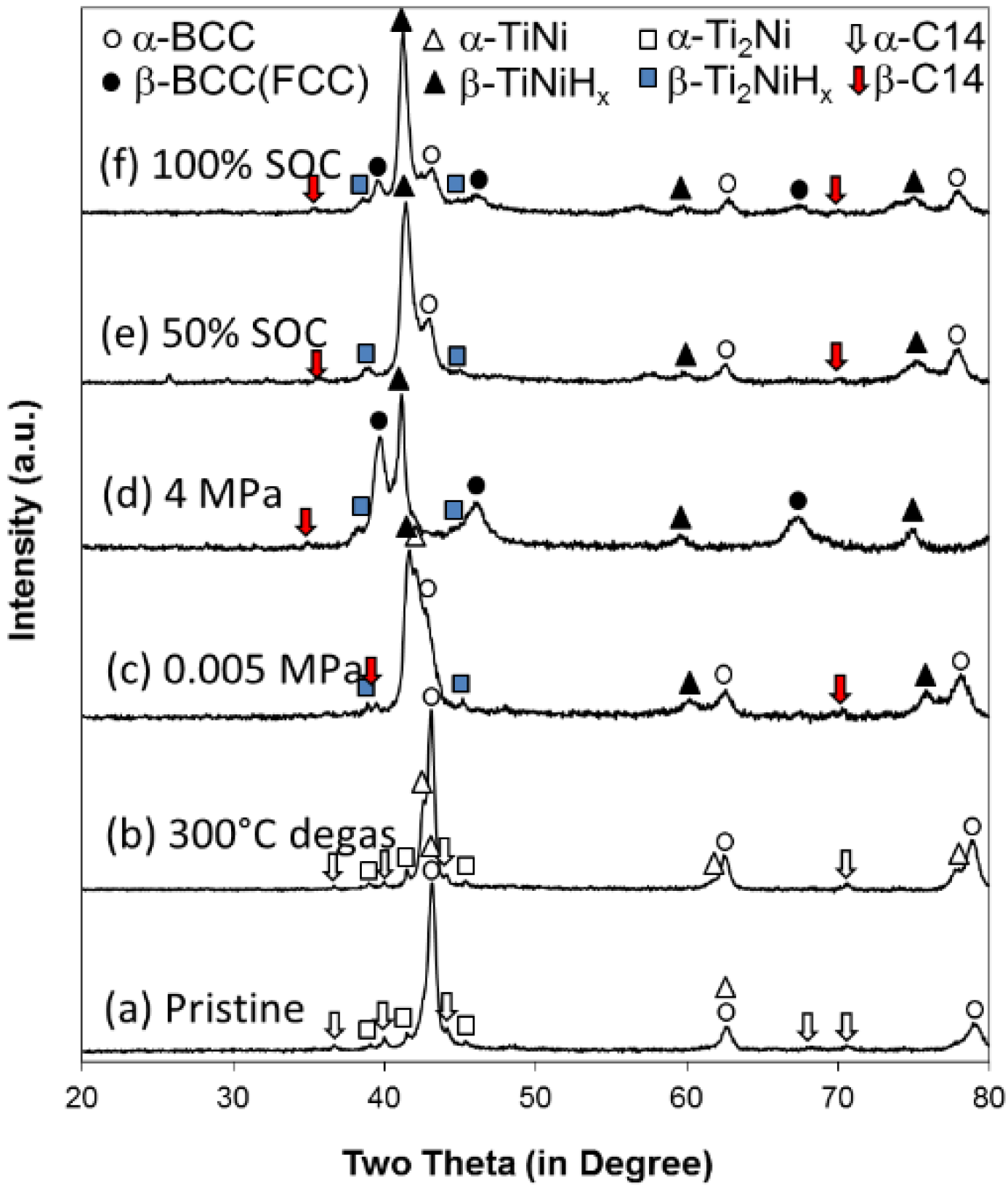
Table 2) was reproduced through conventional induction melting. The XRD patterns from the as-prepared samples show an additional Ti
2Ni phase (
Figure 11a) which was not observed in the sample prepared by arc melting, due to different cooling rates (
Figure 1). The Rietveld refinement indicates percentages of 53.6% BCC, 29.4% TiNi, 10.8% Ti
2Ni, and 6.2% C14 (
Table 8). The sample was activated in a 4 MPa hydrogen environment first and then degassed under vacuum for 8 h at 300 °C. XRD analysis at this stage (
Figure 11b) shows a shift for TiNi peaks to lower angles, which indicates the presence of some hydrogen remaining in the α-TiNi. The Rietveld refinement shows the β-phase (MH) of C14 and Ti
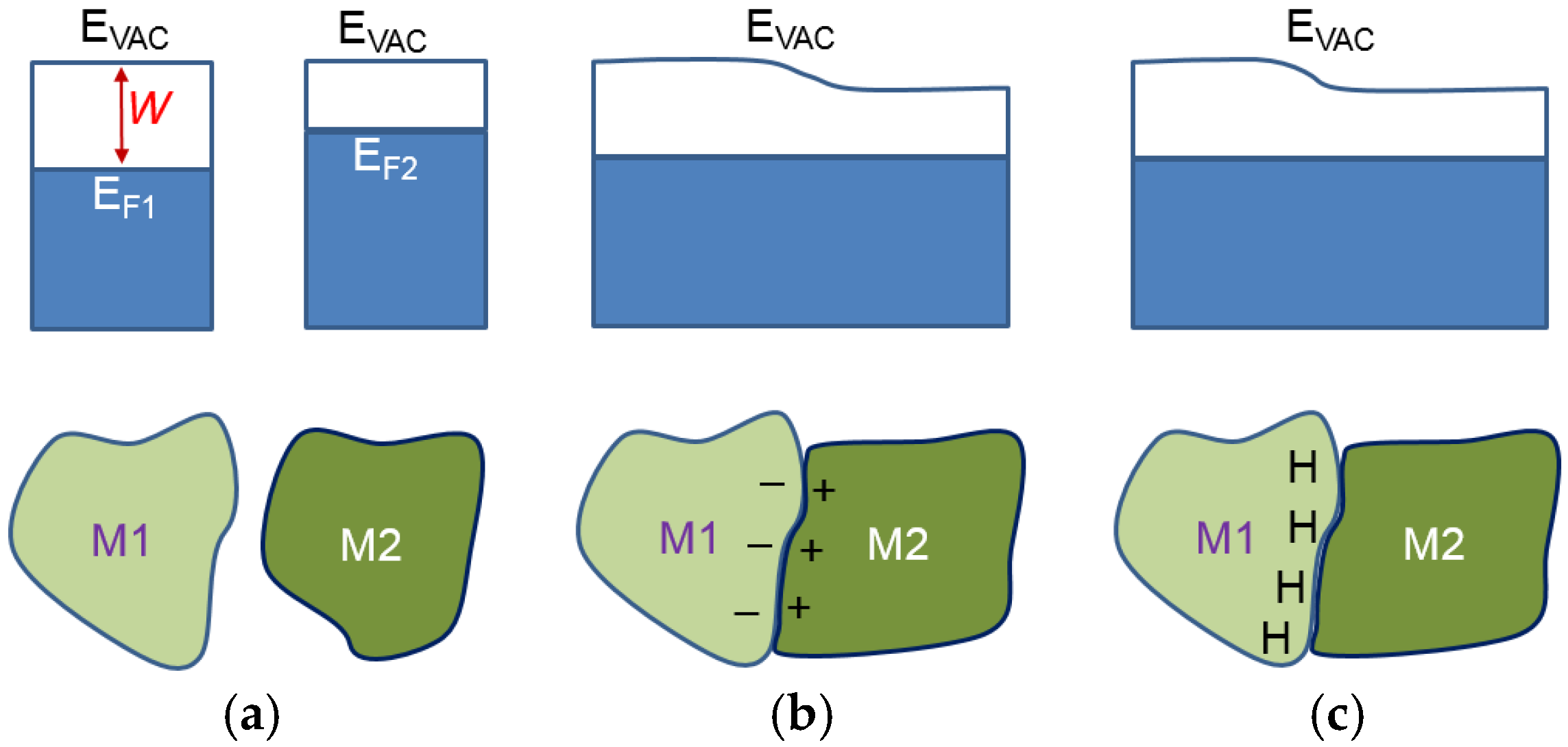
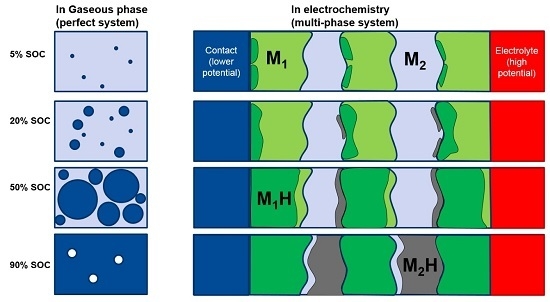
2Ni. There are still MH remaining in the alloy after 8 h of degassing. In order to understand the cause of the remaining hydride after 8 h degassing, a discussion of two dissimilar metals is necessary. When two metallic phases, M1 and M2, with different work functions (differences between the electron potential in vacuum (
EVAC) and the Fermi level (
EF)) are brought into contact (
Figure 12b), there will be a small charge transfer that builds a potential (contact potential), preventing electron flow from the metal with a smaller work function (M2) into the metal with a larger work function (M1). During the initial hydrogenation, the neutral hydrogen (proton plus a nearby electron) will reside on the M1 side of the boundary. The extra electron brought by proton will contribute to the conduction-band and raise the Fermi level, as shown in
Figure 12c [
59]. These protons will stay balanced in equilibrium under vacuum. In the case of MH, the hydrogen storage capability of a hydride former metal is related to its own electron density. For the study in MH from elements, the heat of hydride formation (indicator for M–H bond strength) and the work function for the first row of transition metals (from Sc to Ni) were plotted against their number of 3d electron in
Figure 13. As the number of electrons increases, the work function increases due to the increase in the charge of the nucleus, and the host metal starts to resist incorporation of extra electrons brought by the absorbed hydrogen and consequently weakens the MH bond strength (less negative ∆
H), with the exception of Mn. Mn has an extraordinarily low work function due to its containing of the maximum number of un-paired electrons (five) and also to a lower ∆
H than predicted by the trend. For MH from intermetallic alloys, the situation is the opposite. Comparing the alloys LaNi
2 and LaNi
5, for example, the former has a lower electron density (lower Ni-content), a lower E
F (as M1 in
Figure 13), and a tendency to trap the residual hydrogen near the interface. According to Equation (3), LaNi
2 (33% of La) has a stronger MH bond strength compared to that of LaNi
5 (16% of La). Therefore, we believe the phase with a stronger MH bond will keep the MH (β-phase) during a total degassing in vacuum. In this study, C14 and Ti
2Ni are deemed to be the phases with the strongest MH bond strength, which agrees with the prediction from Equation (3). These pre-occupied sites at the grain boundary on the side with a higher work function will act as the nucleation center for the β-phase growth (as illustrated in
Figure 14). Therefore, the PCT absorption/desorption hysteresis of a single-phase AB
5 MH alloy is always larger than that from a typical multi-phase AB
2 MH alloy [
7].
A sample was removed from the PCT apparatus with an equilibrium hydrogen pressure of 0.005 MPa, a pressure that separates the two plateaus in the PCT isotherms, as shown in
Figure 5. From previous studies in BCC MH alloys, we believe the oxide layer formed at the alloy surface during the sudden exposure to air can prevent the loss of hydrogen into atmosphere [
61]. Its XRD pattern is shown in
Figure 11c, and the Rietveld analysis shows an unchanged BCC phase with 2/3 hydrided TiNi and Ti
2Ni phase (
Table 8) and demonstrates that the higher plateau (from 0.1 MPa to 1 MPa) belongs to the BCC phase. Another sample was removed at the maximum hydrogen pressure (4 MPa). The XRD pattern of this sample shows a 90% transition from BCC (M) to FCC (MH) in the BCC phase and complete α- to β-phase transitions for the other three phases.
Besides the phase transition in the gaseous phase hydrogen reaction, we also performed XRD analysis on two pieces of electrode made from the same P17 alloy by induction melting with 50% and 100% state of charge (SOC). The resulting patterns are shown in
Figure 11e,f. The Rietveld analysis shows a clear α-BCC phase and almost fully hydrided TiNi, Ti
2Ni, and C14 phases for the 50% SOC sample, while a 40% hydrided BCC (β-BCC) phase with the other phases fully hydrided are seen for the 100% SOC. During charging, the phases with stronger MH bond strength (more negative ∆
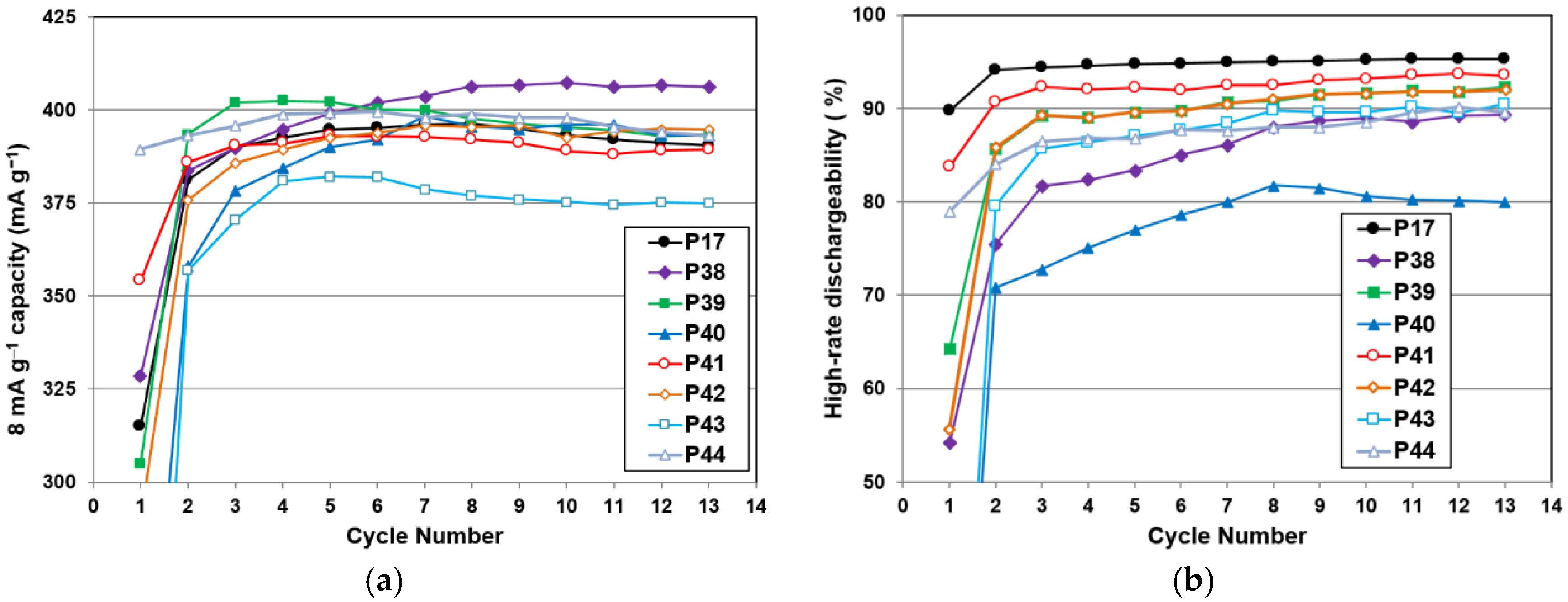
H) were hydrided first, and BCC was charged last, as in the case of the gaseous phase reaction. The BCC phase was not hydrogenated before completion of hydride formation in other non-BCC phases. The BCC phase was never fully charged in the half-cell testing, due to the nature of the open-to-air configuration used in the electrochemical study. This explains the low electrochemical discharge capacity found in
Figure 9, when compared to the gaseous phase maximum capacity.
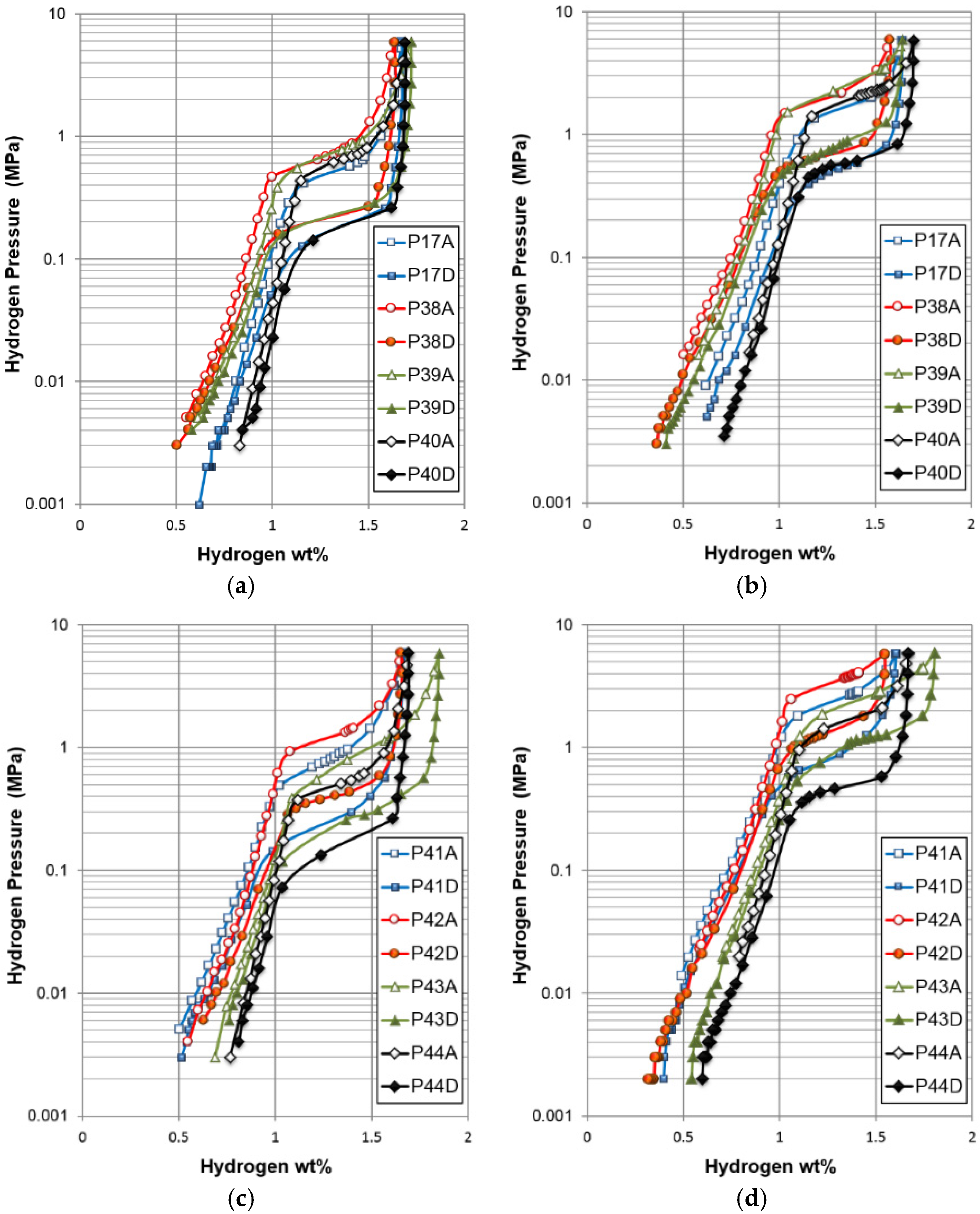
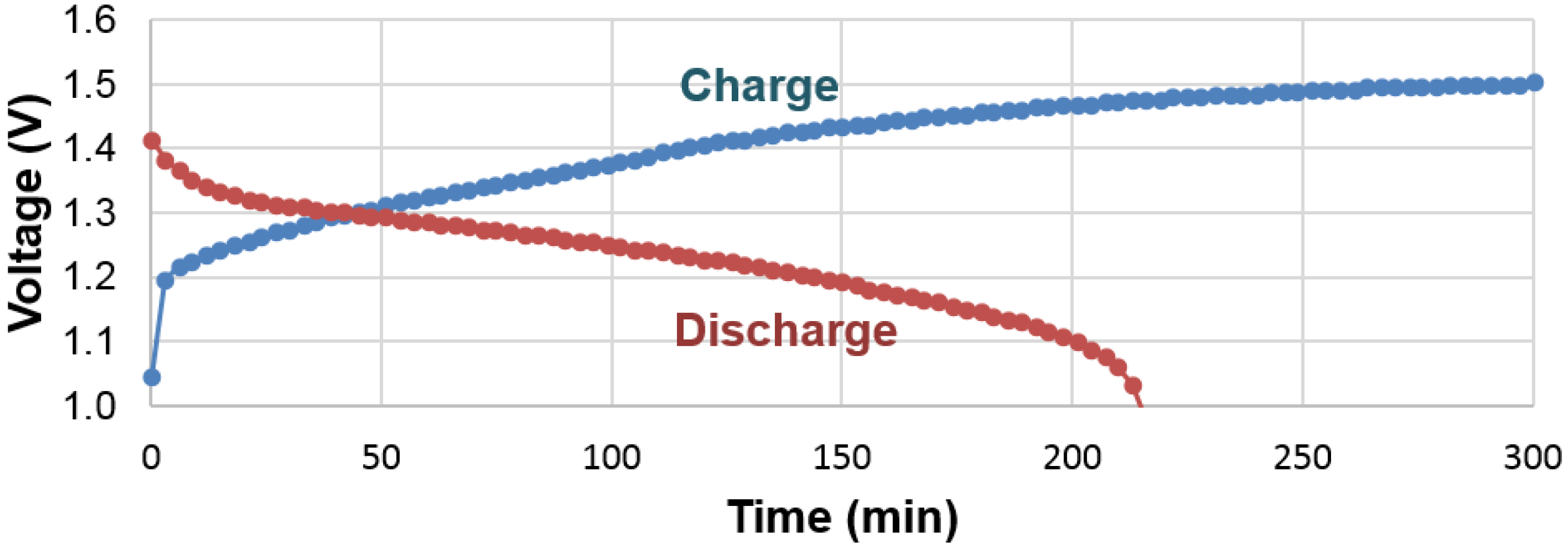
There are some important differences in hydrogen storage between gaseous phase and electrochemistry, especially when the PCT isotherm (
Figure 5) is compared with the electrochemical charge and discharge curves (
Figure 15). While the gaseous hydrogen charging occurs through hydrogen gas molecule adsorption and splitting into two hydrogen atoms at the clean surface of metals (free from oxide), the electrochemical hydrogen charging is implemented by an applied voltage, which drives electrons into the negative electrode and leads to water splitting at the surface (which is typically covered with a thin oxide [
62]). In PCT, hydrogen absorption can begin as soon as any amount of hydrogen gas is available. However, in the case of an electrochemical environment, a minimal voltage is required to generate enough of a strong electric field to split water into protons and hydroxide ions. Also in PCT, the equilibrium pressure depends only on the concentration of hydrogen in the MH; the electrochemical voltage is mainly determined by the surface reaction and thus a sudden change in the charge voltage profile is not seen when the active storing material switches from one phase into another one (at approximately 50% SOC). The discharge processes between the gaseous phase and electrochemistry are also different. For the gaseous phase, the movement of proton is influenced by diffusion and an equilibrium is reached when the same amount of hydrogen gas leaves and enters the metal. The electrochemical case is far more complicated. During discharge, the electron moves away from the MH alloy into the current collector, which forces the proton to move in the opposite direction, reaching the surface. Unless the surface recombination of either proton-hydroxide or proton-oxygen is very slow (which is unlikely) and there is a large number of protons accumulated at the electrolyte interface, the proton should continue to arrive at the interface to reach charge neutrality. Energy is gained from both the reduction in number of electrons in the metal system and the dehydride process. Therefore, there is no sudden drop in the discharge voltage profile when the system finishes discharging for one phase and starts the discharge for the next phase. This finding is very encouraging with regard to the electrochemical applications of MH alloys. In a multi-phase system, as long as there is an electrochemically active phase, the so called “irreversible” or “no participation” phases found in PCT can be fully utilized in the electrochemical environment. This finding can be used to explain the unpredicted high electrochemical capacity (compared to the gaseous phase capacities) observed in multi-phase systems involving the Zr
2Ni
7 phase [
8,
9].
With the knowledge that the visible plateaus of the PCT isotherm in the 0.1–1 MPa range originate entirely from the phase transition from BCC to FCC, the mechanism of this step, at equilibrium pressure in pure BCC alloys [
61,
63], is not well understood. Evidently, with synergetic effects through the non-BCC phases, the intermediate body-centered-tetragonal (BCT) phase is skipped. Although the hysteresis of the BCC phase is still very large (compared to all other MH alloy families), it has been significantly reduced through the synergetic effects of the non-BCC phases. In this study, it is clearly observed that, during hydrogenation, the non-BCC phases hydrogenate first and expand the lattice on the non-BCC phase side of the grain boundary. The lattice of the BCC phase is also pre-expanded before hydrogenation by the stress from the hydride on the other side, which reduces the energy barrier needed to expand the BCC lattice, and thus reduces the hysteresis and changes the FCC β-phase directly.
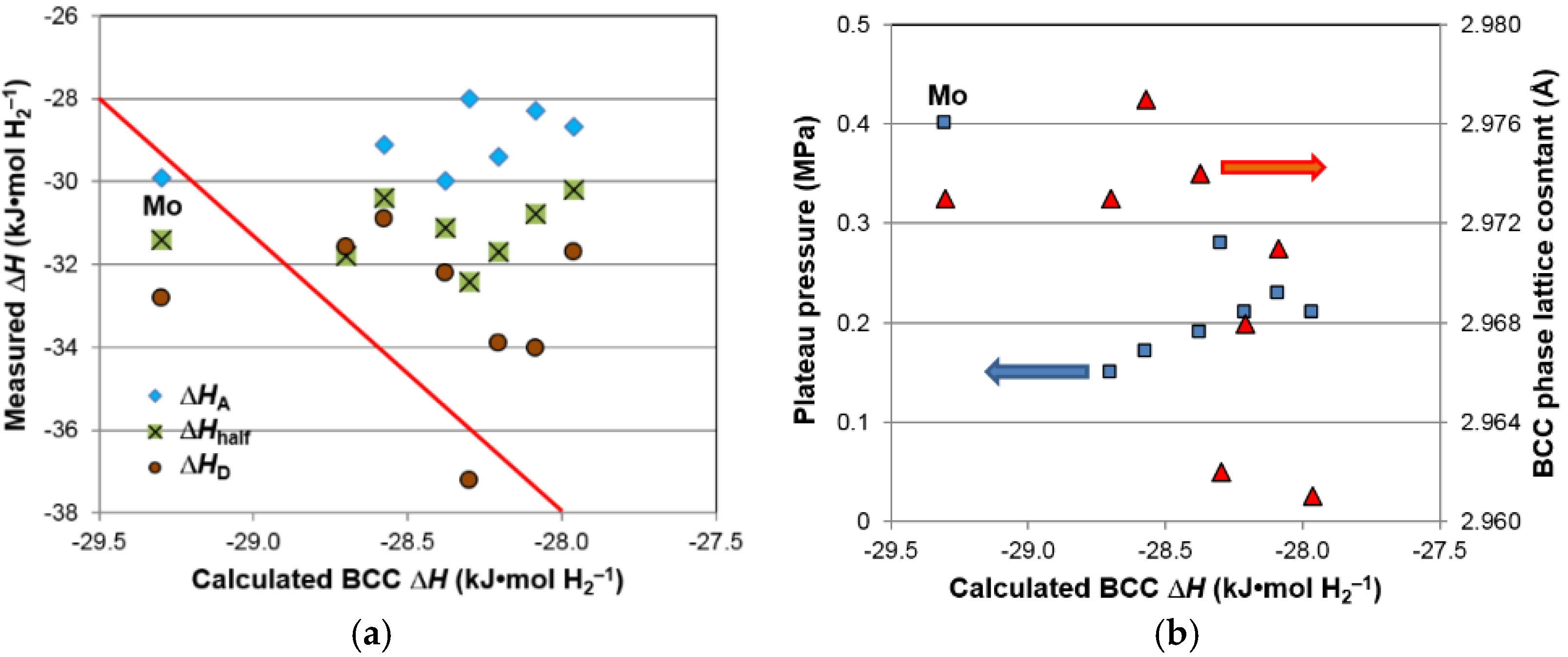
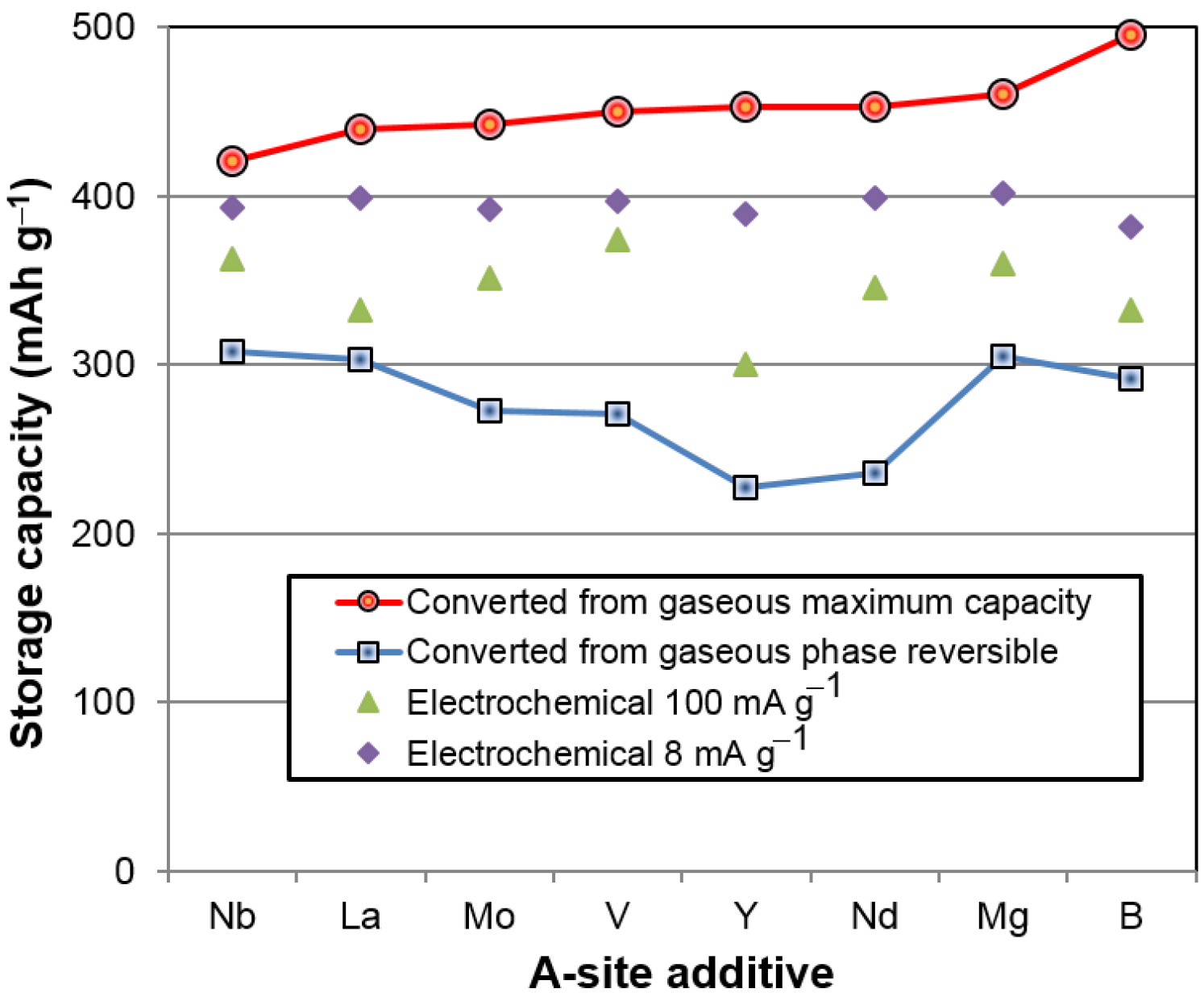
After we identified BCC as the storage phase and the others (C14, TiNi, and Ti
2Ni) as the catalytic phase, a correlation study between the properties of the constituent phases and the hydrogen storage characteristics (both in gaseous phase and electrochemistry) was conducted; the results are summarized in
Table 9. Those with significant correlation (larger
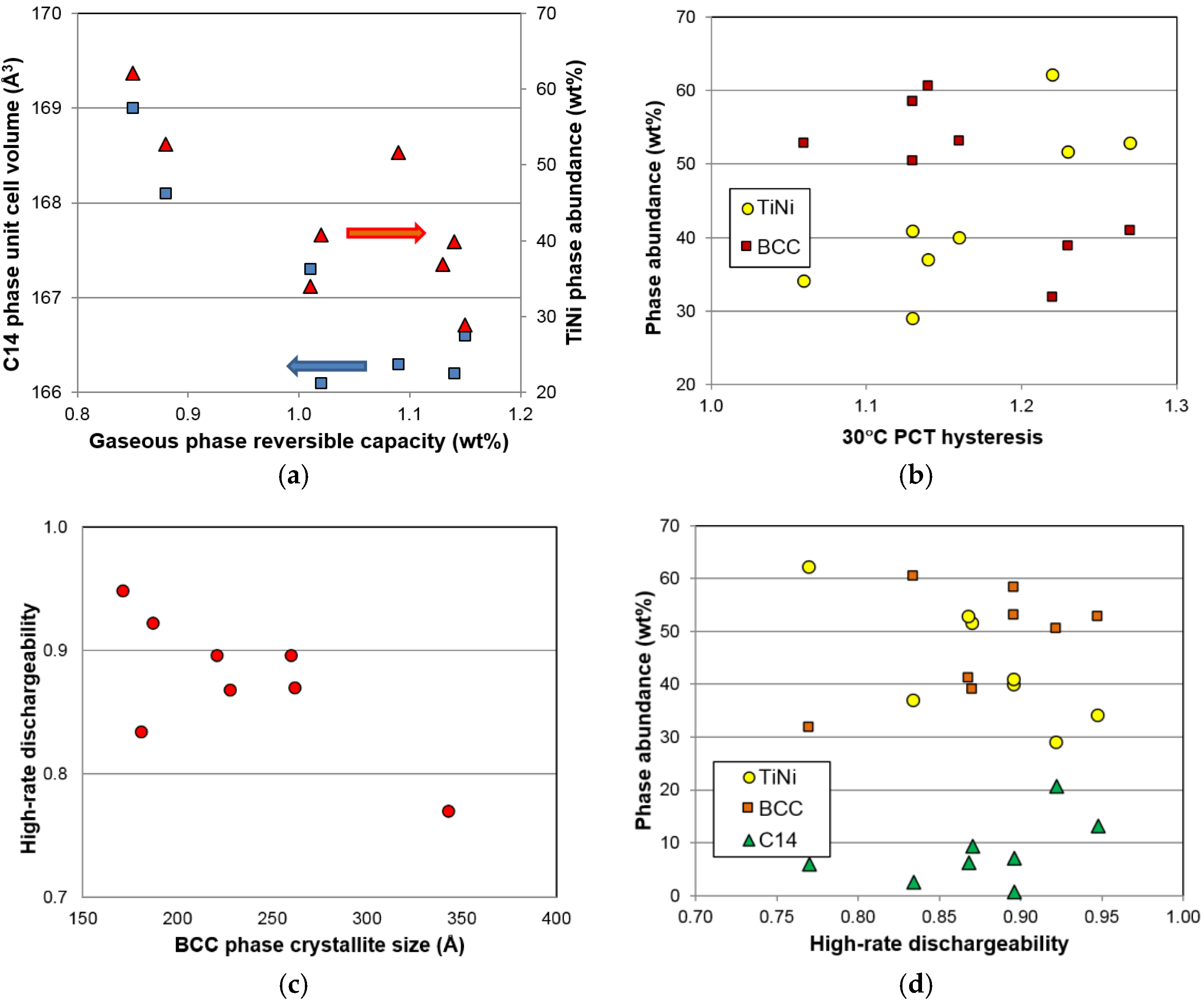
R2) are highlighted. There are two noticeable correlations in the gaseous phase properties. The first is the reversible capacity, which decreases with the increase in TiNi phase abundance and C14 unit cell volume (
Figure 16a). The former has already been explained by the TiNi phase being the largest component in the lower plateau (not seen from the PCT isotherm in
Figure 5) and makes the largest contribution to the irreversible part of the gaseous phase hydrogen storage capacity (
Figure 6). The source of the latter correlation is less clear. C14, although it has a relatively low ∆
H, may be still a catalytic phase. With the decrease in the unit cell volume, hydride from the C14 phase becomes less stable and contributes positively to the reversible capacity of the storage phase (TiNi and BCC). The second noticeable correlation found in the gaseous phase properties is related to the PCT hysteresis—more precisely, the hysteresis of the BCC phase. It has been found that the 30 °C PCT hysteresis increases with increasing TiNi phase abundance and decreasing BCC phase abundance (
Figure 16b). Many studies have been conducted on the PCT hysteresis (for a review see [
64]). It is generally accepted that the PCT hysteresis is the elastic energy needed for the deformation of the lattice near the α-β boundary. Higher PCT hysteresis in an MH alloy increases the difficulty of hydrogenation. From this aspect, the BCC phase facilitates reaction, while TiNi phase retards the hydrogenation of the BCC phase, which is very difficult to observe from the large value of hysteresis since it involves phase changes from BCC to FCC [
6].
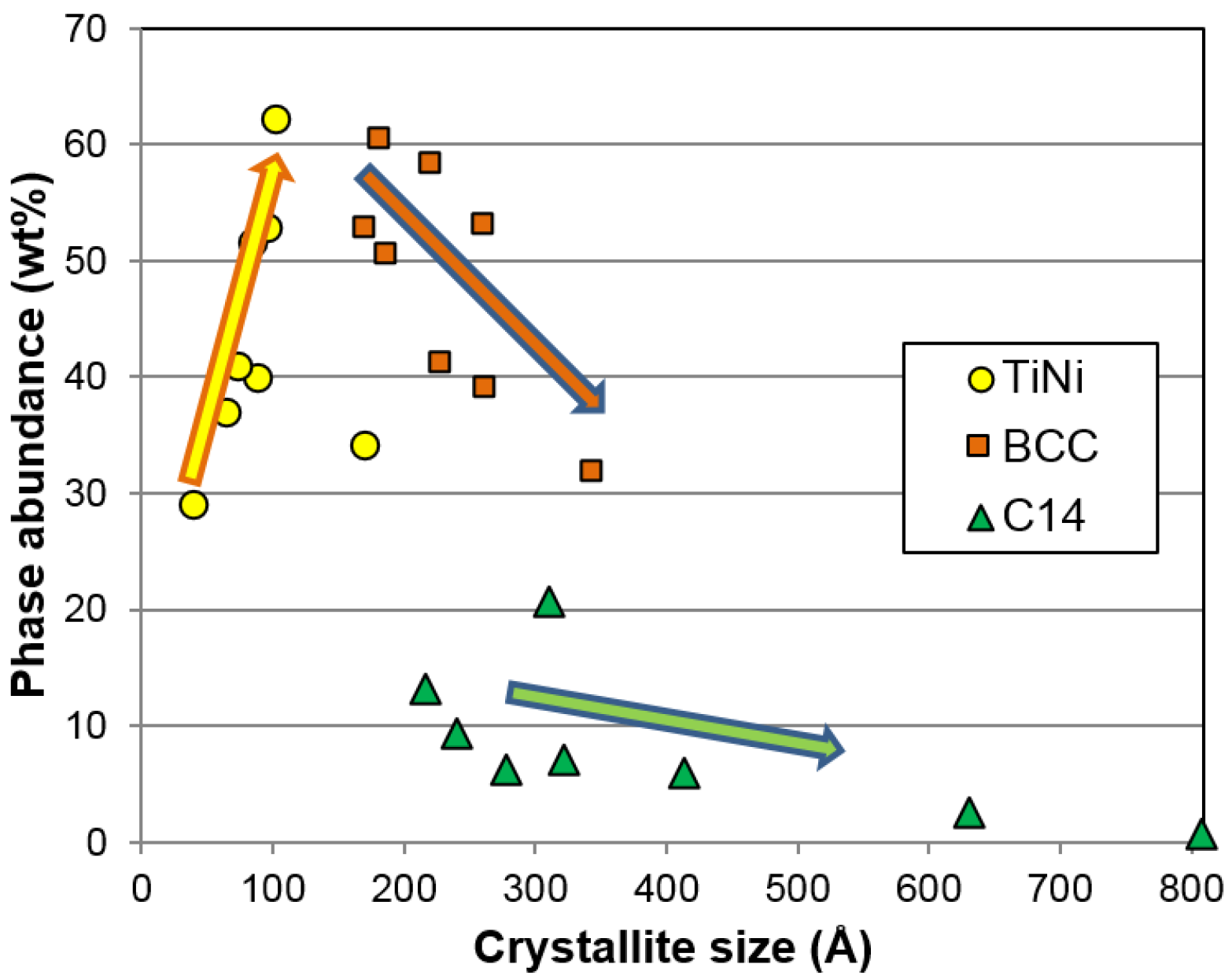
As for correlation to the electrochemical properties, both the BCC crystallite size and the TiNi phase abundance show significant correlation to the high-rate discharge performance. A smaller crystallite in the BCC phase is more desirable, as it gives better high-rate performance (
Figure 16c). It is possible that smaller crystallite sizes will generate more grain boundaries (shown in
Figure 12c) and facilitate more charge transfer between the two phases. The phase abundances from the three major phases are plotted against
HRD in
Figure 16d. The general trends suggest that the BCC and C14 phases are more beneficial to
HRD, while the TiNi phase deteriorates it.