Characterising Lithium-Ion Battery Degradation through the Identification and Tracking of Electrochemical Battery Model Parameters
Abstract
:1. Introduction
2. Model Formulation and Parameters
2.1. Pseudo Two-Dimensional Model Formulation
2.2. Pseudo Two-Dimensional Model Parameters
3. Method for Restricting the Number of Parameters for Identification
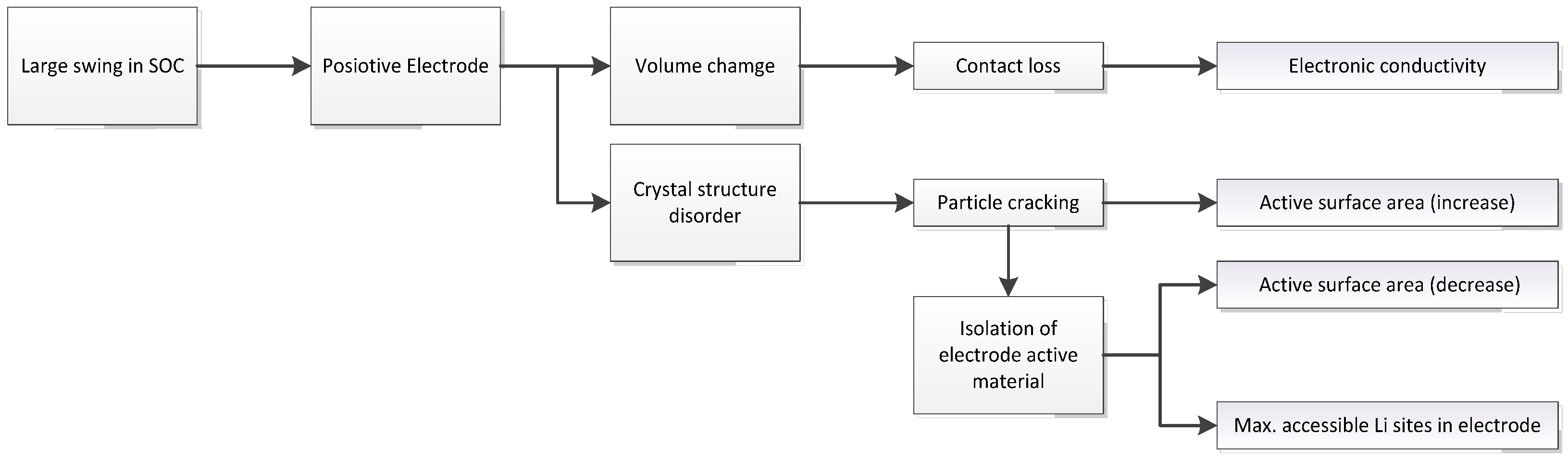
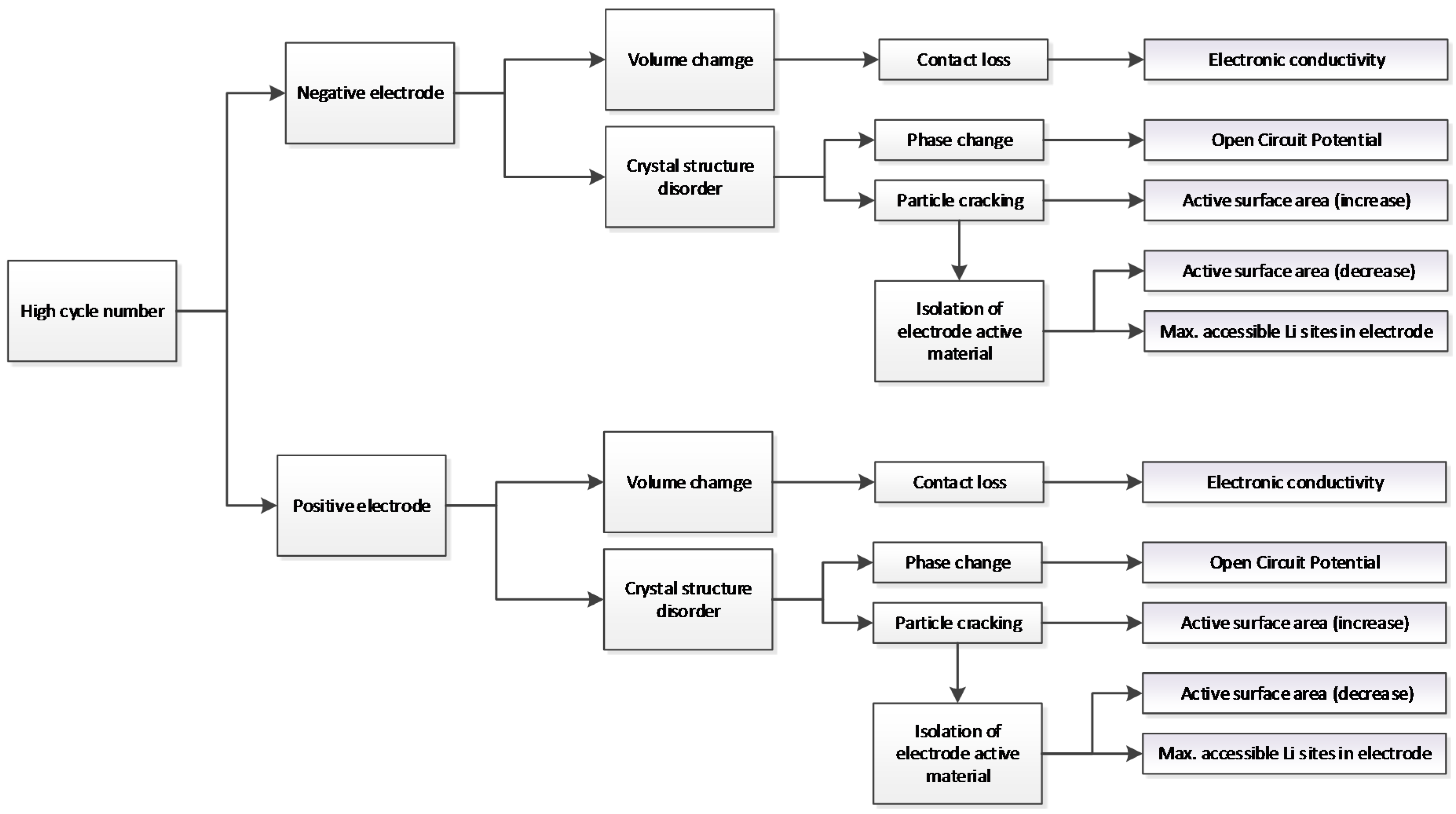
3.1. Expected Parameter Changes Resulting from Battery Degradation
3.2. Reducing the Number of Parameters Selected for Identification
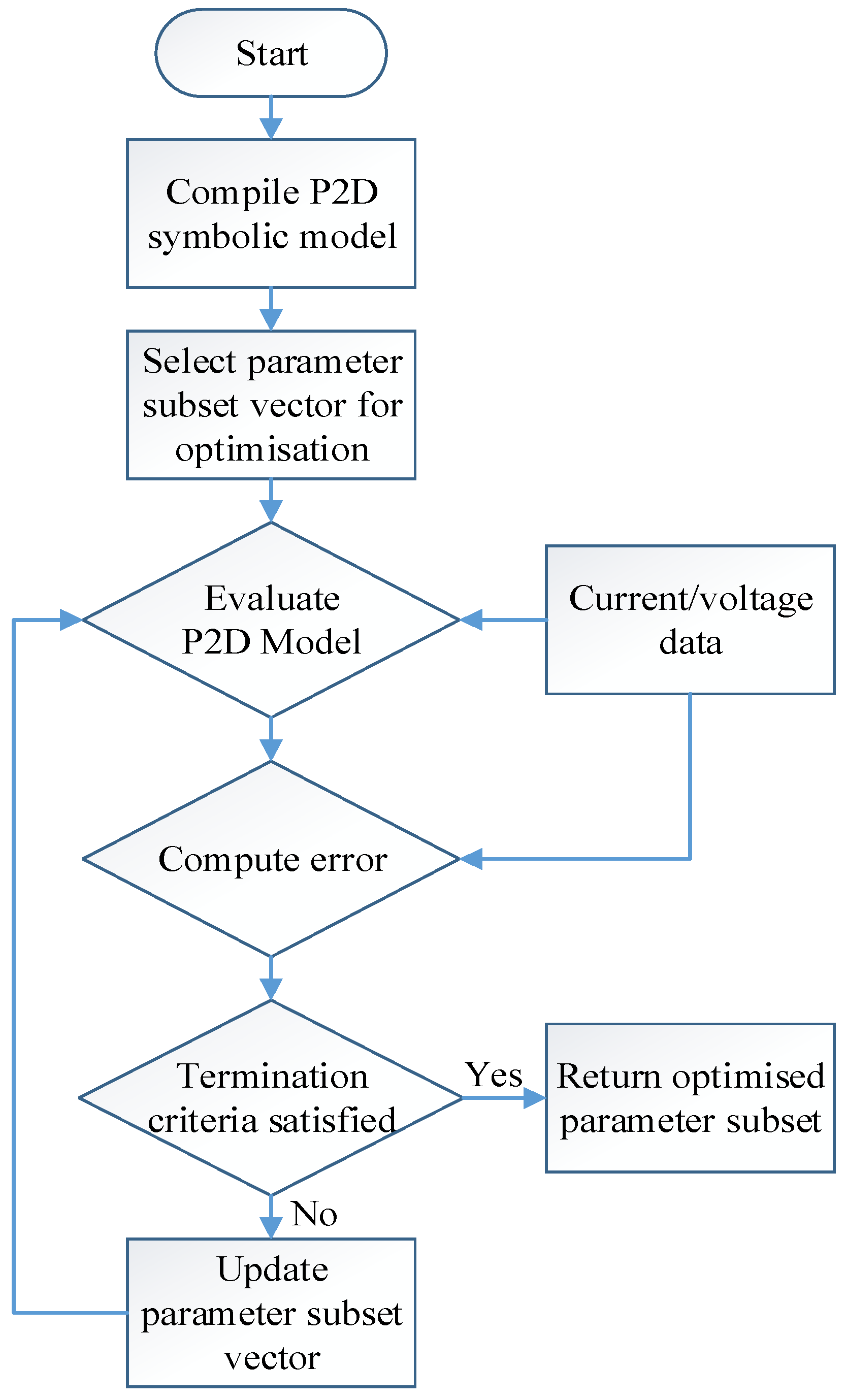
4. Parameter Identification Process
4.1. Optimisation Strategy
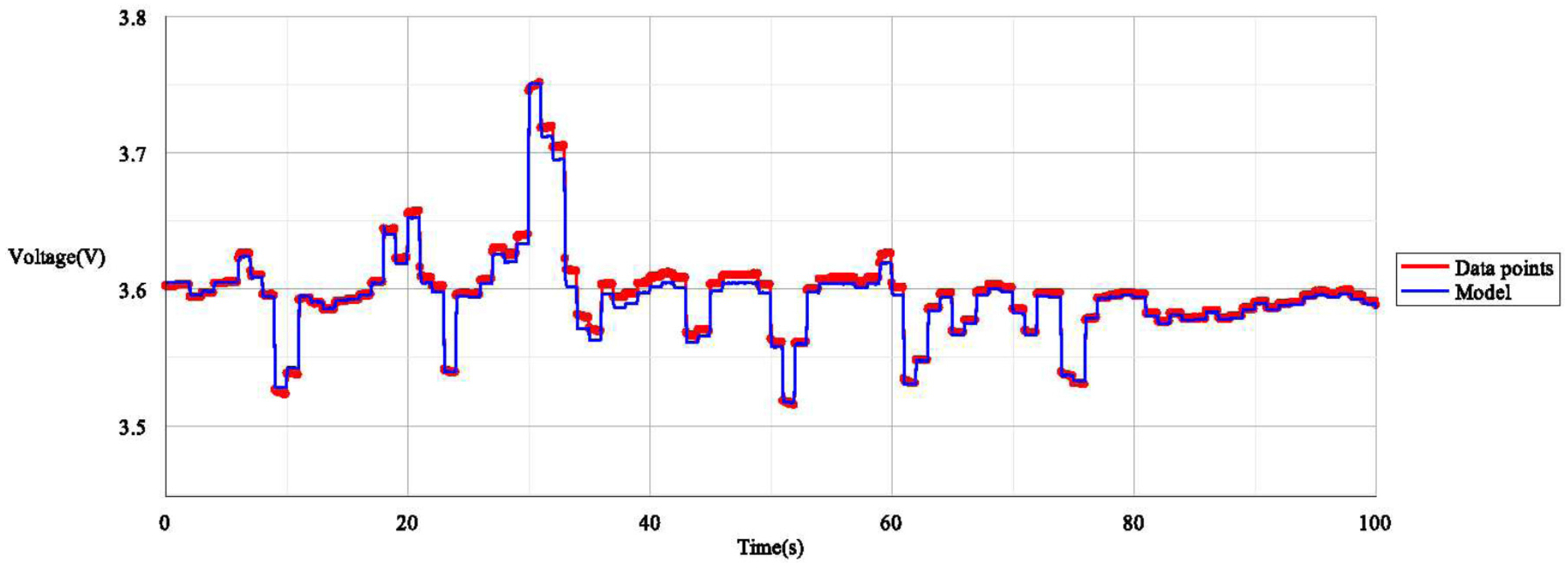
4.2. Verifying the Non-Aged Refined Parameter Set for LiNiCoAlO2
5. Results and Discussions
5.1. Conditions for the Cell Ageing Experiment
5.2. Battery Ageing Characterisation
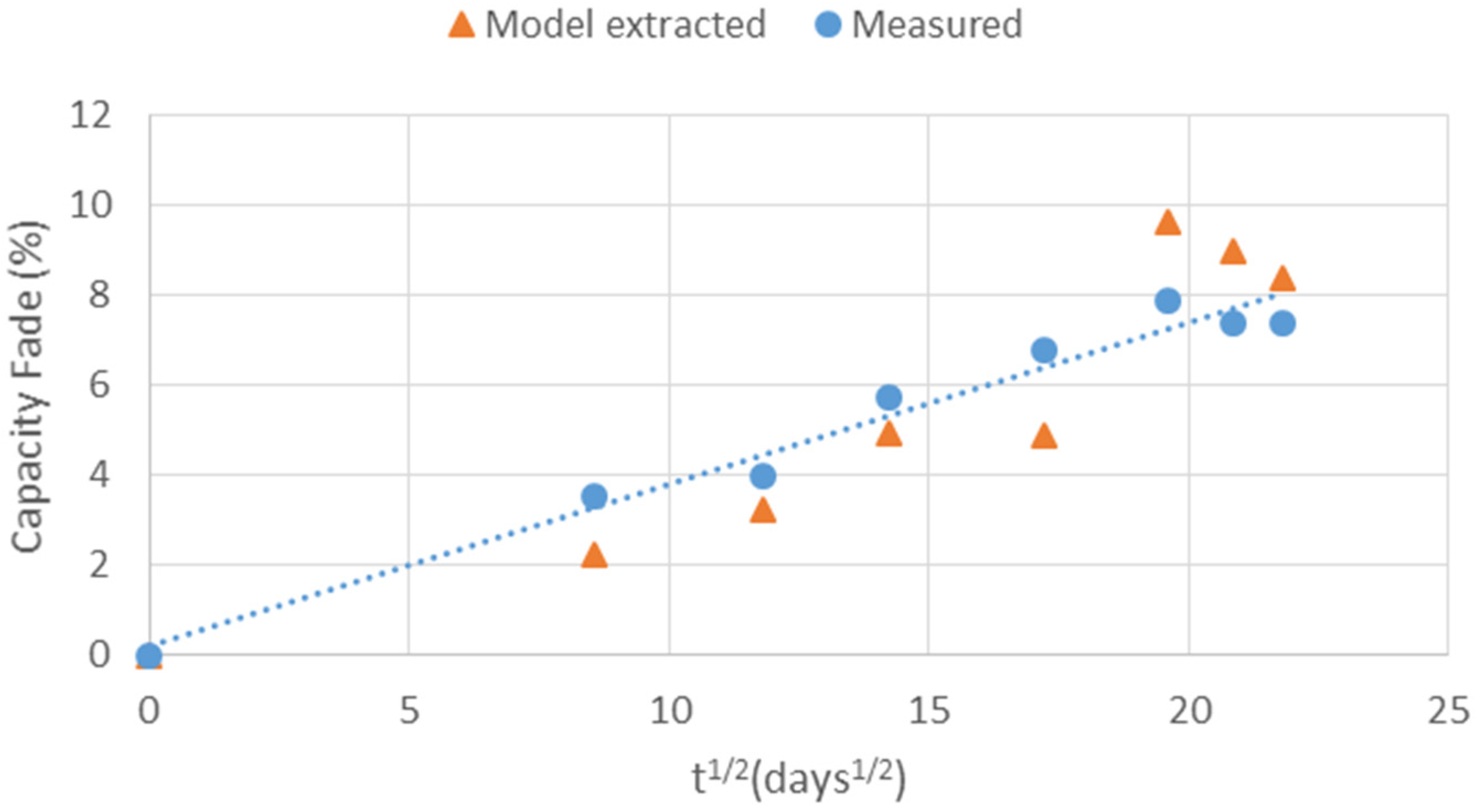
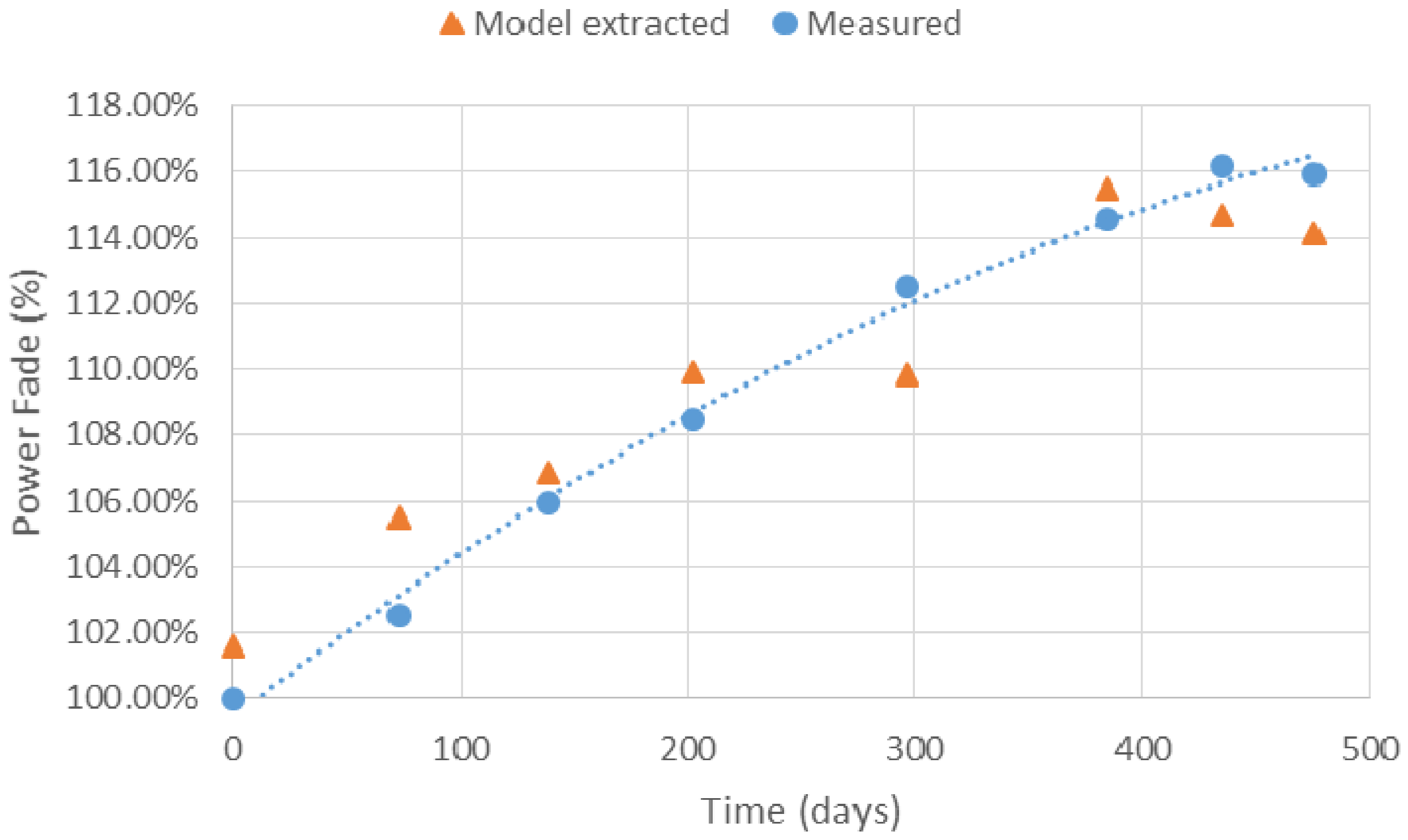
5.3. Battery Ageing Diagnostics Using Parameter Identification
5.4. Analysis of Model Extracted Results
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BMS | Battery management system |
| NCA | LiNiCoAlO2 |
| PHEV | Plug-in hybrid electric vehicle |
| P2D | Pseudo two-dimensional |
| SEI | Solid electrolyte interphase |
| SoC | State of charge |
| SP | Single particle |
| ΔSoC | Change in state of charge |
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Earleywine, M.; Wood, E.; Neubauer, J.; Pesaran, A. Comparison of Plug-In Hybrid Electric Vehicle Battery Life across Geographies and Drive Cycles. In Proceedings of the 2012 SAE World Congress and Exhibition, Detroit, MI, USA, 24–26 April 2012. [CrossRef]
- Uddin, K.; Picarelli, A.; Lyness, C.; Taylor, N.; Marco, J. An Acausal Li-Ion Battery Pack Model for Automotive Applications. Energies 2014, 7, 5675–5700. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef]
- Serrao, L.; Onori, S.; Sciarretta, A.; Guezennec, Y.; Rizzoni, G. Optimal Energy Management of Hybrid Electric Vehicles Including Battery Aging. In Proceedings of the 2011 American Control Conference (ACC), San Francisco, CA, USA, 29 June–1 July 2011; pp. 2125–2130.
- Moura, S.J.; Callaway, D.S.; Fathy, H.K.; Stein, J.L. Impact of Battery Sizing on Stochastic Optimal Power Management in Plug-In Hybrid Electric Vehicles. In Proceedings of the 2008 IEEE International Conference Vehicular Electronics and Safety, Columbus, OH, USA, 22–24 September 2008; pp. 96–102.
- Taylor, J.; Ball, R.; Mcgordon, A.; Marco, J. Sizing Tool for Rapid Optimisation of Pack Configuration at Early-Stage Automotive Product Development. In Proceedings of the EVS28 International Electric Vehicle Symposium and Exhibition, Goyang, Korea, 3–6 May 2015; pp. 1–8.
- Li, Y.; Tan, B.; Wu, Y. Mesoporous Co3O4 Nanowire Arrays for Lithium Ion Batteries with High Capacity and Rate Capability. Nano Lett. 2008, 8, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental investigation of the lithium-ion battery impedance characteristic at various conditions and aging states and its influence on the application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
- Uddin, K.; Perera, S.; Widanage, W.D.; Marco, J. Characterising Li-Ion Battery Degradation through the Identification of Perturbations in Electrochemical Battery Models. In Proceedings of the EVS28 International Electric Vehicle Symposium and Exhibition, Goyang, Korea, 3–6 May 2015; pp. 1–9.
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Schmidt, A.P.; Bitzer, M.; Imre, Á.W.; Guzzella, L. Experiment-driven electrochemical modeling and systematic parameterization for a lithium-ion battery cell. J. Power Sources 2010, 195, 5071–5080. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Guo, Q.; White, R.E. Parameter estimation and model discrimination for a lithium-ion cell. J. Electrochem. Soc. 2007, 154, A198–A206. [Google Scholar] [CrossRef]
- Forman, J.C.; Moura, S.J.; Stein, J.L.; Fathy, H.K. Genetic identification and fisher identifiability analysis of the Doyle–Fuller–Newman model from experimental cycling of a LiFePO4 cell. J. Power Sources 2012, 210, 263–275. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Lyu, C.; Li, J.; Zheng, J. Non-destructive analysis of degradation mechanisms in cycle-aged graphite/LiCoO2 batteries. Energies 2014, 7, 6282–6305. [Google Scholar] [CrossRef]
- Narayanrao, R.; Joglekar, M.M.; Inguva, S. A phenomenological degradation model for cyclic aging of lithium ion cell materials. J. Electrochem. Soc. 2012, 160, A125–A137. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Guo, Q.; Ramadass, P.; White, R.E. Review of models for predicting the cycling performance of lithium ion batteries. J. Power Sources 2006, 156, 620–628. [Google Scholar] [CrossRef]
- Dao, T.S.; Vyasarayani, C.P.; McPhee, J. Simplification and order reduction of lithium-ion battery model based on porous-electrode theory. J. Power Sources 2012, 198, 329–337. [Google Scholar] [CrossRef]
- Smith, K.; Wang, C.-Y. Solid-state diffusion limitations on pulse operation of a lithium ion cell for hybrid electric vehicles. J. Power Sources 2006, 161, 628–639. [Google Scholar] [CrossRef]
- Ye, Y.; Shi, Y.; Cai, N.; Lee, J.; He, X. Electro-thermal modeling and experimental validation for lithium ion battery. J. Power Sources 2012, 199, 227–238. [Google Scholar] [CrossRef]
- Doyle, M.; Newman, J.; Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.-M. Comparison of modeling predictions with experimental data from plastic lithium ion cells. J. Electrochem. Soc. 1996, 143, 1890–1903. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Bohlen, O.; Roscher, M.A.; Bäker, B. Current density and state of charge inhomogeneities in Li-ion battery cells with LiFePO4 as cathode material due to temperature gradients. J. Power Sources 2011, 196, 4769–4778. [Google Scholar] [CrossRef]
- Fu, R.; Xiao, M.; Choe, S.-Y. Modeling, validation and analysis of mechanical stress generation and dimension changes of a pouch type high power Li-ion battery. J. Power Sources 2013, 224, 211–224. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Hicks-Garner, J.; Sherman, E.; Soukiazian, S.; Verbrugge, M.; Tataria, H.; Musser, J.; Finamore, P. Cycle-life model for graphite-LiFePO4 cells. J. Power Sources 2011, 196, 3942–3948. [Google Scholar] [CrossRef]
- Savoye, F.; Venet, P.; Millet, M.; Groot, J. Impact of periodic current pulses on Li-ion battery performance. IEEE Trans. Ind. Electron. 2012, 59, 3481–3488. [Google Scholar] [CrossRef]
- Abraham, D.; Liu, J.; Chen, C.; Hyung, Y.; Stoll, M.; Elsen, N.; MacLaren, S.; Twesten, R.; Haasch, R.; Sammann, E.; et al. Diagnosis of power fade mechanisms in high-power lithium-ion cells. J. Power Sources 2003, 119–121, 511–516. [Google Scholar] [CrossRef]
- Broussely, M.; Herreyre, S.; Biensan, P.; Kasztejna, P.; Nechev, K.; Staniewicz, R. Aging mechanism in Li ion cells and calendar life predictions. J. Power Sources 2001, 97–98, 13–21. [Google Scholar] [CrossRef]
- Liao, L.; Zuo, P.; Ma, Y.; Chen, X.; An, Y.; Gao, Y.; Yin, G. Effects of temperature on charge/discharge behaviors of LiFePO4 cathode for Li-ion batteries. Electrochim. Acta 2012, 60, 269–273. [Google Scholar] [CrossRef]
- Gabrisch, H.; Wilcox, J.; Doeff, M.M. TEM study of fracturing in spherical and plate-like LiFePO4 particles. Electrochem. Solid State Lett. 2008, 11, A25–A29. [Google Scholar] [CrossRef]
- Chen, G.; Song, X.; Richardson, T.J. Electron microscopy study of the LiFePO4 to FePO4 phase transition. Electrochem. Solid State Lett. 2006, 9, A295–A298. [Google Scholar] [CrossRef]
- Aurbach, D. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Woodford, W.H.; Chiang, Y.-M.; Carter, W.C. “Electrochemical Shock” of Intercalation Electrodes: A Fracture Mechanics Analysis. J. Electrochem. Soc. 2010, 157, A1052–A1059. [Google Scholar] [CrossRef]
- Huang, H.-Y.S.; Wang, Y.-X. Dislocation based stress developments in lithium-ion batteries. J. Electrochem. Soc. 2012, 159, A815–A821. [Google Scholar] [CrossRef]
- Bourlot, S.; Blanchard, P.; Robert, S. Investigation of aging mechanisms of high power Li-ion cells used for hybrid electric vehicles. J. Power Sources 2011, 196, 6841–6846. [Google Scholar] [CrossRef]
- Himmelblau, D.M. Applied Nonlinear Programming; McGraw-Hill Companies: New York, NY, USA, 1972. [Google Scholar]
- Chakraborty, U.K. Advances in Differential Evolution; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Storn, R.; Price, K. Differential evolution—A simple and efficient heuristic for global optimization over continuous spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar] [CrossRef]
- Storn, R. On the Usage of Differential Evolution for Function Optimization. In Proceedings of the North American Fuzzy Information Processing Society, Berkeley, CA, USA, 19–22 June 1996; IEEE: New York, NY, USA; pp. 519–523.
- Doyle, M.; Fuentes, Y. Computer Simulations of a Lithium-Ion Polymer Battery and Implications for Higher Capacity Next-Generation Battery Designs. J. Electrochem. Soc. 2003, 150, A706–A713. [Google Scholar] [CrossRef]
- Pinson, M.; Bazant, M. Theory of SEI formation in rechargeable batteries: Capacity fade, accelerated aging and lifetime prediction. J. Electrochem. Soc. 2013, 160, A243–A250. [Google Scholar] [CrossRef]
- Ploehn, H.J.; Ramadass, P.; White, R.E. Solvent diffusion model for aging of lithium-ion battery cells. J. Electrochem. Soc. 2004, 151, A456–A462. [Google Scholar] [CrossRef]
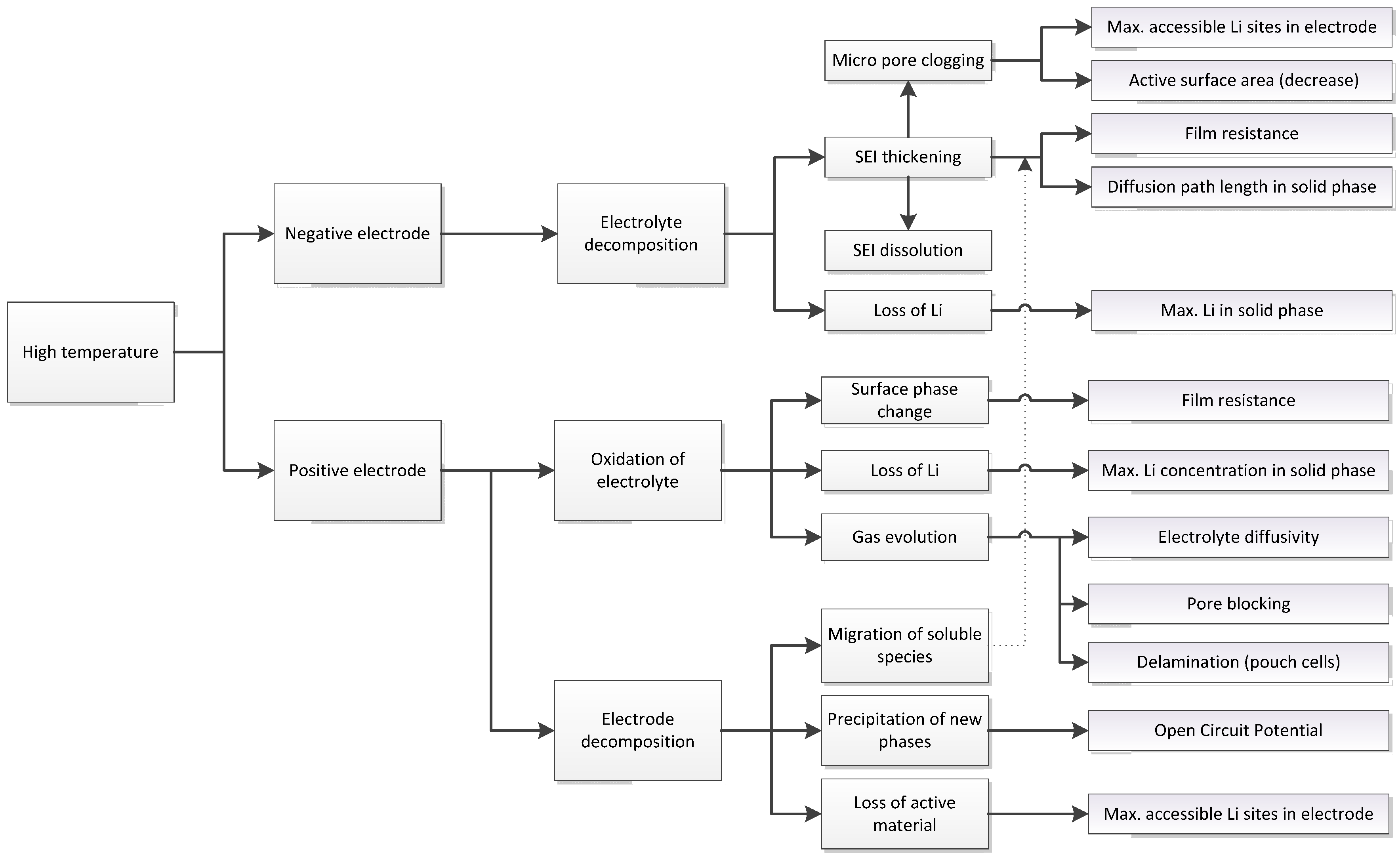
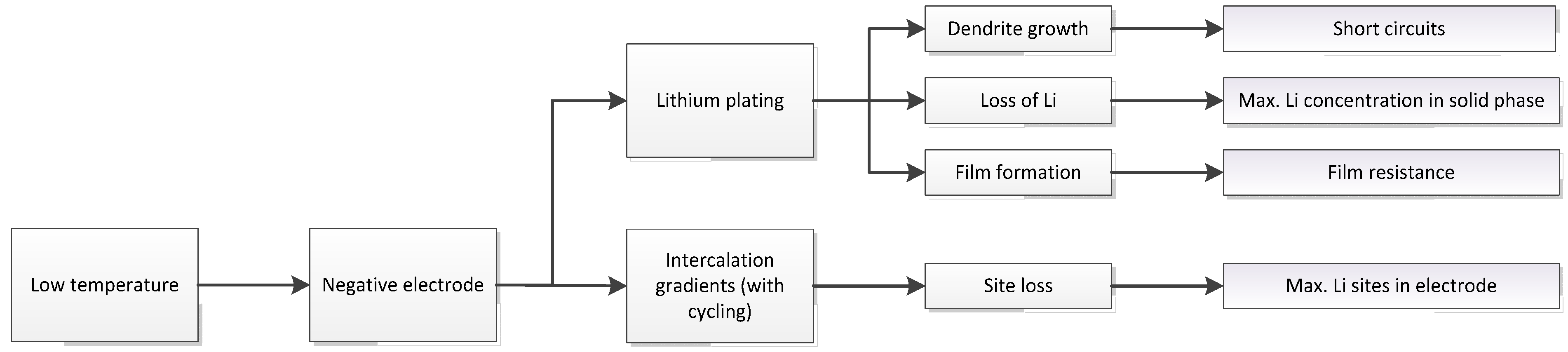
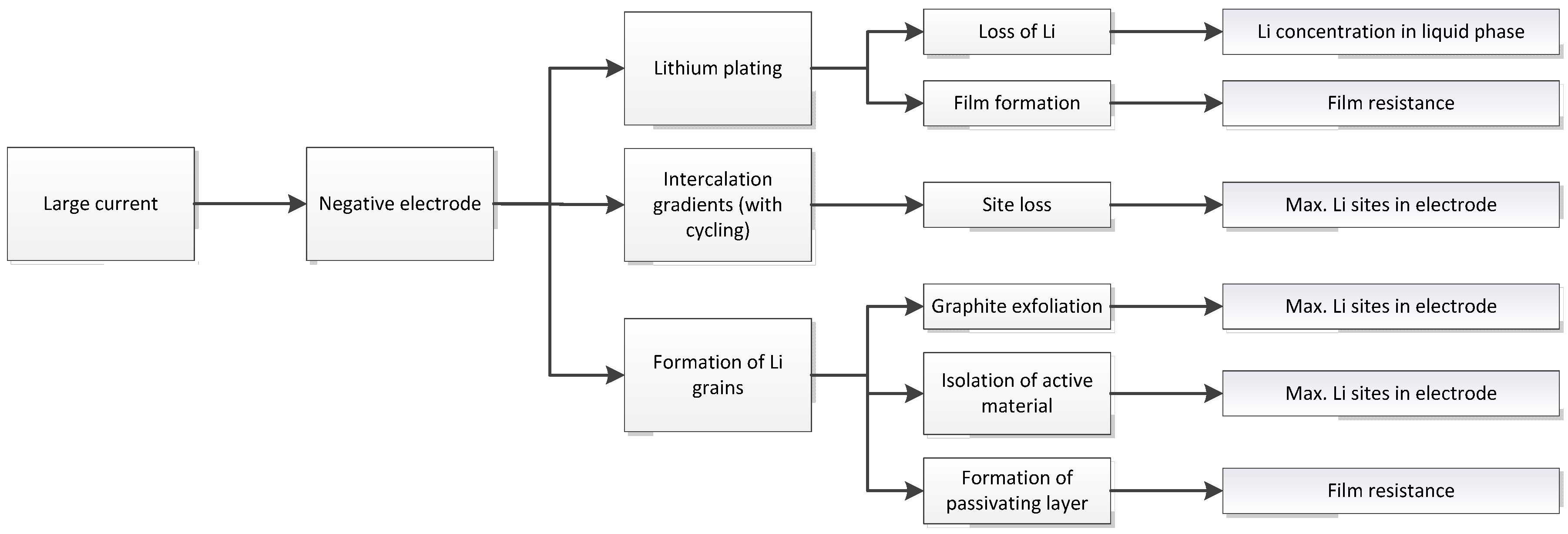

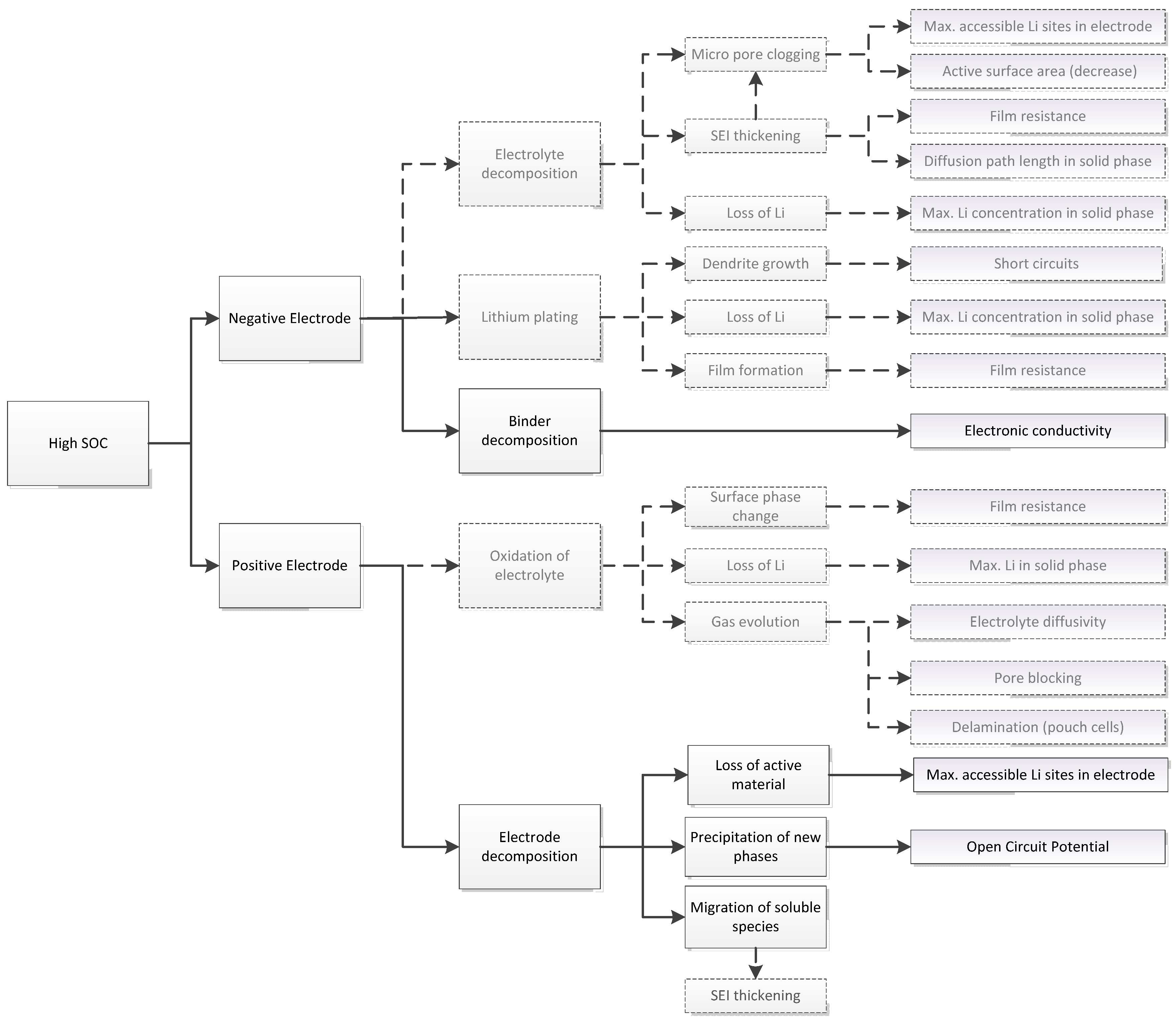

| Relation/Domain | Governing Equations | Boundary Conditions | |
|---|---|---|---|
| Conservation of charge | Electrolyte phase | ||
| Solid Phase | |||
| Conservation of lithium | Electrolyte phase | ||
| Solid Phase | , | ||
| Kinetics | Electrochemical reaction rate | ||
| Overpotential | |||
| Parameter | Symbol | Initial | Refined | Difference (%) |
|---|---|---|---|---|
| Thickness of negative electrode | L− (10−4 cm) | 149.9 | 153.1 | 2.1 |
| Thickness of positive electrode | L+ (10−4 cm) | 134.0 | 129.8 | −3.2 |
| Thickness of separator | Lsep (10−4 cm) | 25.0 | 27.5 | 9.1 |
| Surface area of negative electrode, | A− (cm2) | 428.4 | 437.5 | 2.1 |
| Surface area of positive electrode | A+ (cm2) | 389.61 | 377.4 | −3.2 |
| Surface area of separator | Asep (cm2) | 448.35 | 493.2 | 9.1 |
| Modal radius of negative electrode particle | Rs,− (10−4 cm) | 10.7 | 10.9 | 1.8 |
| Modal radius of positive electrode particle | Rs,+ (10−4 cm) | 5.7 | 7.9 | 27.8 |
| Active material volume fraction of negative electrode | εs,− | 0.3 | 0.31 | 3.2 |
| Active material volume fraction of positive electrode | εs,+ | 0.3 | 0.49 | 38.8 |
| Electrolyte phase volume fraction of negative electrode | εe,− | 0.595 | 0.595 | 0.0 |
| Electrolyte phase volume fraction of positive electrode | εe,+ | 0.63 | 0.63 | 0.0 |
| Volume fraction of separator in liquid phase | εe,sep | 0.5 | 0.45 | −11.1 |
| Volume fraction of inactive material in negative electrode | εf,− | 0.105 | 0.04 | −162.5 |
| Volume fraction of inactive material in positive electrode | εf,+ | 0.07 | 0.99 | 92.9 |
| Maximum li-concentration in negative electrode | (10−3 mol·cm−3) | 30.6 † | 30.6 | 0.0 |
| Maximum li-concentration in positive electrode | (10−3 mol·cm−3) | 51.6 † | 51.0 | −1.2 |
| Average electrolyte concentration | ce,0 (10−3 mol·cm−3) | 1.2 * | 1.04 | −15.4 |
| Stoichiometry of negative electrode at 0% SoC | x−,0 | 0.126 * | 0.126 | 0.0 |
| Stoichiometry of positive electrode at 0% SoC | x+,0 | 0.936 * | 0.936 | 0.0 |
| Stoichiometry of negative electrode at 100% SoC | x−,100 | 0.676 * | 0.676 | 0.0 |
| Stoichiometry of positive electrode at 100% SoC | x+,100 | 0.442 * | 0.442 | 0.0 |
| Diffusion coefficient of negative electrode in solid phase | Ds,− (10−12 cm2·s−1) | 1.14 | 1.14 | 0.0 |
| Diffusion coefficient of positive electrode in solid phase | Ds,+ (10−12 cm2·s−1) | 3.7 * | 3.7 | 0.0 |
| Diffusion coefficient in liquid phase | De (10−6 cm2·s−1) | 2.6 * | 2.6 | 0.0 |
| Conductivity of negative electrode in solid phase | σs,− (S·cm−1) | 1.0 * | 1 | 0.0 |
| Conductivity of positive electrode in solid phase | σs,+ (S·cm−1) | 1.0 † | 1 | 0.0 |
| Charge transfer coefficient in negative electrode | ke,− (10−6 cm2.5·mol−0.5·s−1) | 5.03 | 5.03 | 0.0 |
| Charge transfer coefficient in positive electrode | ke,+ (10−6 cm2.5·mol−0.5·s−1) | 2.33 | 2.33 | 0.0 |
| Anodic charge transfer coefficient | αa | 0.5 | 0.5 | 0.0 |
| Cathodic charge transfer coefficient | αc | 0.5 | 0.5 | 0.0 |
| Li transference number | 0.36 | 0.36 | 0.0 | |
| Electrolyte activity coefficient | 1.0 | 1 | 0.0 | |
| Bruggeman porosity exponent | p | 1.5 | 1.5 | 0.0 |
| Resistivity of film layers (including SEI) | - | 0.1 | 0.001 | −990 |
| Resistivity of the current collector | Rf (Ω·cm2) | 20 | 10 | −100 |
| Negative electrode potential, coefficients | - | - | - | |
| Positive electrode potential, , coefficients | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, K.; Perera, S.; Widanage, W.D.; Somerville, L.; Marco, J. Characterising Lithium-Ion Battery Degradation through the Identification and Tracking of Electrochemical Battery Model Parameters. Batteries 2016, 2, 13. https://doi.org/10.3390/batteries2020013
Uddin K, Perera S, Widanage WD, Somerville L, Marco J. Characterising Lithium-Ion Battery Degradation through the Identification and Tracking of Electrochemical Battery Model Parameters. Batteries. 2016; 2(2):13. https://doi.org/10.3390/batteries2020013
Chicago/Turabian StyleUddin, Kotub, Surak Perera, W. Dhammika Widanage, Limhi Somerville, and James Marco. 2016. "Characterising Lithium-Ion Battery Degradation through the Identification and Tracking of Electrochemical Battery Model Parameters" Batteries 2, no. 2: 13. https://doi.org/10.3390/batteries2020013







