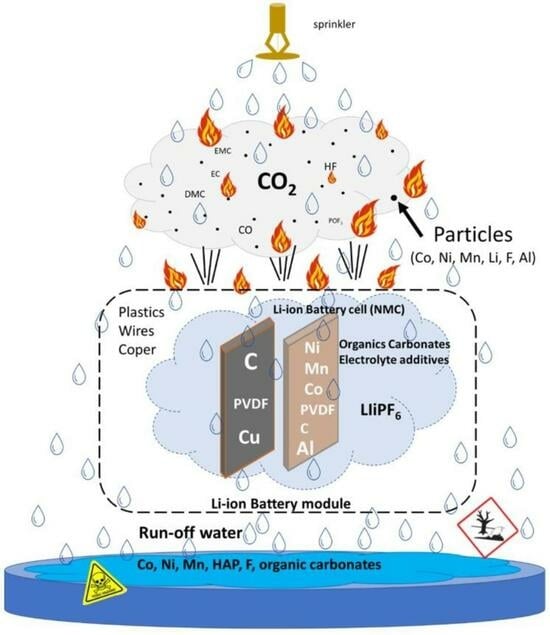
Assessment of Run-Off Waters Resulting from Lithium-Ion Battery Fire-Fighting Operations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Samples
2.2. Abuse Tests Set Up
2.3. Water Sampling
2.4. Water Analysis
2.4.1. Inductively Couple Plasma Optical Emission Spectroscopy
2.4.2. Inductively Couple Plasma Mass Spectrometry
2.4.3. Ion Chromatography
2.4.4. Liquid Chromatography
2.4.5. Gas Chromatography
2.5. Particle Morphology Characterization
3. Results
3.1. Test Conditions and TR Characteristics
3.2. Characterization of Water Contamination
3.2.1. Halogens and Metals
3.2.2. Poly Aromatic Hydrocarbons (PAHs)
3.2.3. Organic Carbonates
3.2.4. Particle Size Analysis
3.2.5. pH Measurement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giger, W. The Rhine red, the fish dead—The 1986 Schweizerhalle disaster, a retrospect and long-term impact assessment. Environ. Sci. Pollut. Res. 2009, 16, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Schwabach, A. The Sandoz spill: The failure of international law to protect the Rhine from pollution. Ecol. LQ 1989, 16, 443. [Google Scholar]
- Zhang, H.; Duan, H.; Zuo, J.; Song, M.; Zhang, Y.; Yang, B.; Niu, Y. Characterization of post-disaster environmental management for Hazardous Materials Incidents: Lessons learnt from the Tianjin warehouse explosion, China. J. Environ. Manag. 2017, 199, 21–30. [Google Scholar] [CrossRef] [PubMed]
- EPRI. Battery Firewater Composition and Risk Assessment. 2020. Available online: https://www.epri.com/research/products/3002020017/ (accessed on 2 February 2024).
- ISO TR 26368; Environmental Damage Limitation from Fire Fighting Water Run-Off (under Revision). ISO: Geneva, Switzerland, 2012.
- Larsson, F.; Andersson, P.; Blomqvist, P.; Lorén, A.; Mellander, B.-E. Characteristics of lithium-ion batteries during fire tests. J. Power Sources 2014, 271, 414–420. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, D.; Maleki, A.; Kohzadi, S.; Liu, Y.; Chen, Y.; Liu, R.; Gao, G.; Zhi, J. Characterization of Thermal-Runaway Particles from Lithium Nickel Manganese Cobalt Oxide Batteries and Their Biotoxicity Analysis. ACS Appl. Energy Mater. 2021, 4, 10713–10720. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Yan, W. Identification and characteristic analysis of powder ejected from a lithium ion battery during thermal runaway at elevated temperatures. J. Hazard. Mater. 2020, 400, 123169. [Google Scholar] [CrossRef]
- Bordes, A.; Marlair, G.; Zantman, A.; Herreyre, S.; Papin, A.; Desprez, P.; Lecocq, A. New insight on the risk profile pertaining to lithium-ion batteries under thermal runaway as affected by system modularity and subsequent oxidation regime. J. Energy Storage 2022, 52, 104790. [Google Scholar] [CrossRef]
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- McNamee, M.; Marlair, G.; Truchot, B.; Meacham, B.J. Research Roadmap: Environmental Impact of Fires in the Built Environment; Fire Protection Research Foundation: Norfolk County, MA, USA, 2020. [Google Scholar]
- Rappsilber, T.; Yusfi, N.; Krüger, S.; Hahn, S.-K.; Fellinger, T.-P.; von Nidda, J.K.; Tschirschwitz, R. Meta-analysis of heat release and smoke gas emission during thermal runaway of lithium-ion batteries. J. Energy Storage 2023, 60, 106579. [Google Scholar] [CrossRef]
- Vilic, A. Environmental Risk Assessment of Fire-Water Runoff from Vehicle Fire-Development of a Predictive Model Intended for the Fire-Rescue Service; Fire Protection Research Foundation: Norfolk County, MA, USA, 2019. [Google Scholar]
- Noiton, D.; Fowles, J.; Davies, H. The ecotoxicity of Fire Water Run-off, Part II: Analytical Results, New Zealand Fire Service Commission Research Report Number 18. 2001. ISBN 0-908920-61. Available online: https://www.researchgate.net/publication/272508692_Fire_Research_The_Ecotoxicity_of_Fire-Water_Runoff_Part_II_Analytical_Results_ESR#fullTextFileContent (accessed on 3 March 2024).
- EMPA. Minimization of Fire Risks from Electric Vehicles in Underground Traffic Infrastructures. 2018. Available online: https://plus.empa.ch/images/2020-08-17_Brandversuch-Elektroauto/AGT_2018_006_EMob_RiskMin_Undergr_Infrastr_Final_Report_V1.0.pdf (accessed on 2 February 2024).
- Held, M.; Tuchschmid, M.; Zennegg, M.; Figi, R.; Schreiner, C.; Mellert, L.D.; Welte, U.; Kompatscher, M.; Hermann, M.; Nachef, L. Thermal runaway and fire of electric vehicle lithium-ion battery and contamination of infrastructure facility. Renew. Sustain. Energy Rev. 2022, 165, 112474. [Google Scholar] [CrossRef]
- RIVM. Risico’s Van Rook Door Branden Van Li-Ionbatterijen. 2021. Available online: https://www.rivm.nl/bibliotheek/rapporten/2021-0019.pdf (accessed on 2 February 2024).
- Quant, M.; Willstrand, O.; Mallin, T.; Hynynen, J. Ecotoxicity Evaluation of Fire-Extinguishing Water from Large-Scale Battery and Battery Electric Vehicle Fire Tests. Environ. Sci. Technol. 2023, 57, 4821–4830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Li, W.; Li, C.; Ouyang, M. Size distribution and elemental composition of vent particles from abused prismatic Ni-rich automotive lithium-ion batteries. J. Energy Storage 2019, 26, 100991. [Google Scholar] [CrossRef]
- Dyer, J.A.; Scrivner, N.C.; Dentel, S.K. A practical guide for determining the solubility of metal hydroxides and oxides in water. Environ. Prog. 1998, 17, 1–8. [Google Scholar] [CrossRef]
- Winberry, W.T.; Murphy, N.T.; Riggan, R. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air; EPA, National Service Center for Environmental Publications: Washington, DC, USA, 1988.
- Geiser, M.; Kreyling, W.G. Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 2010, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef] [PubMed]
- Rivero Arze, A.; Mouneyrac, C.; Chatel, A.; Manier, N. Comparison of uptake and elimination kinetics of metallic oxide nanomaterials on the freshwater microcrustacean Daphnia magna. Nanotoxicology 2021, 15, 1168–1179. [Google Scholar] [CrossRef]
- Rivero Arze, A.; Manier, N.; Chatel, A.; Mouneyrac, C. Characterization of the nano–bio interaction between metallic oxide nanomaterials and freshwater microalgae using flow cytometry. Nanotoxicology 2020, 14, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants (accessed on 2 February 2024).
- Available online: https://www.canada.ca/fr/sante-canada/services/publications/vie-saine/recommandations-pour-qualite-eau-potable-canada-chlore-document-technique/page-3-recommandations-pour-qualite-eau-potable-canada-chlore-document-technique.html (accessed on 2 February 2024).
- Ottawa City Service. Guide Discharging Waste Water Ind. Facilities. 2011. Available online: https://nchca.ca/wp-content/uploads/2017/06/Sewer-Use-Program_English_2011.pdf (accessed on 22 March 2024).
- LCPP. Étude de l’Impact de Feux de Véhicules Electriques (RENAULT) sur les Intervenants des Services de Secours. 2015. Available online: http://iuv.sdis86.net/wp-content/uploads/2015/09/Rapport-LCPP-brulages-vehicules-electriques-Renault.pdf (accessed on 2 February 2024).
- Alberto Project. Available online: https://alberoprojekt.de/index_htm_files/WP%201.4%20Contamination%20of%20extinguishing%20water%20after%20fires%20of%20Li-Ion%20Batteries.pdf (accessed on 2 February 2024).
- Merseyside Fire & Rescue Service. SIGNIFICANT INCIDENT REPORT—018965—15092020—Orsted BESS, Carnegie Road, Liverpool, L137HY. 2021. Available online: https://planningregister.cherwell.gov.uk/Document/Download?module=PLA&recordNumber=154109&planId=1951104&imageId=30&isPlan=False&fileName=Appendix%202%20%20-%20Liverpool%20BESS%20Significant%20Investigation%20Report%20%281%29.pdf (accessed on 28 March 2024).
- BEA-RI. Rapport d’Enquête Technique sur l’Incendie au Sein du Poste de Transformation RTE. de Perles et Castelet (09). 2021. Available online: https://www.igedd.developpement-durable.gouv.fr/IMG/pdf/rapportperlesvdif_cle286783.pdf (accessed on 2 February 2024).
- Tesla. Model 3 Emergency Response Guide. 2018. Available online: https://www.tesla.com/sites/default/files/downloads/2017_Model_3_Emergency_Response_Guide_en.pdf (accessed on 2 February 2024).
- Zhang, L.; Duan, Q.; Liu, Y.; Xu, J.; Sun, J.; Xiao, H.; Wang, Q. Experimental investigation of water spray on suppressing lithium-ion battery fires. Fire Saf. J. 2021, 120, 103117. [Google Scholar] [CrossRef]
- Available online: https://echa.europa.eu/fr/home (accessed on 2 February 2024).
- Available online: https://substances.ineris.fr/fr/substance/1301/3 (accessed on 2 February 2024).
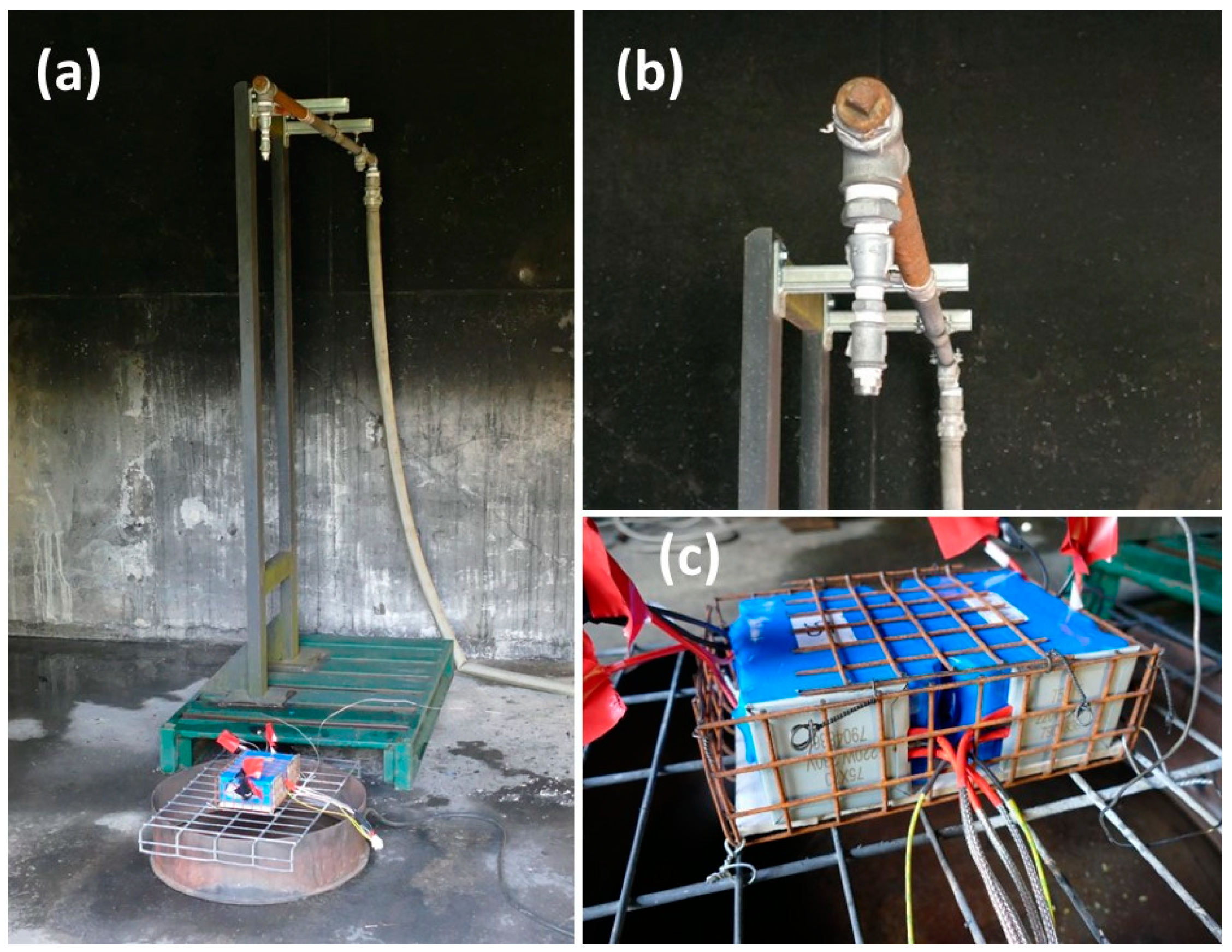

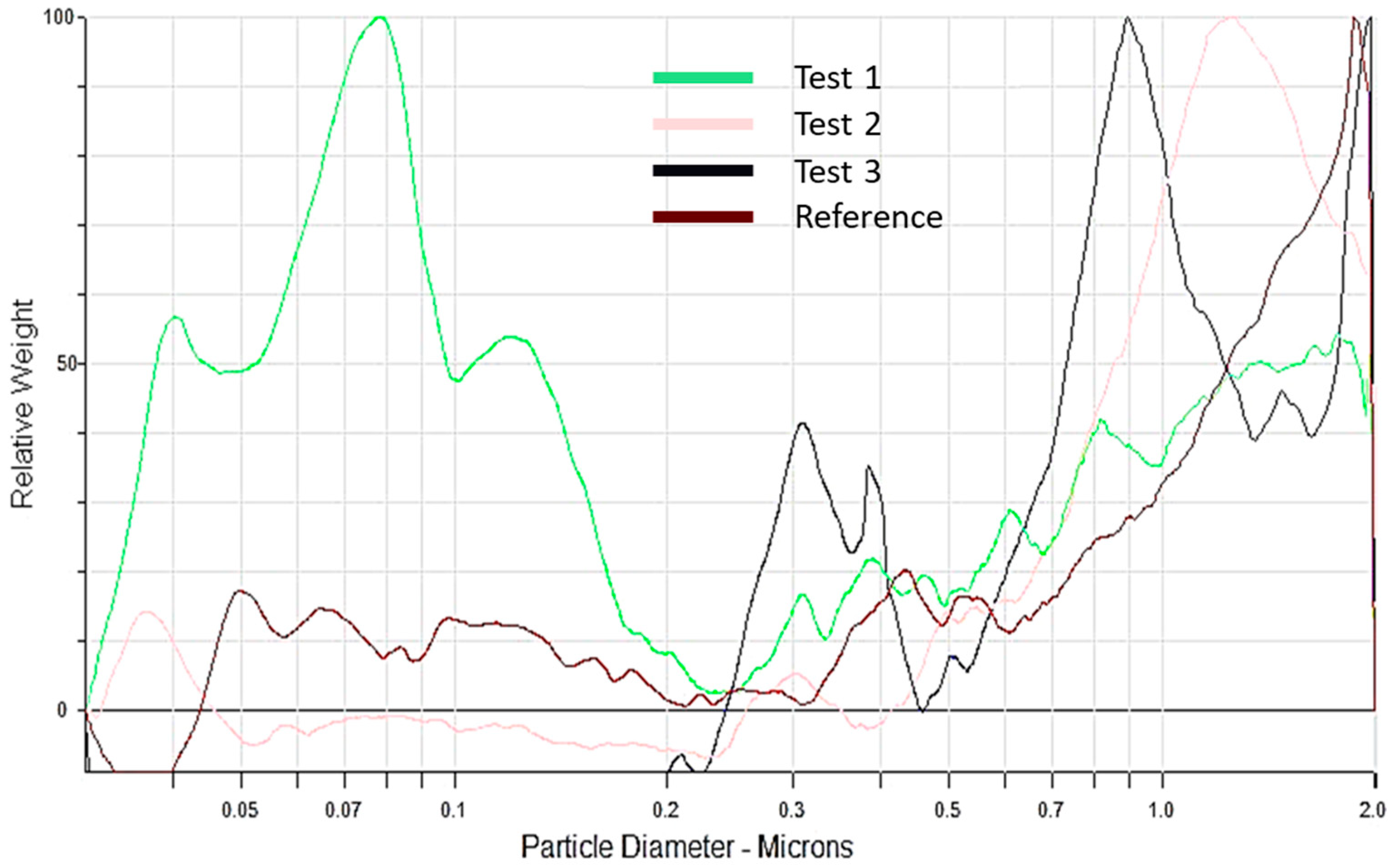
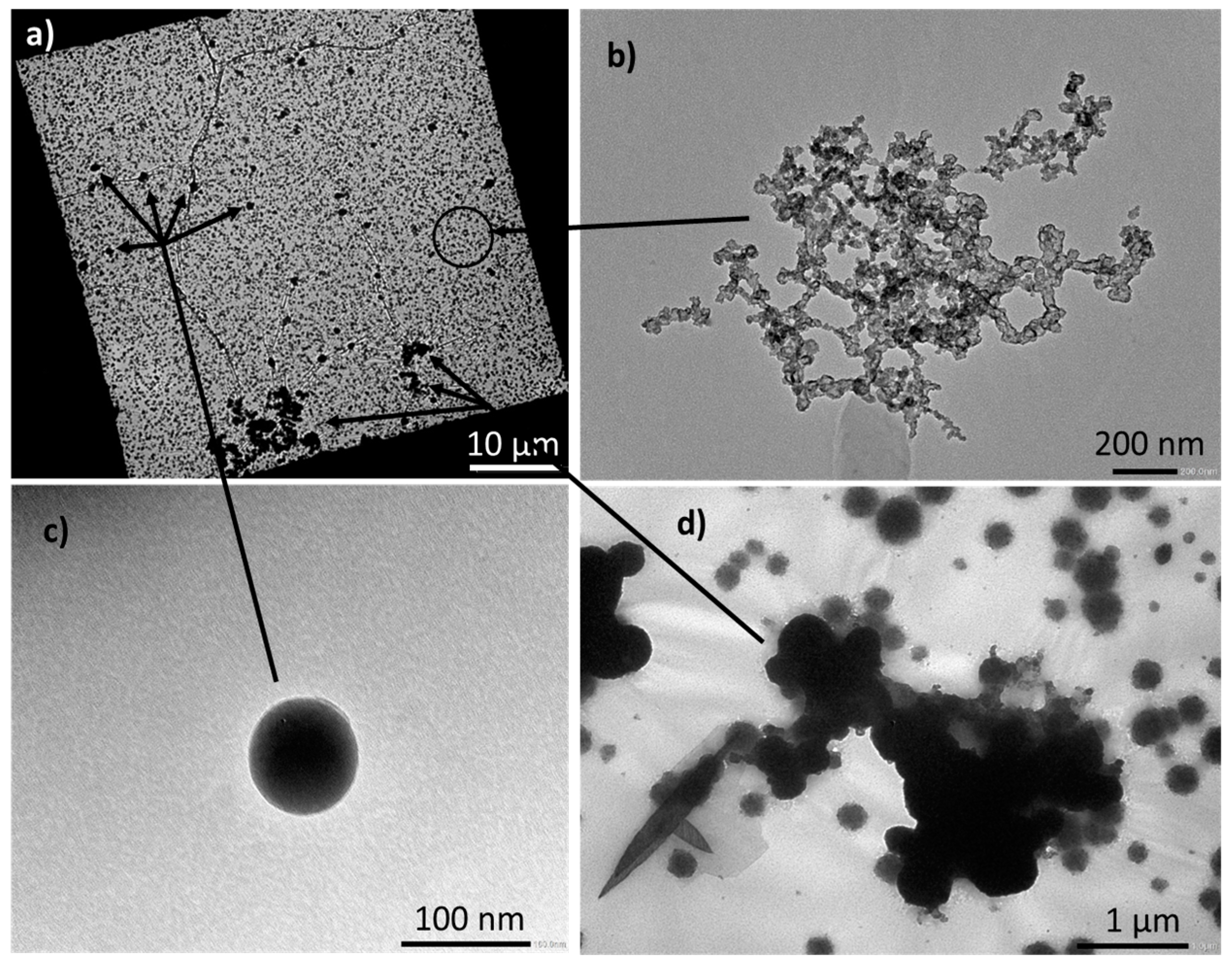
| Module Type | Module Energy | Heating Method | Reaction | Module State after Test | Sprinkler Flow | Amount of Water Delivered | |
|---|---|---|---|---|---|---|---|
| Test 1 | Prismatic cell assembly NMC (module A) | 500 Wh | Gas burner | Venting + moderate fire | Upper plastic burnt Mechanical integrity conserved No module casing opening | 10 L/m2/min | 7 L |
| Test 2 | Prismatic cell assembly NMC (module A) | 500 Wh | Gas burner | Jet fire + explosion | Module casing ejected All cells fully burnt with casing damaged | 10 L/m2/min | 7 L |
| Test 3 | Cylindrical cell assembly NMC (module B) | 900 Wh | Thermal pad | Jet fire + explosion | All cells burnt Some jelly rolls visible | 10 L/m2/min | 9 L |
| QL | Uncertainty | Reference | Test 1 (Module A) | Test 2 (Module A) | Test 3 (Module B) | |
|---|---|---|---|---|---|---|
| Ions | ||||||
| F− (mg/L) | 0.05 | 8% | 0.25 | 142 | 91.6 | 93.7 |
| Cl− (mg/L) | 0.01 | 3% | 24.9 | 33.4 | 36.5 | 203 |
| Metals | ||||||
| Al (mg/L) | 0.17 | 15% | 0.91 | 74.2 | 29.3 | 73.9 |
| Co (mg/L) | 0.03 | 10% | <LQ | 0.42 | 12.8 | 7.07 |
| Cu (mg/L) | 0.03 | 10% | 0.04 | 0.30 | 0.26 | 4.18 |
| Fe (mg/L) | 0.08 | 9% | 0.30 | 5.92 | 4.59 | 0.30 |
| Li (mg/L) | 1.67 | 15% | <LQ | 44.5 | 27.8 | 360 |
| Mn (mg/L) | 0.03 | 10% | <LQ | 1.22 | 17.0 | 5.82 |
| Na (mg/L) | 1.67 | 14% | 13.0 | 15.6 | 16.3 | 26.2 |
| Ni (mg/L) | 0.08 | 12% | <LQ | 3.25 | 49.0 | 40.1 |
| P (mg/L) | 0.17 | 17% | <LQ | 201 | 113 | 5.80 |
| PAH (Polycyclic Aromatic Hydrocarbons) | |||||
|---|---|---|---|---|---|
| QL | Reference | Test 1 (Module A) | Test 2 (Module A) | Test 3 (Module B) | |
| Naphtalène (ng/L) | 10.0 | <LQ | 1279.2 | 2792.2 | 3114.6 |
| Acénaphtylène (ng/L) | 40.0 | <LQ | 2421.7 | 2405.1 | 1193.4 |
| méthyl-1.naphtalène (ng/L) | 10.0 | <LQ | 26.8 | 459.4 | 667.1 |
| méthyl-2.naphtalène (ng/L) | 10.0 | <LQ | 203.2 | <LQ | 2058.4 |
| Acénaphtène (ng/L) | 2.0 | <LQ | 34.1 | 110.6 | 275.7 |
| Fluorene (ng/L) | 2.0 | <LQ | 74.1 | 752.3 | 1055.0 |
| Phénanthrène (ng/L) | 4.0 | 5.7 | 360.9 | 3026.8 | 2581.6 |
| Anthracène (ng/L) | 2.0 | <LQ | 10.6 | 330.5 | 303.3 |
| Fluoranthène (ng/L) | 2.0 | 10.8 | 57.7 | 1280.9 | 349.8 |
| Pyrène (ng/L) | 2.0 | 7.2 | 45.1 | 1279.8 | 20.5 |
| méthyl-2.fluoranthène (ng/L) | 4.0 | <LQ | 7.3 | 45.1 | 21.3 |
| B(a)A (ng/L) | 2.0 | <LQ | 24.8 | 185.7 | 131.8 |
| Chrysene (ng/L) | 2.0 | <LQ | 32.5 | 212.3 | 40.8 |
| Retene (ng/L) | 2.0 | <LQ | 104.9 | 170.7 | 19.8 |
| B(e)P (ng/L) | 2.0 | <LQ | 7.5 | 306.3 | 50.4 |
| B(j)F (ng/L) | 20.0 | <LQ | <LQ | 106.3 | <LQ |
| B(b)F (ng/L) | 2.0 | <LQ | 34.6 | 259.6 | 5.8 |
| B(k)F (ng/L) | 2.0 | <LQ | 8.3 | 81.0 | 8.2 |
| B(a)P (ng/L) | 2.0 | <LQ | 13.0 | 163.9 | 20.8 |
| D(a.h)A (ng/L) | 2.0 | <LQ | <LQ | 36.7 | 4.5 |
| benzo(ghi)P (ng/L) | 2.0 | <LQ | 13.3 | 169.6 | 4.1 |
| Indèno (ng/L) | 4.0 | <LQ | 35.2 | 162.1 | 11.8 |
| Coronene (ng/L) | 2.0 | <LQ | 4.0 | 54.0 | <LQ |
| Species | QL | Reference | Test 1 (Module A) | Test 2 (Module A) | Test 3 (Module B) |
|---|---|---|---|---|---|
| DMC (µg/mL) | 8.8 | n/a | n/a | n/a | n/a |
| EMC (µg/mL) | 8.3 | n/a | 138 | 59 | n/a |
| VC (µg/mL) | 9.4 | n/a | n/a | n/a | n/a |
| DEC (µg/mL) | 8.1 | n/a | n/a | n/a | n/a |
| FEC (µg/mL) | 10.2 | n/a | n/a | n/a | n/a |
| EC (µg/mL) | 7.7 | n/a | 1082 | 461 | n/a |
| PC (µg/mL) | 10 | n/a | n/a | n/a | n/a |
| Test 1 (Module A) | Test 2 (Module A) | Test 3 (Module B) | |
|---|---|---|---|
| pH | 5.2 | 5.9 | 11 |
| pH Limit Values | Drinkable Water | Industrial Effluent Value for Discharge in Sewage Systems |
|---|---|---|
| EPA [27] (USA) | 6.5 to 9 | |
| Canada [28,29] | 7 to 10.5 | 6–9 |
| Switzerland [16,17] | 6.8 to 8.2 | 6.5 to 9 |
| Substance | Test 1 (Module A) | Test 2 (Module A) | Test 3 (Module B) | PNEC Freshwater |
|---|---|---|---|---|
| Al (mg/L) | 8.7 | 5.9 | 6.2 | - |
| Co (mg/L) | 0.05 | 2.6 | 0.6 | 0.00106 |
| Cu (mg/L) | 0.04 | 0.05 | 0.3 | 0.0063 |
| Fe (mg/L) | Test 1 (module A) | Test 2 (module A) | Test 3 (module B) | |
| Li (mg/L) | pH | 5.2 | 5.9 | 11 |
| Mn (mg/L) | 0.1 | 3.4 | 0.5 | 0.034 |
| Na (mg/L) | 1.8 | 3.3 | 2.2 | - |
| Ni (mg/L) | 0.4 | 9.8 | 3.3 | - |
| P (mg/L) | 23.5 | 22.6 | 0.5 | - |
| Fluorides (mg/L) | 16.6 | 18.3 | 7.8 | 0.89 |
| Chlorides (mg/L) | 3.9 | 7.3 | 16.9 | - |
| EMC (mg/L) | 16.1 | 11.8 | n/a | 0.062 |
| EC (mg/L) | 126.2 | 92.2 | n/a | 5.9 |
| Naphthalene (mg/L) | 0.00015 | 0.00056 | 0.00026 | 0.0024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordes, A.; Papin, A.; Marlair, G.; Claude, T.; El-Masri, A.; Durussel, T.; Bertrand, J.-P.; Truchot, B.; Lecocq, A. Assessment of Run-Off Waters Resulting from Lithium-Ion Battery Fire-Fighting Operations. Batteries 2024, 10, 118. https://doi.org/10.3390/batteries10040118
Bordes A, Papin A, Marlair G, Claude T, El-Masri A, Durussel T, Bertrand J-P, Truchot B, Lecocq A. Assessment of Run-Off Waters Resulting from Lithium-Ion Battery Fire-Fighting Operations. Batteries. 2024; 10(4):118. https://doi.org/10.3390/batteries10040118
Chicago/Turabian StyleBordes, Arnaud, Arnaud Papin, Guy Marlair, Théo Claude, Ahmad El-Masri, Thierry Durussel, Jean-Pierre Bertrand, Benjamin Truchot, and Amandine Lecocq. 2024. "Assessment of Run-Off Waters Resulting from Lithium-Ion Battery Fire-Fighting Operations" Batteries 10, no. 4: 118. https://doi.org/10.3390/batteries10040118