Crystal Structures and Magnetic Properties of Diaquatetrapyridinenickel(II) and Diaquatetrapyridinecobalt(II) Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Materials
2.3. Preparations
2.4. Crystallography
2.5. Computation
3. Results and Discussion
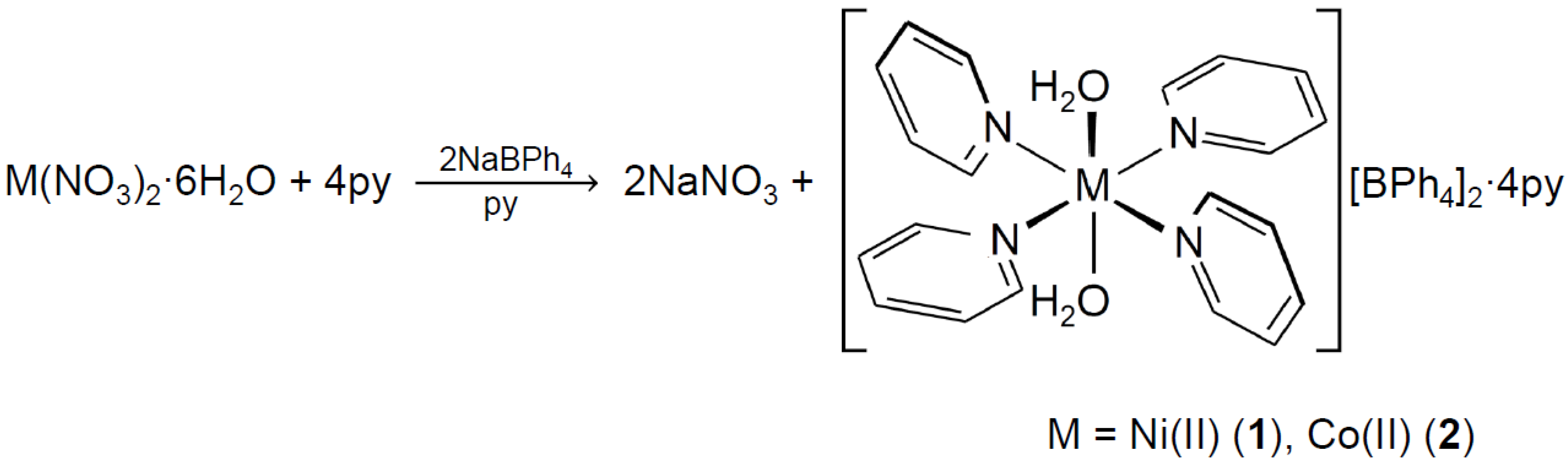
3.1. Preparation of Complexes 1 and 2
3.2. Crystal Structures of Complexes 1 and 2
3.2.1. Crystal Structure of [Ni(H2O)2(py)4][BPh4]2·4py (1)
3.2.2. Crystal Structure of [Co(H2O)2(py)4][BPh4]2·4py (2)
3.3. Magnetic Properties of Complexes 1 and 2
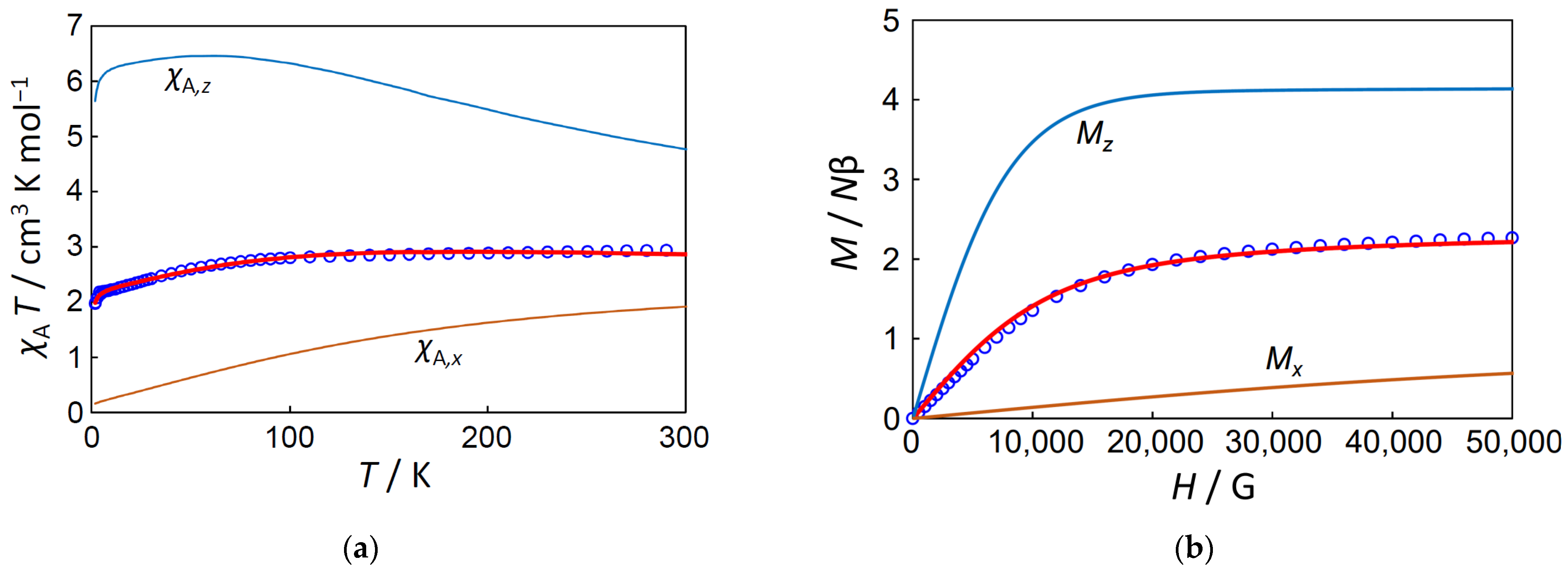
3.3.1. Magnetic Properties of 1
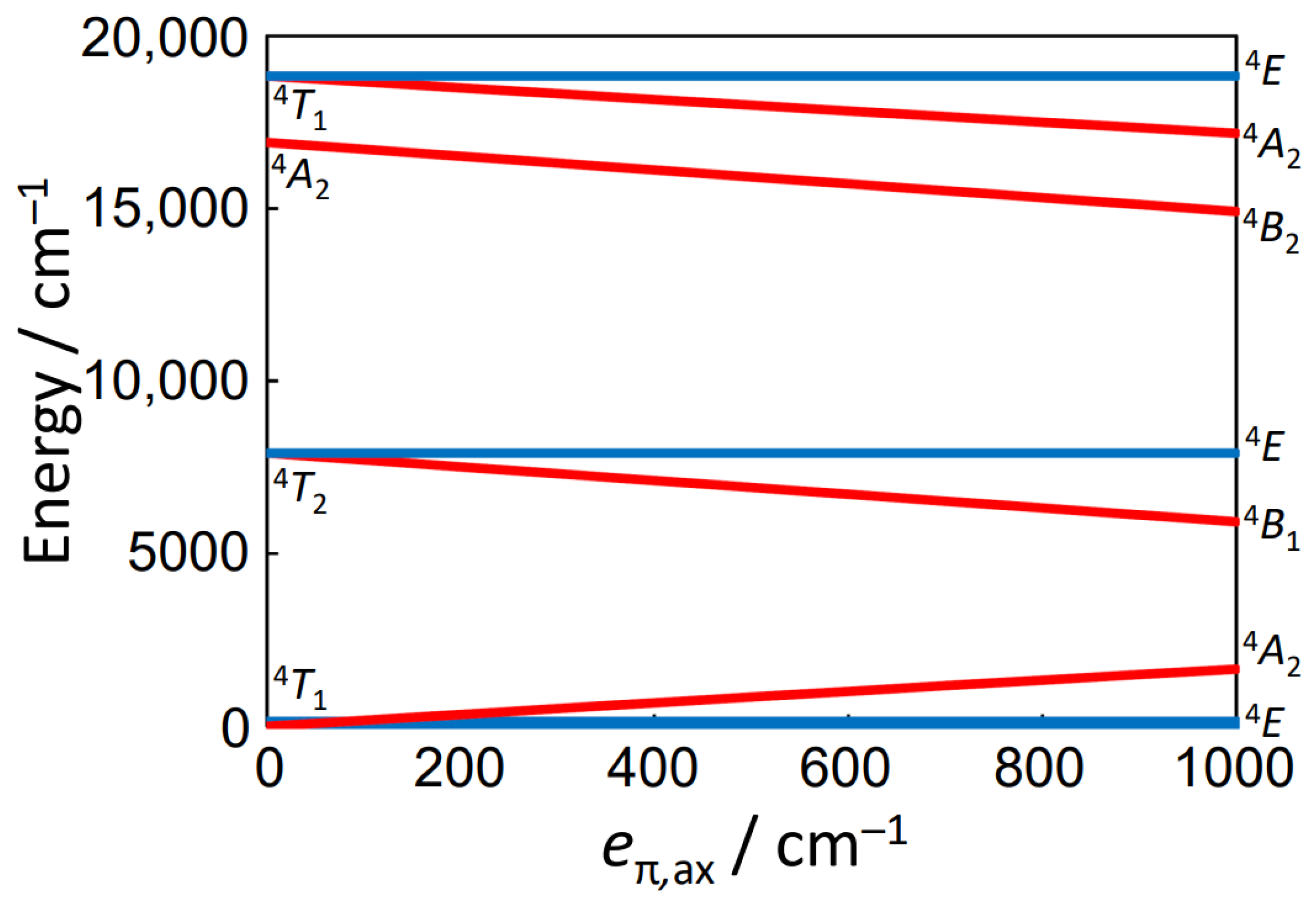
3.3.2. Magnetic Properties of [Co(H2O)2(py)4][BPh4]2·4py (2)
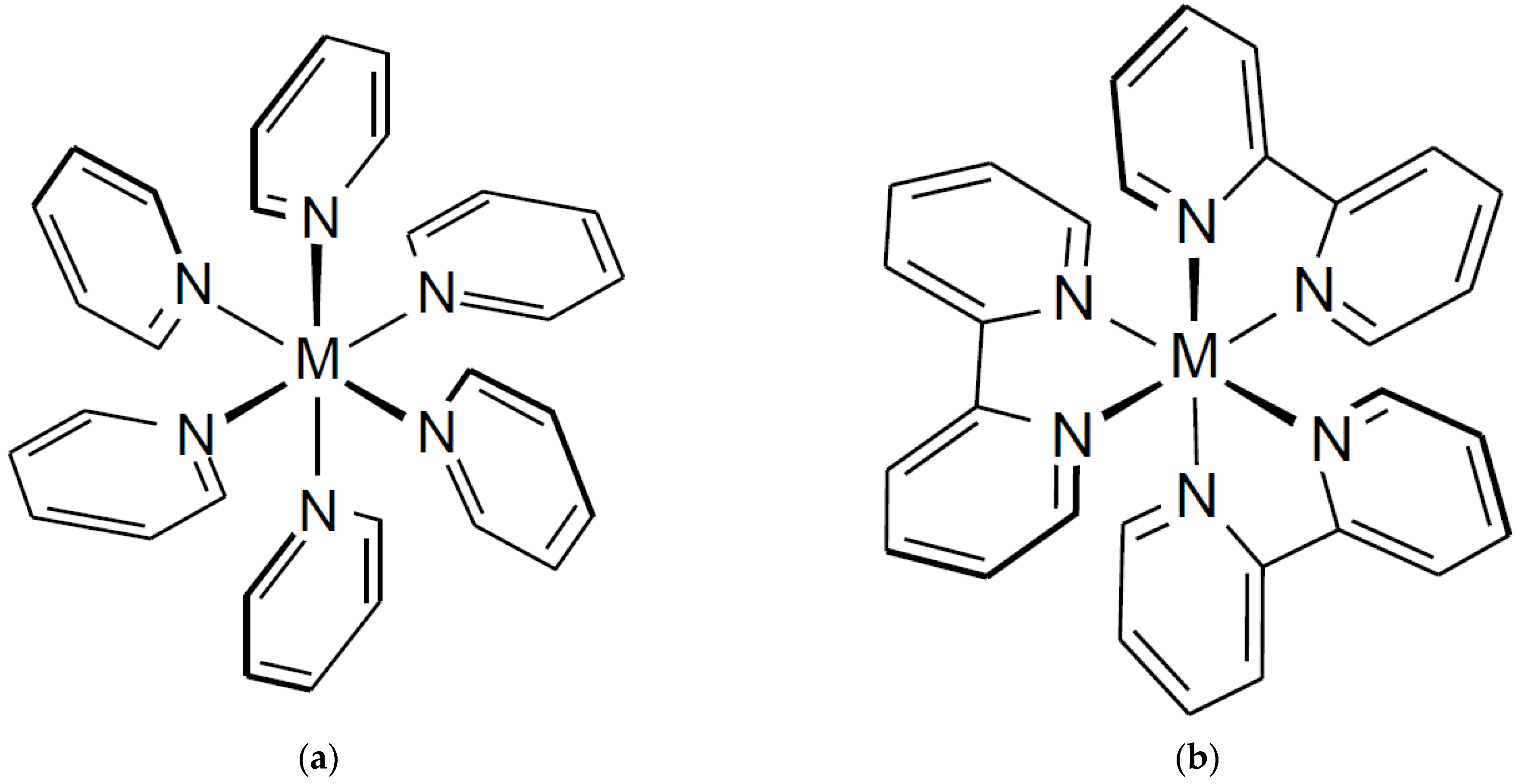
3.4. Structural Consideration for Diaquatetrapyridine Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, S. Pyridine: A useful ligand in transition metal complexes. In Pyridine; Pandey, P.P., Ed.; IntechOpen Limited: London, UK, 2018; Chapter 5; pp. 57–74. [Google Scholar]
- Raj, D.; Padhi, S.K. The sporadic l-pyridine bridge in transition metal complexes: A real bond or an interaction? Coord. Chem. Rev. 2022, 450, 214238. [Google Scholar] [CrossRef]
- Pardey, A.J.; Longo, C. Catalysis by rhodium complexes bearing pyridine ligands: Mechanistic aspects. Coord. Chem. Rev. 2010, 254, 254–272. [Google Scholar] [CrossRef]
- Doedens, R.J.; Dahl, L.F. Structure of the hexapyridineiron(II) salt of the tetranuclear iron carbonyl anion, [Fe4(CO)13]–2, with comments concerning the nonisolation of the corresponding neutral tetranuclear iron carbonyl, Fe4(CO)14. J. Am. Chem. Soc. 1966, 88, 4847–4855. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. The early years of 2,2′-bipyridine—A ligand in its own lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, A.; Sakabe, N.; Tanaka, J. The crystal structure of tris(2,2′-bipyridyl)nickel(II) sulphate hydrate, [Ni(C10H8N2)3]SO4.7.5H2O. Acta Cryst. 1976, B32, 1121–1127. [Google Scholar] [CrossRef]
- Stebler, M.; Gutierrez, A.; Ludi, A.; Buergi, H.B. Synthesis and crystal structure of tris(2,2′-bipyridine)rhenium(2+) perrhenate. Inorg. Chem. 1987, 26, 1449–1451. [Google Scholar] [CrossRef]
- Constable, E.C.; Raithby, P.R.; Smit, D.N. The X-ray crystal structure of tris (2,2′-bipyridine)osmium(II) hexafluorophosphate. Polyhedron 1989, 8, 367–369. [Google Scholar] [CrossRef]
- Harrowfield, J.M.; Sobolev, A.N. The crystal structure of tris (2,2′-bipyridine) ruthenium (II) perchlorate. Aust. J. Chem. 1994, 47, 763–767. [Google Scholar] [CrossRef]
- Dick, S. Crystal structure of tris(2,2′-bipyridine)iron(II) bis(hexafluorophosphate), (C10H8N2)3Fe(PF6)2. Z. Krist.-New Cryst. Struct. 1998, 213, 356. [Google Scholar] [CrossRef] [Green Version]
- Benabdallah, J.; Setifi, Z.; Setifi, F.; Boughzala, H.; Titi, A. Crystal structure of tris(2,2′-bipyridine)cobalt(II)bis(1,1,3,3-tetracyano-2-ethoxypropenide). Acta Cryst. 2019, E75, 142–145. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakiyama, H. Development of MagSaki software for magnetic analysis of dinuclear high-spin cobalt(II) complexes in an axially distorted octahedral field. J. Chem. Softw. 2001, 7, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Sakiyama, H. Development of MagSaki(A) software for the magnetic analysis of dinuclear high-spin cobalt(II) complexes considering anisotropy in exchange interaction. J. Comput. Chem. Jpn. 2007, 6, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Sakiyama, H. Development of MagSaki(Tri) software for the magnetic analysis of trinuclear high-spin cobalt(II) complexes. J. Comput. Chem. Jpn. Int. Ed. 2015, 1, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Sakiyama, H. Development of MagSaki(Tetra) software for the magnetic analysis of tetranuclear high-spin cobalt(II) complexes. J. Comput. Chem. Jpn. Int. Ed. 2016, 2, 1–4. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Sakiyama, H. Theoretical equations of Zeeman energy levels for distorted metal complexes with 3T1 ground terms. Magnetochemistry 2019, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Sakiyama, H.; Abiko, T.; Yoshida, K.; Shomura, K.; Mitsuhashi, R.; Koyama, Y.; Mikuriya, M.; Koikawa, M.; Mitsumi, M. Detailed magnetic analysis and successful deep-neural-network-based conformational prediction for [VO(dmso)5][BPh4]2. RSC Adv. 2020, 10, 9678. [Google Scholar] [CrossRef] [Green Version]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Murray, K.S.; Hitchman, M.A.; Rowbottom, G.L. Metal–ligand bonding parameters and magnetic properties of some previously reported tetragonal nickel(II) complexes. J. Chem. Soc. Dalton Trans. 1987, 4, 825–830. [Google Scholar] [CrossRef]
- Sakiyama, H.; Sudo, R.; Abiko, T.; Yoshioka, D.; Mitsuhashi, R.; Omote, M.; Mikuriya, M.; Yoshitake, M.; Koikawa, M. Magneto-structural correlation of hexakis-dmso cobalt(II) complex. Dalton Trans. 2017, 46, 16306. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; VCH Publishers, Inc.: New York, NY, USA, 1993; pp. 38–41. [Google Scholar]
- Sakiyama, H.; Ito, R.; Kumagai, H.; Inoue, K.; Sakamoto, M.; Nishida, Y.; Yamasaki, M. Dinuclear cobalt(II) complexes of an acyclic phenol-based dinucleating ligand with four methoxyethyl chelating arms—First magnetic analysis in an axially distorted octahedral field. Eur. J. Inorg. Chem. 2001, 2001, 2027–2032. [Google Scholar] [CrossRef]
- Mikuriya, M.; Naka, Y.; Inaoka, A.; Okayama, M.; Yoshioka, D.; Sakiyama, H.; Handa, M.; Tsuboi, M. Mixed-Valent Trinuclear CoIII-CoII-CoIII Complex with 1,3-Bis(5-chlorosalicylideneamino)-2-propanol. Molecules 2022, 27, 4211. [Google Scholar] [CrossRef]
- Figgis, B.N.; Gerloch, M.; Lewis, J.; Mabbs, F.E.; Webb, G.A. The magnetic behaviour of cubic-field 4T1g terms in lower symmetry. J. Chem. Soc. A 1968, 2086–2093. [Google Scholar] [CrossRef]
- Figgis, B.N.; Hitchman, M.A. Ligand Field Theory and Its Application; Wiley-VCH: New York, NY, USA, 2000; pp. 70–73. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Hoshikawa, R.; Waki, K.; Sakiyama, H. Enumeration of conformers for octahedral [M(AB2)6] complexes and conformational prediction for a related metal complex. MATCH Commun. Math. Comput. Chem. 2021, 85, 499–508. [Google Scholar]
- Sakiyama, H.; Waki, K. Enumeration of edge-orienting conformers for octahedral [MX6–n(AB2)n] complexes (n = 1–5). MATCH Commun. Math. Comput. Chem. 2021, 85, 509–522. [Google Scholar]



| Compound | Complex 1 | Complex 2 |
|---|---|---|
| Empirical formula | C88H84B2N8NiO2 | C88H84B2CoN8O2 |
| Formula weight | 1365.96 | 1366.18 |
| Crystal system | orthorhombic | orthorhombic |
| Space group | Pna21 | Pna21 |
| a/Å | 28.4603(10) | 28.5839(14) |
| b/Å | 11.4004(5) | 11.3675(5) |
| c/Å | 22.6229(6) | 22.6811(8) |
| V/Å3 | 7340.2(5) | 7369.7(6) |
| Z | 4 | 4 |
| Crystal dimensions/mm | 0.24 × 0.14 × 0.12 | 0.22 × 0.17 × 0.10 |
| T/K | 100 | 100 |
| λ/Å | 0.71073 | 0.71073 |
| ρcalcd/g cm−3 | 1.236 | 1.231 |
| µ/mm−1 | 0.320 | 0.288 |
| F(000) | 2888 | 2884 |
| 2θmax/◦ | 55 | 55 |
| No. of reflections measured | 15,854 | 14,855 |
| No. of independent reflections | 11,529 (Rint = 0.0651) | 9696 (Rint = 0.1526) |
| Data/restraints/parameters | 15,854/5/926 | 14,855/5/926 |
| R1 (I > 2.00σ(I)) 1 | 0.0467 | 0.0786 |
| wR2 (All reflections) 2 | 0.1012 | 0.1816 |
| Goodness of fit indicator | 1.006 | 0.992 |
| Highest peak, deepest hole/e Å−3 | 0.250, −0.446 | 0.569, −0.873 |
| CCDC deposition number | 2219439 | 2219440 |
| Atom–Atom | Distance/Å | Atom–Atom | Distance/Å |
|---|---|---|---|
| Ni(1)–O(1) | 2.052(3) | Ni(1)–O(2) | 2.028(3) |
| Ni(1)–N(1) | 2.163(3) | Ni(1)–N(2) | 2.157(3) |
| Ni(1)–N(3) | 2.164(3) | Ni(1)–N(4) | 2.157(3) |
| O(1)∙∙∙N(5) | 2.726(4) | O(1)∙∙∙N(6) | 2.763(5) |
| O(2)∙∙∙N(7) | 2.687(4) | O(2)∙∙∙N(8) | 2.676(4) |
| Atom–Atom–Atom | Angle/° | Atom–Atom–Atom | Angle/° |
|---|---|---|---|
| O(1)–Ni(1)–O(2) | 178.58(13) | O(1)–Ni(1)–N(1) | 90.40(12) |
| O(1)–Ni(1)–N(2) | 90.39(13) | O(1)–Ni(1)–N(3) | 87.88(13) |
| O(1)–Ni(1)–N(4) | 90.54(13) | O(2)–Ni(1)–N(1) | 90.99(12) |
| O(2)–Ni(1)–N(2) | 89.94(12) | O(2)–Ni(1)–N(3) | 90.73(12) |
| O(2)–Ni(1)–N(4) | 89.16(12) | N(1)–Ni(1)–N(2) | 89.21(10) |
| N(1)–Ni(1)–N(3) | 178.18(12) | N(1)–Ni(1)–N(4) | 89.35(12) |
| N(2)–Ni(1)–N(3) | 91.37(12) | N(2)–Ni(1)–N(4) | 178.29(13) |
| N(3)–Ni(1)–N(4) | 90.09(10) |
| Atom–Atom | Distance/Å | Atom–Atom | Distance/Å |
|---|---|---|---|
| Co(1)–O(1) | 2.048(4) | Co(1)–O(2) | 2.016(4) |
| Co(1)–N(1) | 2.218(6) | Co(1)–N(2) | 2.213(6) |
| Co(1)–N(3) | 2.221(6) | Co(1)–N(4) | 2.190(6) |
| O(1)∙∙∙N(5) | 2.761(8) | O(1)∙∙∙N(6) | 2.716(8) |
| O(2)∙∙∙N(7) | 2.676(8) | O(2)∙∙∙N(8) | 2.682(8) |
| Atom–Atom–Atom | Angle/° | Atom–Atom–Atom | Angle/° |
|---|---|---|---|
| O(1)–Co(1)–O(2) | 178.2(3) | O(1)–Co(1)–N(1) | 87.9(2) |
| O(1)–Co(1)–N(2) | 90.1(2) | O(1)–Co(1)–N(3) | 90.0(2) |
| O(1)–Co(1)–N(4) | 90.6(2) | O(2)–Co(1)–N(1) | 90.4(2) |
| O(2)–Co(1)–N(2) | 89.6(2) | O(2)–Co(1)–N(3) | 91.7(2) |
| O(2)–Co(1)–N(4) | 89.7(2) | N(1)–Co(1)–N(2) | 91.9(2) |
| N(1)–Co(1)–N(3) | 177.88(19) | N(1)–Co(1)–N(4) | 89.64(18) |
| N(2)–Co(1)–N(3) | 88.64(18) | N(2)–Co(1)–N(4) | 178.4(2) |
| N(3)–Co(1)–N(4) | 89.9(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakiyama, H.; Yamamoto, Y.; Hoshikawa, R.; Mitsuhashi, R. Crystal Structures and Magnetic Properties of Diaquatetrapyridinenickel(II) and Diaquatetrapyridinecobalt(II) Complexes. Magnetochemistry 2023, 9, 14. https://doi.org/10.3390/magnetochemistry9010014
Sakiyama H, Yamamoto Y, Hoshikawa R, Mitsuhashi R. Crystal Structures and Magnetic Properties of Diaquatetrapyridinenickel(II) and Diaquatetrapyridinecobalt(II) Complexes. Magnetochemistry. 2023; 9(1):14. https://doi.org/10.3390/magnetochemistry9010014
Chicago/Turabian StyleSakiyama, Hiroshi, Yuya Yamamoto, Ryusei Hoshikawa, and Ryoji Mitsuhashi. 2023. "Crystal Structures and Magnetic Properties of Diaquatetrapyridinenickel(II) and Diaquatetrapyridinecobalt(II) Complexes" Magnetochemistry 9, no. 1: 14. https://doi.org/10.3390/magnetochemistry9010014





