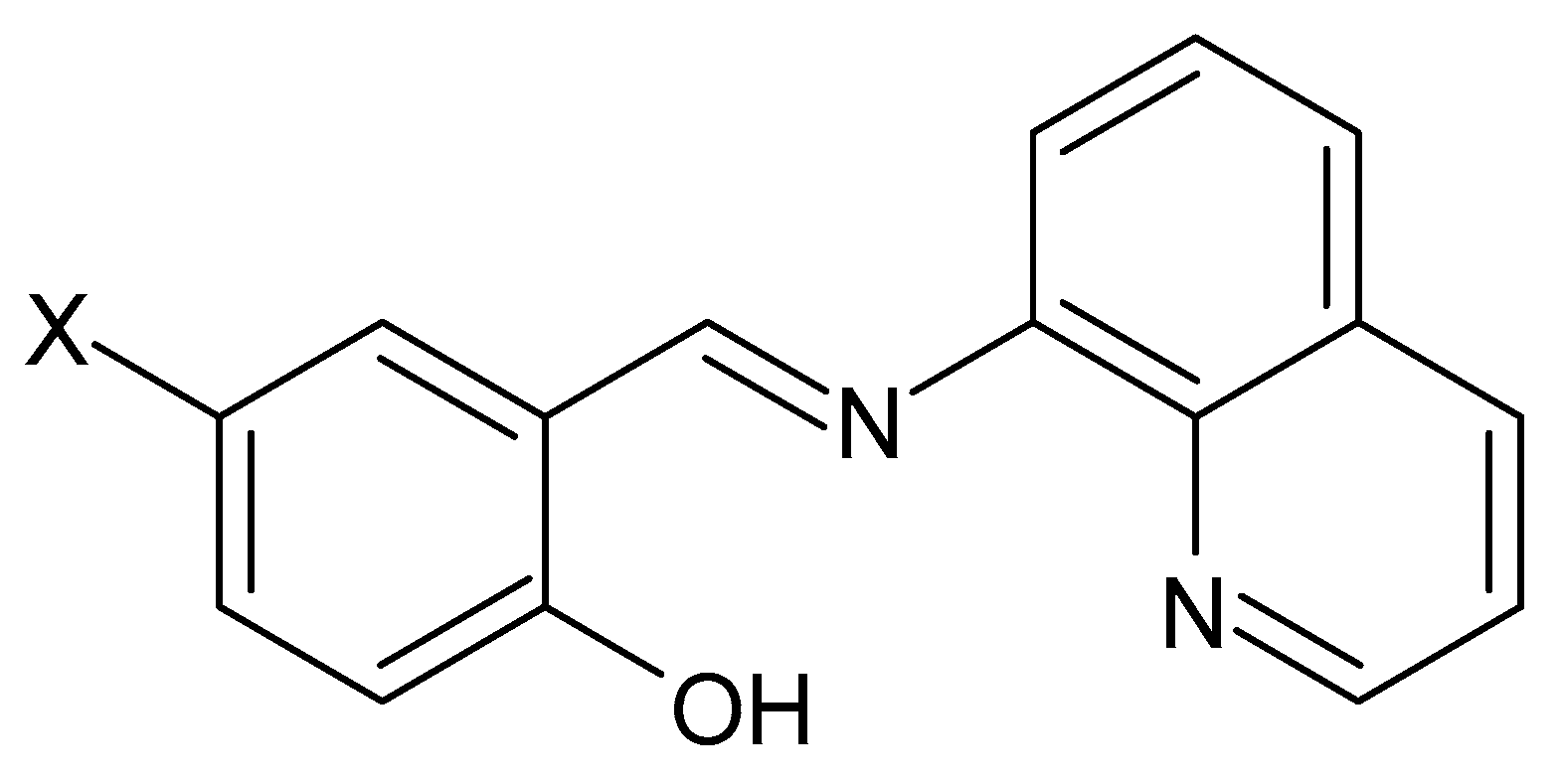
Correlation between Supramolecular Connectivity and Magnetic Behaviour of [FeIII(5-X-qsal)2]+-Based Salts Prone to Exhibit SCO Transition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Magnetic Characterisation
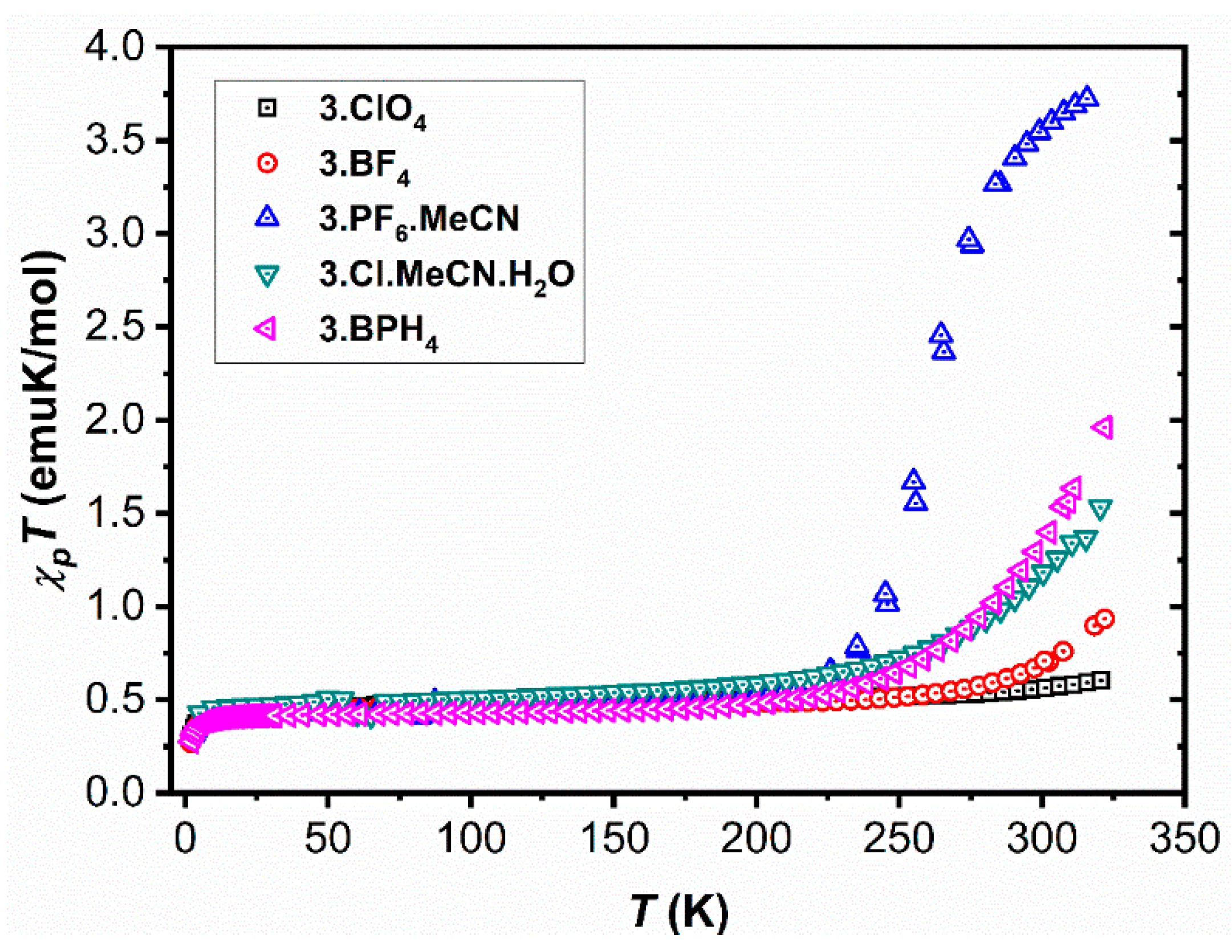
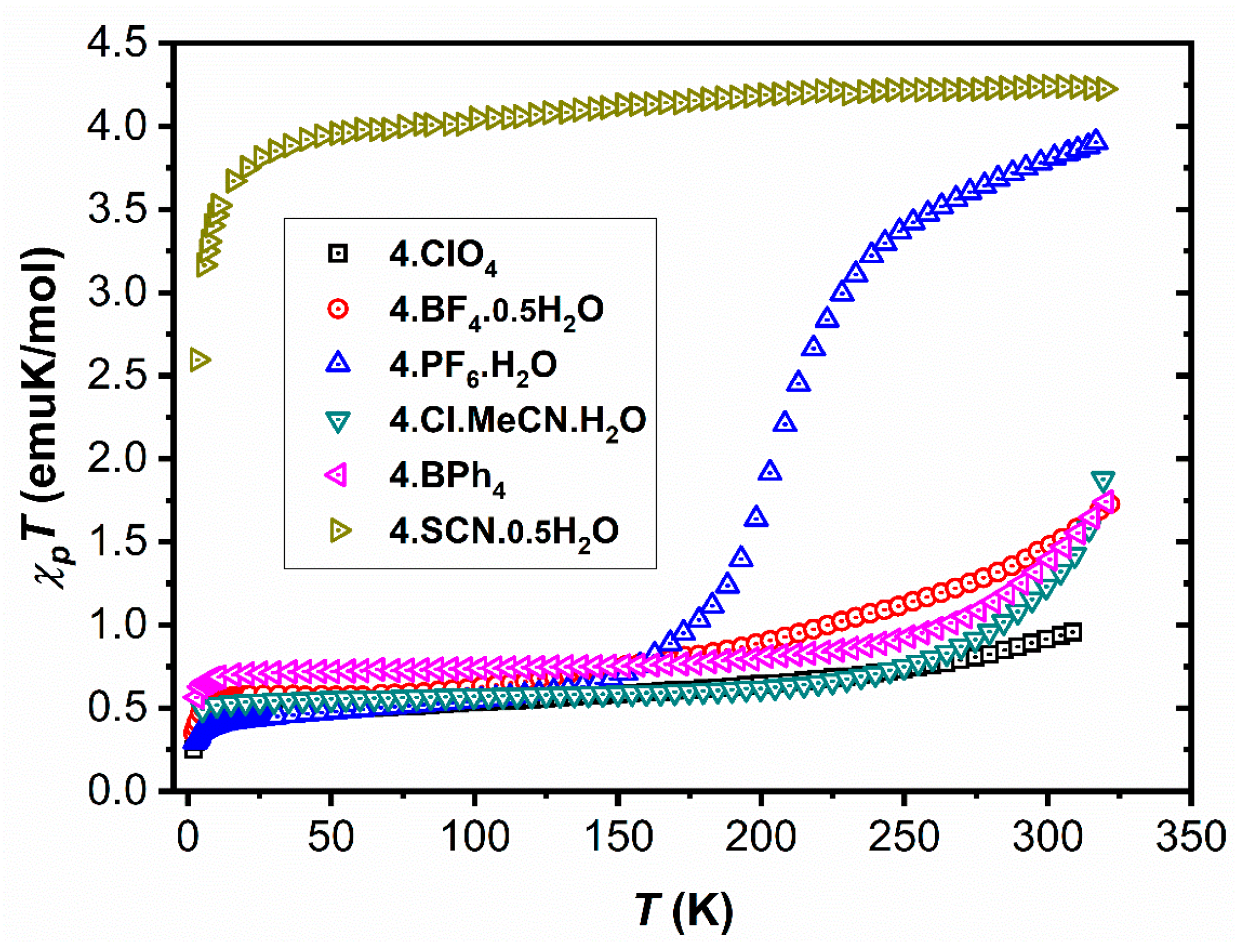
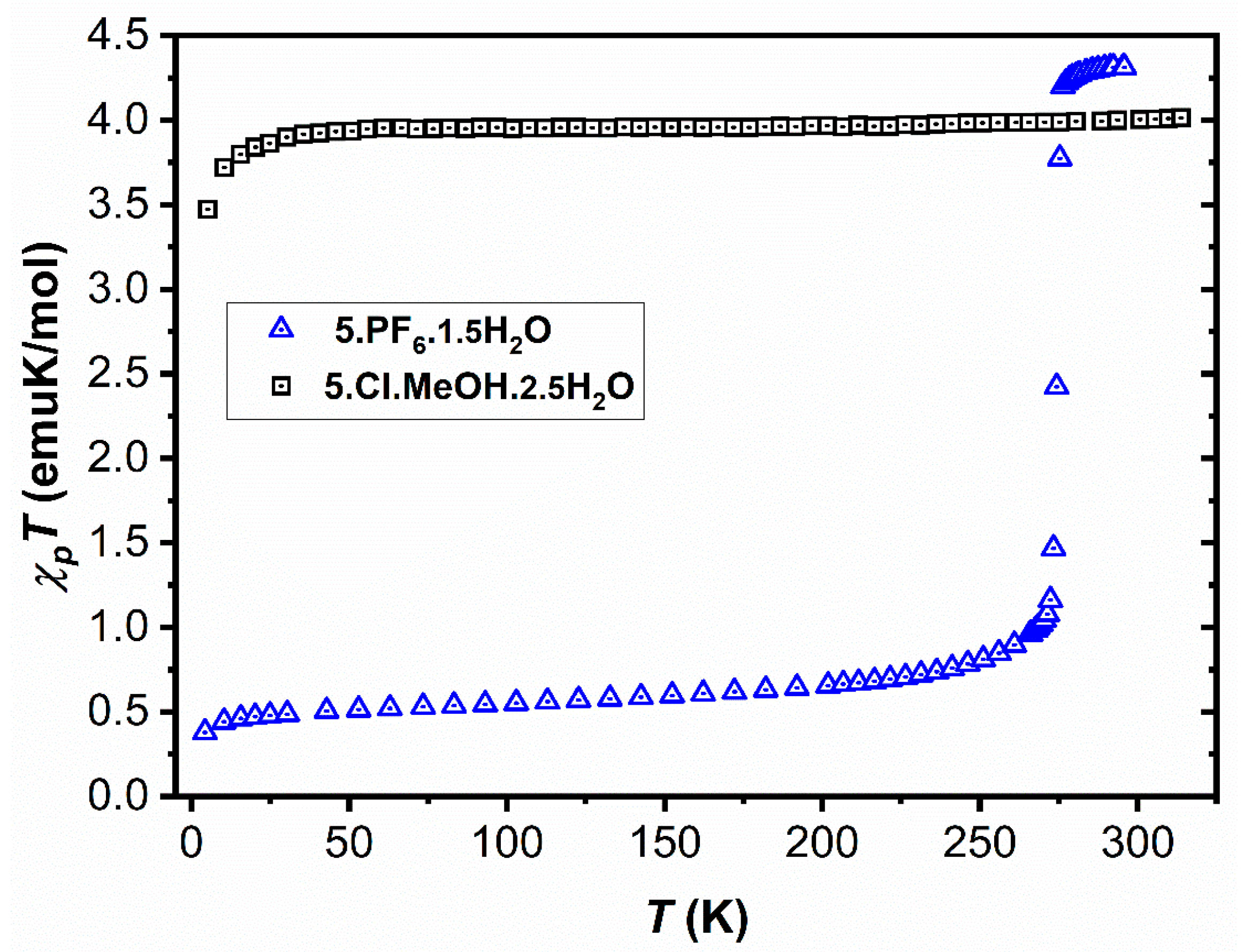
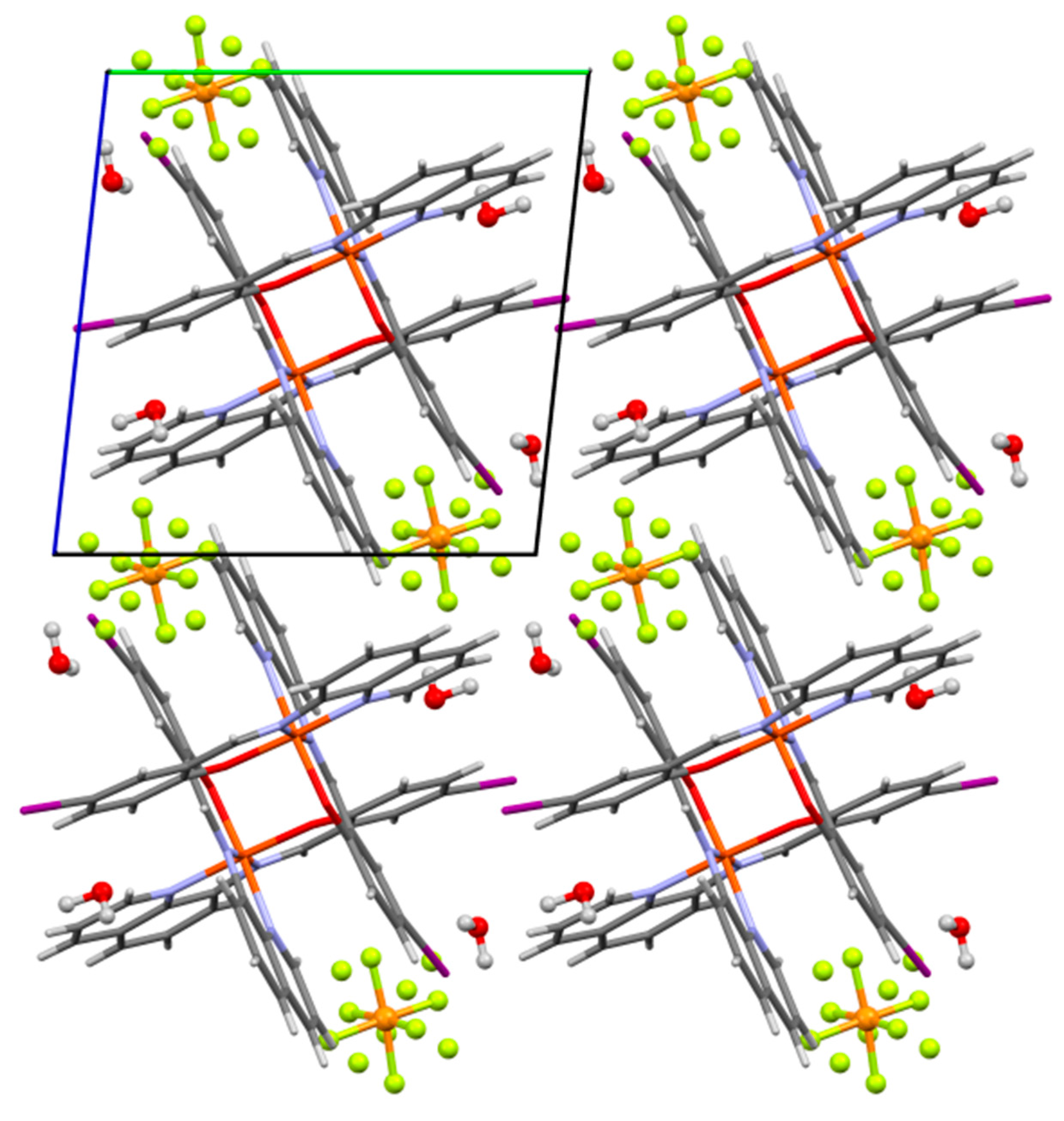
2.2. Structural Characterization
2.2.1. Dimer Chains
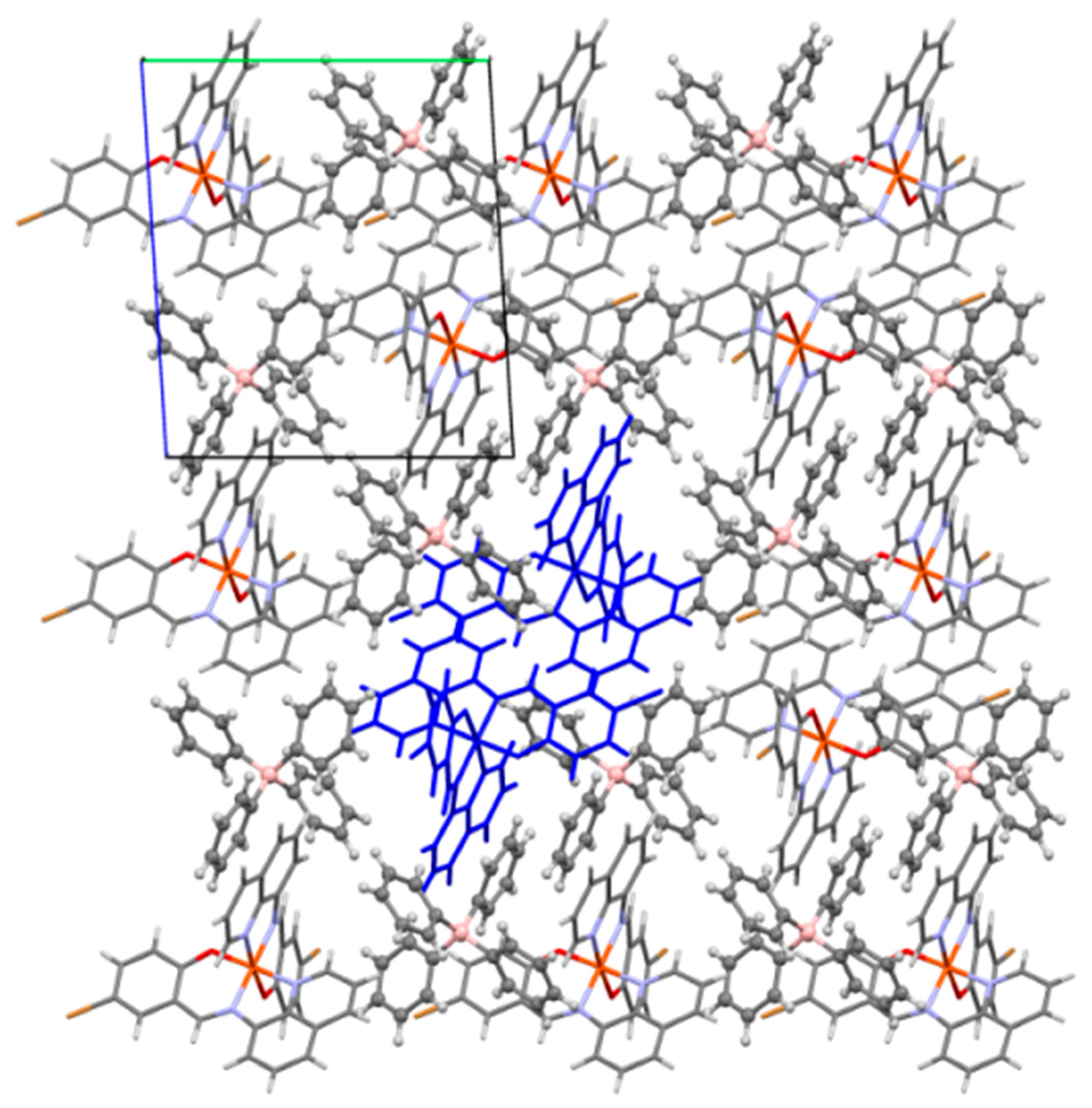
2.2.2. Dimer Layers
2.2.3. Type I Dimer Layers
2.2.4. Type II Dimer Layers
2.2.5. Chain Layers
2.2.6. [Fe(5-X-qsal)2]+ Based Salts Reported in the Literature
3. Materials and Methods
3.1. Synthesis
3.2. X-ray Crystallography
3.3. Magnetic Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. Spin Crossover—An Overall Perspective. Top. Curr. Chem. 2004, 233, 1–47. [Google Scholar]
- Halcrow, M.A. Spin-Crossover Materials: Properties and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Koningsbruggen, P.; Maeda, Y.; Oshio, H. Spin Crossover in Transition Metal Compounds I; Gütlich, P., Goodwin, H.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 233, pp. 259–324. [Google Scholar]
- Zhao, X.H.; Zhang, S.L.; Shao, D.; Wang, X.Y. Spin Crossover in [Fe(2-Picolylamine)3]2+ Adjusted by Organosulfonate Anions. Inorg. Chem. 2015, 54, 7857–7867. [Google Scholar] [CrossRef]
- Shiga, T.; Saiki, R.; Akiyama, L.; Kumai, R.; Natke, D.; Renz, F.; Cameron, J.M.; Newton, G.N.; Oshio, H. A Brønsted-Ligand-Based Iron Complex as a Molecular Switch with Five Accessible States. Angew. Chem. Int. Ed. 2019, 58, 5658–5662. [Google Scholar] [CrossRef] [PubMed]
- Fukuroi, K.; Takahashi, K.; Mochida, T.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Mori, H. Synergistic Spin Transition between Spin Crossover and Spin-Peierls-like Singlet Formation in the Halogen-Bonded Molecular Hybrid System: [Fe(Iqsal)2][Ni(dmit)2]⋅CH3CN⋅H2O. Angew. Chem. Int. Ed. 2014, 53, 1983–1986, reprinted in Angew. Chem. 2014, 126, 2014. [Google Scholar] [CrossRef]
- Jeon, I.-R.; Mathonière, C.; Clérac, R.; Rouzières, M.; Jeannin, O.; Trzop, E.; Collet, E.; Fourmigué, M. Photoinduced reversible spin-state switching of an Fe III complex assisted by a halogen-bonded supramolecular network. Chem. Commun. 2017, 53, 10283–10286. [Google Scholar] [CrossRef]
- Phonsri, W.; Macedo, D.S.; Vignesh, K.R.; Rajaraman, G.; Davies, C.G.; Jameson, G.N.L.; Moubaraki, B.; Ward, J.S.; Kruger, P.E.; Chastanet, G.; et al. Halogen Substitution Effects on N2O Schiff Base Ligands in Unprecedented Abrupt FeII Spin Crossover Complexes. Chem. Eur. J. 2017, 23, 7052–7065. [Google Scholar] [CrossRef]
- Takahashi, K.; Okai, M.; Mochida, T.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Shiota, Y.; Yoshizawa, K.; Konaka, H.; et al. Contribution of Coulomb Interactions to a Two-Step Crystal Structure Phase Transformation Coupled with a Significant Change in Spin Crossover Behavior for a Series of Charged FeII Complexes from 2,6-Bis(2-methylthiazol-4-yl)pyridine. Inorg. Chem. 2018, 57, 1277–1287. [Google Scholar] [CrossRef] [Green Version]
- Harding, D.J.; Sertphon, D.; Harding, P.; Murray, K.S.; Moubaraki, B.; Cashion, J.D.; Adams, H. Fe III Quinolylsalicylaldimine Complexes: A Rare Mixed-Spin-State Complex and Abrupt Spin Crossover. Chem. Eur. J. 2013, 19, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Togo, T.; Amolegbe, S.A.; Yamaguchi, R.; Kuroda-Sowa, T.; Nakaya, M.; Shimayama, K.; Nakamura, M.; Hayami, S. Crystal Structure and Spin-crossover Behavior of Iron(III) Complex with Nitroprusside. Chem. Lett. 2013, 42, 1542–1544. [Google Scholar] [CrossRef]
- Faulmann, C.; Chahine, J.; Valade, L.; Chastanet, G.; Létard, J.-F.; de Caro, D. Photomagnetic Studies of Spin-Crossover-and Photochromic-Based Complexes. Eur. J. Inorg. Chem. 2013, 2013, 1058–1067. [Google Scholar] [CrossRef]
- Takahashi, K.; Sato, T.; Mori, H.; Tajima, H.; Sato, O. Correlation between the magnetic behaviors and dimensionality of intermolecular interactions in Fe(III) spin crossover compounds. Phys. B 2010, 405, S65–S68. [Google Scholar] [CrossRef]
- Oshio, H.; Kitazaki, K.; Mishiro, J.; Kato, N.; Maeda, Y.; Takashima, Y. New spin-crossover iron(III) complexes with large hysteresis effects and time dependence of their magnetism. J. Chem. Soc. Dalton Trans. 1987, 1341–1347. [Google Scholar] [CrossRef]
- Hayami, S.; Hiki, K.; Kawahara, T.; Maeda, Y.; Urakami, D.; Inoue, K.; Ohama, M.; Kawata, S.; Sato, O. Photo-Induced Spin Transition of Iron(III) Compounds with π–π Intermolecular Interactions. Chem. Eur. J. 2009, 15, 3497–3508. [Google Scholar] [CrossRef]
- Phonsri, W.; Harding, D.J.; Harding, P.; Murray, K.S.; Moubaraki, B.; Gass, I.A.; Cashion, J.D.; Jameson, G.N.L.; Adams, H. Stepped spin crossover in Fe(III) halogen substituted quinolylsalicylaldimine complexes. Dalton Trans. 2014, 43, 17509–17518. [Google Scholar] [CrossRef] [PubMed]
- Harding, D.J.; Phonsri, W.; Harding, P.; Murray, K.S.; Moubaraki, B.; Jameson, G.N.L. Abrupt two-step and symmetry breaking spin crossover in an iron(iii) complex: An exceptionally wide [LS–HS] plateau. Dalton Trans. 2015, 44, 15079–15082. [Google Scholar] [CrossRef]
- Phonsri, W.; Harding, P.; Liu, L.; Telfer, S.G.; Murray, K.S.; Moubaraki, B.; Ross, T.M.; Jameson, G.N.L.; Harding, D.J. Solvent modified spin crossover in an iron(iii) complex: Phase changes and an exceptionally wide hysteresis. Chem. Sci. 2017, 8, 3949–3959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, D.J.; Phonsri, W.; Harding, P.; Gass, I.A.; Murray, K.S.; Moubaraki, B.; Cashion, J.D.; Liu, L.; Telfer, S.G. Abrupt spin crossover in an iron(iii) quinolylsalicylaldimine complex: Structural insights and solvent effects. Chem. Commun. 2013, 49, 6340–6342. [Google Scholar] [CrossRef] [PubMed]
- Phukkaphan, N.; Cruickshank, D.L.; Murray, K.S.; Phonsri, W.; Harding, P.; Harding, D.J. Hysteretic spin crossover driven by anion conformational change. Chem. Commun. 2017, 53, 9801–9804. [Google Scholar] [CrossRef] [PubMed]
- Sertphon, D.; Harding, D.J.; Harding, P.; Murray, K.S.; Moubaraki, B. Adams, Steric Trapping of the High Spin State in FeIII Quinolylsalicylaldimine Complexes. Aust. J. Chem. 2014, 67, 1574–1580. [Google Scholar] [CrossRef]
- Díaz-Torres, R.; Phonsri, W.; Murray, K.S.; Liu, L.; Ahmed, M.; Neville, S.M.; Harding, P.; Harding, D.J. Spin Crossover in Iron(III) Quinolylsalicylaldiminates: The Curious Case of [Fe(qsal-F)2](Anion). Inorg. Chem. 2020, 59, 13784–13791. [Google Scholar] [CrossRef]
- Sertphon, D.; Harding, D.J.; Harding, P.; Murray, K.S.; Moubaraki, B.; Cashion, J.D.; Adams, H. Anionic Tuning of Spin Crossover in FeIII–Quinolylsalicylaldiminate Complexes. Eur. J. Inorg. Chem. 2013, 2013, 788–795. [Google Scholar] [CrossRef]
- Djukic, B.; Jenkins, H.A.; Seda, T.; Lemaire, M.T. Structural and magnetic properties of homoleptic iron(III) complexes containing N-(8-quinolyl)-salicylaldimine [Fe(Qsal)2]+X− {X = I or (Qsal)FeCl3}. Transit. Met. Chem. 2013, 38, 207–212. [Google Scholar] [CrossRef]
- Hayami, S.; Gu, Z.; Yoshiki, H.; Fujishima, A.; Sato, O. Iron(III) Spin-Crossover Compounds with a Wide Apparent Thermal Hysteresis around Room Temperature. J. Am. Chem. Soc. 2001, 123, 11644–11650. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- SMART and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2008.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.; Giacovazzo, G.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R.J. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J.; Wing, X. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, B.J.C.; Pereira, L.C.J.; Gama, V.d.; Santos, I.C.; Cerdeira, A.C.; Waerenborgh, J.C. Correlation between Supramolecular Connectivity and Magnetic Behaviour of [FeIII(5-X-qsal)2]+-Based Salts Prone to Exhibit SCO Transition. Magnetochemistry 2022, 8, 1. https://doi.org/10.3390/magnetochemistry8010001
Vieira BJC, Pereira LCJ, Gama Vd, Santos IC, Cerdeira AC, Waerenborgh JC. Correlation between Supramolecular Connectivity and Magnetic Behaviour of [FeIII(5-X-qsal)2]+-Based Salts Prone to Exhibit SCO Transition. Magnetochemistry. 2022; 8(1):1. https://doi.org/10.3390/magnetochemistry8010001
Chicago/Turabian StyleVieira, Bruno J. C., Laura C. J. Pereira, Vasco da Gama, Isabel C. Santos, Ana C. Cerdeira, and João C. Waerenborgh. 2022. "Correlation between Supramolecular Connectivity and Magnetic Behaviour of [FeIII(5-X-qsal)2]+-Based Salts Prone to Exhibit SCO Transition" Magnetochemistry 8, no. 1: 1. https://doi.org/10.3390/magnetochemistry8010001






