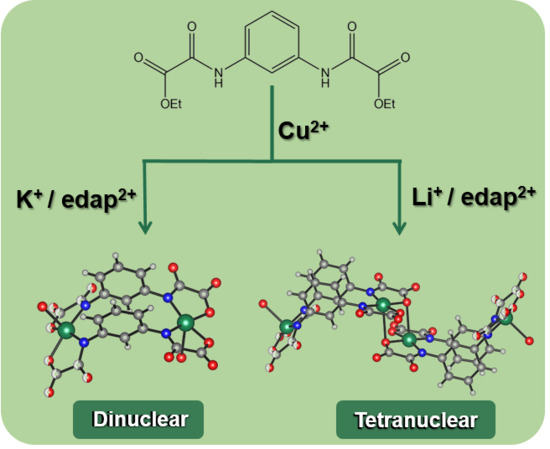
Heterobimetallic One-Dimensional Coordination Polymers MICuII (M = Li and K) Based on Ferromagnetically Coupled Di- and Tetracopper(II) Metallacyclophanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
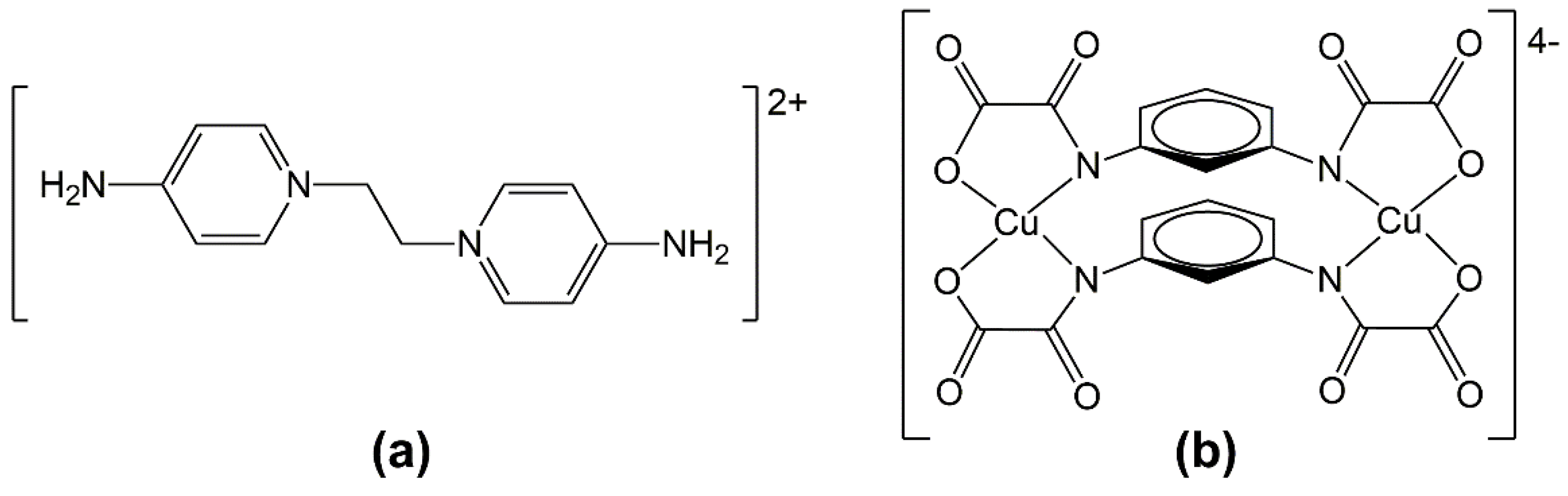
2.2. Synthesis of [EDAP{Li6(H2O)8[(Cu2(μ-mpba)2)2(H2O)2]}]n (1)
2.3. Synthesis of [(EDAP)2{K(H2O)4[Cu2(μ-mpba)2(H2O)2]}Cl·2H2O]n (2)
2.4. Crystallographic Data Collection and Refinement
2.5. Magnetic Measurements
3. Results and Discussion
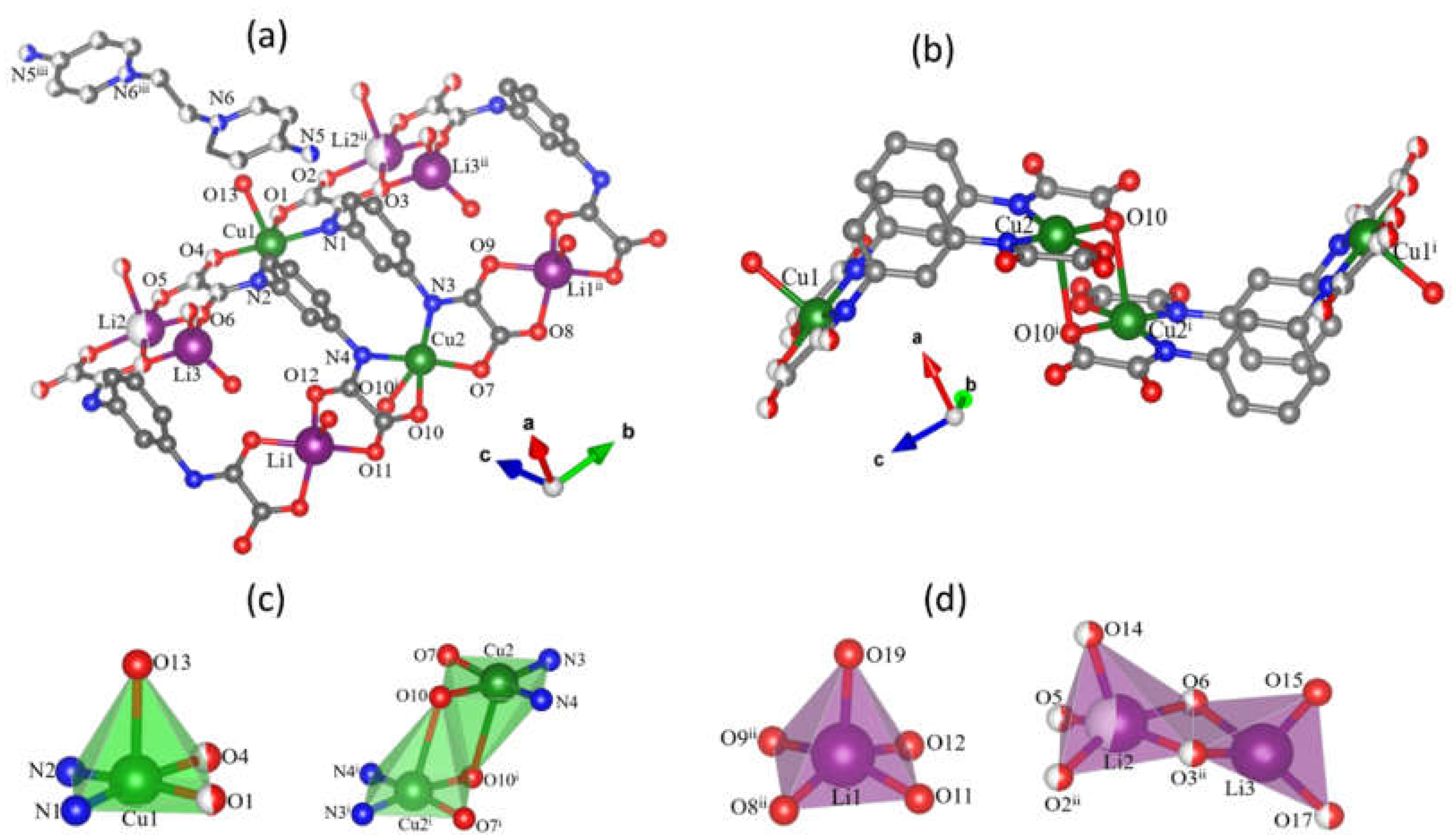
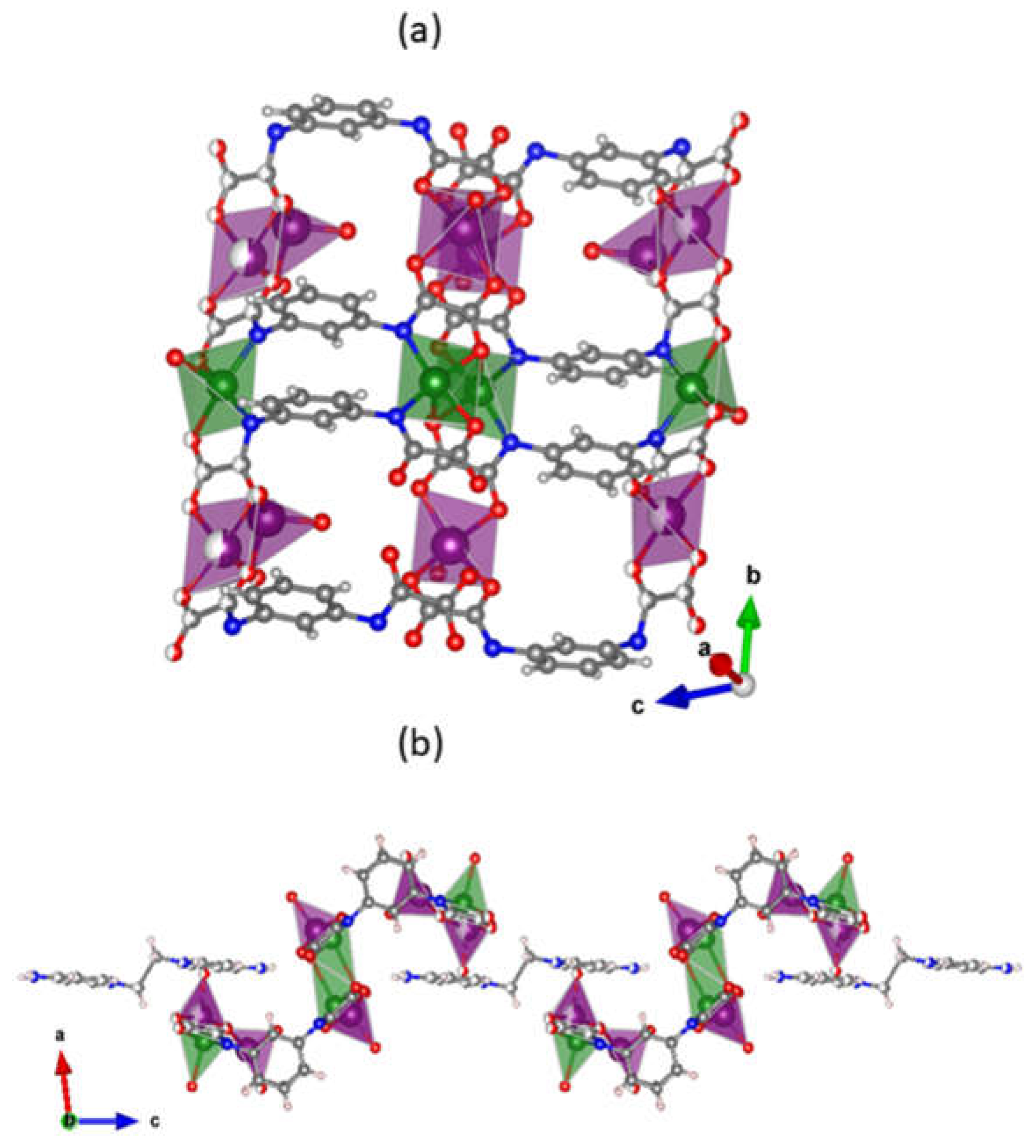
3.1. Description of the Crystal Structures of 1 and 2
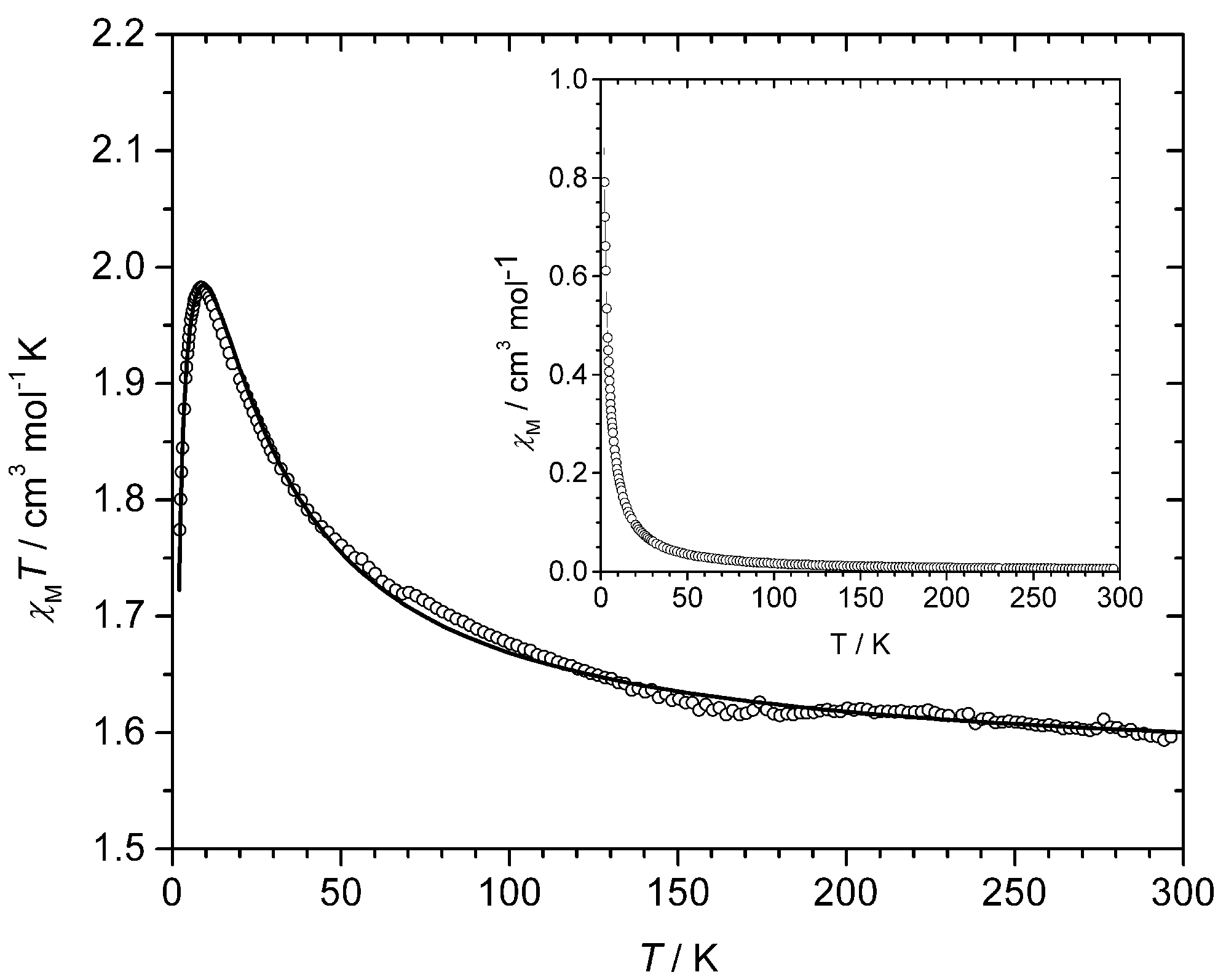
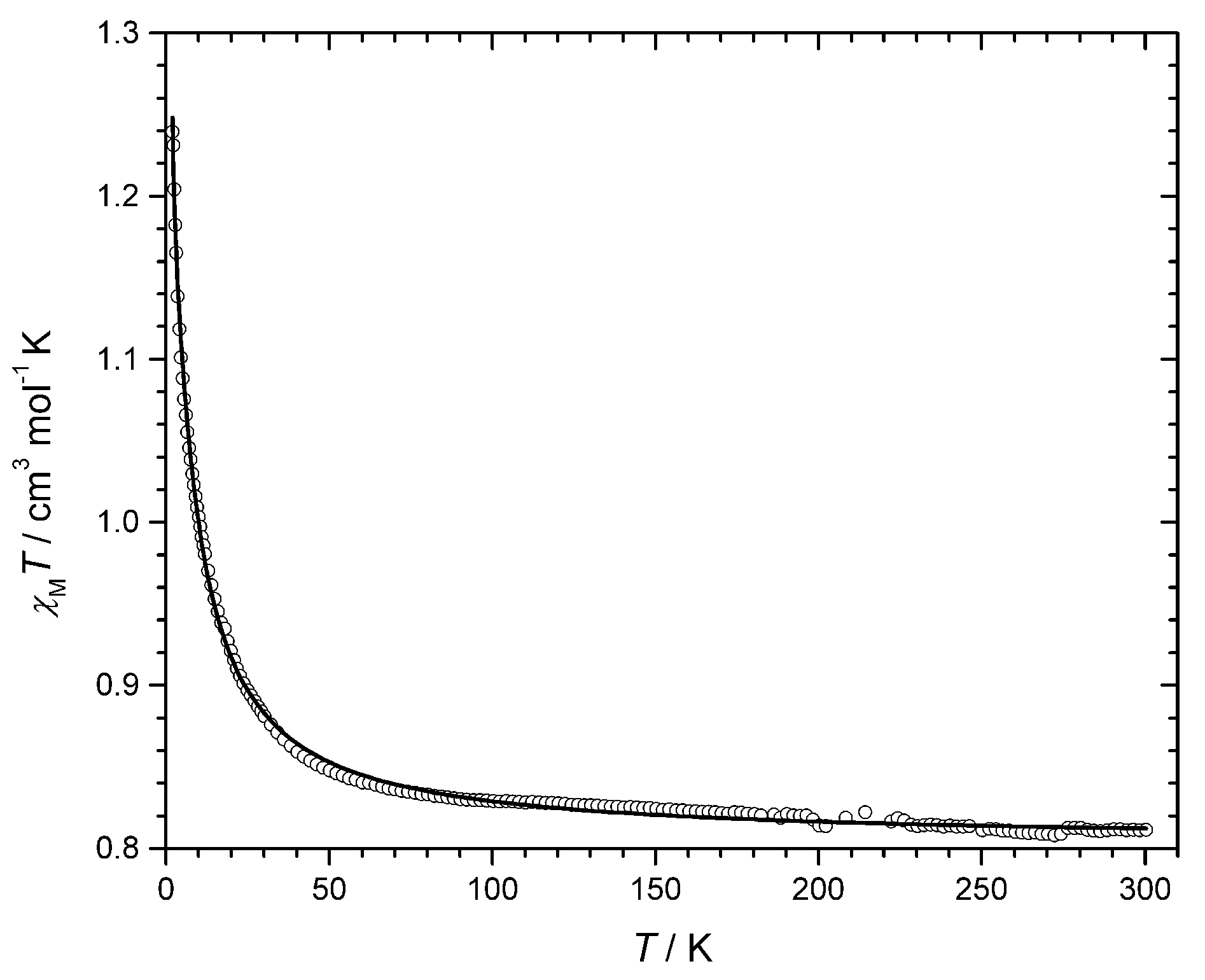
3.2. Magnetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Stadler, R. Electrons in Molecules. From Basic Principles to Molecular Electronics. By Jean-Pierre Launay and Michel Verdaguer. Angew. Chem. Int. Ed. 2014, 53, 6307. [Google Scholar] [CrossRef]
- Ruiz, R.; Lloret, F.; Julve, M.; Faus, J.; Carmen Muñoz, M.; Solans, X. Structure and magnetic properties of a linear oximato-bridged tetranuclear copper(II) complex. Inorg. Chim. Acta 1998, 268, 263–269. [Google Scholar] [CrossRef]
- Teipel, S.; Griesar, K.; Haase, W.; Krebs, B.A. A New-Type of μ4-Oxo-Bridged Copper Tetramer-Synthesis, X-Ray Molecular-Structure, Magnetism and Spectral Properties of (μ4-Oxo)Tetrakis(μ-Bromo)bis(μ-2,6-bis(Morpholinomethyl)-4-Methylphenolato)Tetracopper(II) and (μ4-Oxo)Tetrakis(μ-Benzoato)bis(μ-2,6-bis(Morpholinomethyl)-4-Methylphenolato)Tetracopper(II). Inorg. Chem. 1994, 33, 456–464. [Google Scholar]
- Colacio, E.; Ghazi, M.; Kivekäs, R.; Moreno, J.M. Helical-Chain Copper(II) Complexes and a Cyclic Tetranuclear Copper(II) Complex with Single Syn−Anti Carboxylate Bridges and Ferromagnetic Exchange Interactions. Inorg. Chem. 2000, 39, 2882–2890. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Massera, C.; Roubeau, O.; Gamez, P.; Lanfredi, A.M.M.; Reedijk, J. An Unusual Open Cubane Structure in a μ1,1-Azido- and Alkoxo-Bridged Tetranuclear Copper(II) Complex, [Cu4L2(μ1,1-N3)2]·5H2O (H3L = N,N′-(2-Hydroxylpropane-1,3-diyl)bis-salicylideneimine). Inorg. Chem. 2004, 43, 6842–6847. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.E.; Moubaraki, B.; Fallon, G.D.; Murray, K.S. Tetranuclear copper(II) complexes incorporating short and long metal-metal separations: Synthesis, structure and magnetism. J. Chem. Soc. Dalton Trans. 2000, 5, 713–718. [Google Scholar] [CrossRef]
- Murugesu, M.; Clérac, R.; Pilawa, B.; Mandel, A.; Anson, C.E.; Powell, A.K. Ferromagnetic interactions mediated by syn–anti carboxylate bridging in tetranuclear copper(II) compounds. Inorg. Chim. Acta 2002, 337, 328–336. [Google Scholar] [CrossRef]
- Santana, M.D.; García, G.; Julve, M.; Lloret, F.; Pérez, J.; Liu, M.; Sanz, F.; Cano, J.; López, G. Oxamidate-Bridged Dinuclear Five-Coordinate Nickel(II) Complexes: A Magneto−Structural Study. Inorg. Chem. 2004, 43, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Faus, J.; Julve, M.; Lloret, F.; Muñoz, M.C.; Cano, J.; Ottenwaelder, X.; Journaux, Y.; Carrasco, R.; Blay, G.; et al. Long-Range Magnetic Coupling through Extended π-Conjugated Aromatic Bridges in Dinuclear Copper(II) Metallacyclophanes. J. Am. Chem. Soc. 2003, 125, 10770–10771. [Google Scholar] [CrossRef] [PubMed]
- Chiari, B.; Helms, J.H.; Piovesana, O.; Tarantelli, T.; Zanazzi, P.F. Exchange interaction in multinuclear transition-metal complexes. 9. Magnetostructural correlations in one-atom acetato-bridged copper(II) dimers. Inorg. Chem. 1986, 25, 2408–2413. [Google Scholar] [CrossRef]
- Fernández, I.; Ruiz, R.; Faus, J.; Julve, M.; Lloret, F.; Cano, J.; Ottenwaelder, X.; Journaux, Y.; Muñoz, M.C. Ferromagnetic Coupling through Spin Polarization in a Dinuclear Copper(II) Metallacyclophane. Angew. Chem. Int. Ed. 2001, 40, 3039–3042. [Google Scholar] [CrossRef]
- Pardo, E.; Morales-Osorio, I.; Julve, M.; Lloret, F.; Cano, J.; Ruiz-García, R.; Pasán, J.; Ruiz-Pérez, C.; Ottenwaelder, X.; Journaux, Y. Magnetic Anisotropy of a High-Spin Octanuclear Nickel(II) Complex with a meso-Helicate Core. Inorg. Chem. 2004, 43, 7594–7596. [Google Scholar] [CrossRef] [PubMed]
- Dul, M.C.; Pardo, E.; Lescouëzec, R.; Journaux, Y.; Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cangussu, D.; et al. Supramolecular coordination chemistry of aromatic polyoxalamide ligands: A metallosupramolecular approach toward functional magnetic materials. Coord. Chem. Rev. 2010, 254, 2281–2296. [Google Scholar] [CrossRef] [Green Version]
- Simões, T.R.G.; do Pim, W.D.; Silva, I.F.; Oliveira, W.X.C.; Pinheiro, C.B.; Pereira, C.L.M.; Lloret, F.; Julve, M.; Stumpf, H.O. Solvent-driven dimensionality control in molecular systems containing CuII, 2,2′-bipyridine and an oxamato-based ligand. CrystEngComm 2013, 15, 10165–10170. [Google Scholar] [CrossRef]
- Qu, X.; Song, X.; Li, W.; Xu, Y.; Li, L.; Liao, D.; Jiang, Z. Structural and Magnetic Properties of Two Copper(II) Complexes Based on Dinuclear Copper(II) Metallacyclophane. Eur. J. Inorg. Chem. 2008, 8, 1287–1292. [Google Scholar] [CrossRef]
- Pereira, C.L.M.; Pedroso, E.F.; Stumpf, H.O.; Novak, M.A.; Ricard, L.; Ruiz-García, R.; Rivière, E.; Journaux, Y. A CuIICoII Metallacyclophane-Based Metamagnet with a Corrugated Brick-Wall Sheet Architecture. Angew. Chem. Int. Ed. 2004, 43, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Cangussu, D.; Pardo, E.; Dul, M.C.; Lescouëzec, R.; Herson, P.; Journaux, Y.; Pedroso, E.F.; Pereira, C.L.M.; Stumpf, H.O.; Carmen Muñoz, M.; et al. Rational design of a new class of heterobimetallic molecule-based magnets: Synthesis, crystal structures, and magnetic properties of oxamato-bridged M3′M2 (M′ = LiI and MnII; M = NiII and CoII) open-frameworks with a three-dimensional honeycomb architecture. Inorg. Chim. Acta 2008, 361, 3394–3402. [Google Scholar]
- Lisnard, L.; Chamoreau, L.-M.; Li, Y.; Journaux, Y. Solvothermal Synthesis of Oxamate-Based Helicate: Temperature Dependence of the Hydrogen Bond Structuring in the Solid. Cryst. Growth Des. 2012, 12, 4955–4962. [Google Scholar] [CrossRef]
- Pardo, E.; Burguete, P.; Ruiz-Garcia, R.; Julve, M.; Beltran, D.; Journaux, Y.; Amoros, P.; Lloret, F. Ordered mesoporous silicas as host for the incorporation and aggregation of octanuclear nickel(II) single-molecule magnets: A bottom-up approach to new magnetic nanocomposite materials. J. Mater. Chem. 2006, 16, 2702–2714. [Google Scholar] [CrossRef]
- Pardo, E.; Bernot, K.; Lloret, F.; Julve, M.; Ruiz-García, R.; Pasán, J.; Ruiz-Pérez, C.; Cangussu, D.; Costa, V.; Lescouëzec, R.; et al. Solid-State Anion–Guest Encapsulation by Metallosupramolecular Capsules Made from Two Tetranuclear Copper(II) Complexes. Eur. J. Inorg. Chem. 2007, 29, 4569–4573. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Stiriba, S.-E.; Vallejo, J.; Castro, I.; Julve, M.; Lloret, F.; Amorós, P.; Pasán, J.; et al. Reversible Solvatomagnetic Switching in a Spongelike Manganese(II)–Copper(II) 3D Open Framework with a Pillared Square/Octagonal Layer Architecture. Chem. Eur. J. 2012, 18, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J.; Serra-Crespo, P.; de Lange, M.; Gascon, J.; Kapteijn, F.; Julve, M.; Cano, J.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; et al. Selective Gas and Vapor Sorption and Magnetic Sensing by an Isoreticular Mixed-Metal–Organic Framework. J. Am. Chem. Soc. 2012, 134, 15301–15304. [Google Scholar] [CrossRef] [PubMed]
- Grancha, T.; Acosta, A.; Cano, J.; Ferrando-Soria, J.; Seoane, B.; Gascon, J.; Pasán, J.; Armentano, D.; Pardo, E. Cation Exchange in Dynamic 3D Porous Magnets: Improvement of the Physical Properties. Inorg. Chem. 2015, 54, 10834–10840. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.X.C.; Ribeiro, M.A.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Cangussu, D.; Julve, M.; Stumpf, H.O.; Pereira, C.L.M. Palladium(II)–Copper(II) Assembling with Bis(2-pyridylcarbonyl)amidate and Bis(oxamate) Type Ligands. Cryst. Growth Des. 2015, 15, 1325–1335. [Google Scholar] [CrossRef]
- Da Cunha, T.T.; Oliveira, W.X.C.; Pinheiro, C.B.; Pedroso, E.F.; Nunes, W.C.; Pereira, C.L.M. Alkaline Ion-Modulated Solid-State Supramolecular Organization in Mixed Organic/Metallorganic Compounds Based on 1,1′-Ethylenebis(4-aminopyridinium) Cations and Bis(oxamate)cuprate(II) Anions. Cryst. Growth Des. 2016, 16, 900–907. [Google Scholar] [CrossRef]
- Abdulmalic, M.A.; Aliabadi, A.; Petr, A.; Kataev, V.; Ruffer, T. The formation of overlooked compounds in the reaction of methyl amine with the diethyl ester of o-phenylenebis(oxamic acid) in MeOH. Dalton Trans. 2013, 42, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Barroso, S.; Blay, G.; Fernández, I.; Pedro, J.R.; Ruiz-García, R.; Pardo, E.; Lloret, F.; Muñoz, M.C. Chemistry and reactivity of mononuclear manganese oxamate complexes: Oxidative carbon–carbon bond cleavage of vic-diols by dioxygen and aldehydes catalyzed by a trans-dipyridine manganese(III) complex with a tetradentate o-phenylenedioxamate ligand. J. Mol. Catal. A Chem. 2006, 243, 214–220. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Ribeiro, M.A.; Pinheiro, C.B.; Nunes, W.C.; Julve, M.; Journaux, Y.; Stumpf, H.O.; Pereira, C.L.M. Magneto-Structural Study of an Oxamato-Bridged PdIICoII Chain: X-ray Crystallographic Evidence of a Single-Crystal-to-Single-Crystal Phase Transition. Eur. J. Inorg. Chem. 2012, 34, 5685–5693. [Google Scholar] [CrossRef]
- Pardo, E.; Lloret, F.; Carrasco, R.; Muñoz, M.C.; Temporal-Sánchez, T.; Ruiz-García, R. Chemistry and reactivity of dinuclear iron oxamate complexes: Alkane oxidation with hydrogen peroxide catalysed by an oxo-bridged diiron(III) complex with amide and carboxylate ligation. Inorg. Chim. Acta 2004, 357, 2713–2720. [Google Scholar] [CrossRef]
- CrysAlisPRO. Rigaku Oxford Diffraction: Yarnton, Oxfordshire, England, 2018. Available online: https://www.rigaku.com/en/products/smc/crysalis (accessed on 2 May 2018).
- Sheldrick, G.M. XPREP. Bruker-AXS, Madison, Wisconsin, USA. Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/single-crystal-x-ray-diffraction/sc-xrd-software/learn-more.html (accessed on 2 May 2018).
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92-a program for automatic solution of crystal structures by direct methods. J. Appl. Crystallogr. 1994, 27, 435. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar]
- Farrugia, L. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Unamuno, I.; Gutiérrez-Zorrilla, J.M.; Luque, A.; Román, P.; Lezama, L.; Calvo, R.; Rojo, T. Ion-Pair Charge-Transfer Complexes Based on (o-Phenylenebis(oxamato))cuprate(II) and Cyclic Diquaternary Cations of 1,10-Phenanthroline and 2,2‘-Bipyridine: Synthesis, Crystal Structure, and Physical Properties. Inorg. Chem. 1998, 37, 6452–6460. [Google Scholar] [CrossRef] [PubMed]
- Reis, N.V.; Barros, W.P.; Oliveira, W.X.C.; Pereira, C.L.M.; Rocha, W.R.; Pinheiro, C.P.; Lloret, F.; Julve, M.; Stumpf, H.O. Crystal Structure and Magnetic Properties of an Oxamato-Bridged Heterobimetallic Tetranuclear [NiIICuII]2 Complex of the Rack Type. Eur. J. Inorg. Chem. 2018, 3–4, 477–484. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pinheiro, C.B.; da Costa, M.M.; Fontes, A.P.S.; Nunes, W.C.; Lloret, F.; Julve, M.; Pereira, C.L.M. Crystal Engineering Applied to Modulate the Structure and Magnetic Properties of Oxamate Complexes Containing the [Cu(bpca)]+ Cation. Cryst. Growth Des. 2016, 16, 4094–4107. [Google Scholar] [CrossRef]
- Pardo, E.; Bernot, K.; Julve, M.; Lloret, F.; Cano, J.; Ruiz-García, R.; Delgado, F.S.; Ruiz-Pérez, C.; Ottenwaelder, X.; Journaux, Y. Spin control in ladderlike hexanuclear copper(ii) complexes with metallacyclophane cores. Inorg. Chem. 2004, 43, 2768–2770. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Cangussu, D.; Lescouëzec, R.; Journaux, Y.; Pasán, J.; Delgado, F.S.; Ruiz-Pérez, C.; Ruiz-García, R.; Cano, J.; Julve, M.; et al. Molecular-Programmed Self-Assembly of Homo- and Heterometallic Tetranuclear Coordination Compounds: Synthesis, Crystal Structures, and Magnetic Properties of Rack-Type CuII2MII2 Complexes (M = Cu and Ni) with Tetranucleating Phenylenedioxamato Bridging Ligands. Inorg. Chem. 2009, 48, 4661–4673. [Google Scholar] [PubMed]
- Ferrando-Soria, J.; Khajavi, H.; Serra-Crespo, P.; Gascon, J.; Kapteijn, F.; Julve, M.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; Journaux, Y.; et al. Highly selective chemical sensing in a luminescent nanoporous magnet. Adv. Mater. 2012, 24, 5625–5629. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, G.M.; do Pim, W.D.; Reis, D.O.; Simões, T.R.G.; Pradie, N.A.; Stumpf, H.O. Characterization of compounds derived from copper-oxamate and imidazolium by X-ray absorption and vibrational spectroscopies. Spectrochim. Acta A 2015, 142, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Grancha, T.; Tourbillon, C.; Ferrando-Soria, J.; Julve, M.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; Fabelo, O.; Pardo, E. Self-assembly of a chiral three-dimensional manganese(II)–copper(II) coordination polymer with a double helical architecture. CrystEngComm 2013, 15, 9312–9315. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Ma, Z.Y.; Wang, D.M.; Xie, C.Z. A New Linear Tetranuclear Copper(II) Complex: Synthesis, Structural Characterization, and Magnetic Behavior. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2011, 41, 763–767. [Google Scholar] [CrossRef]
- Pardo, E.; Ruiz-García, R.; Lloret, F.; Julve, M.; Cano, J.; Pasán, J.; Ruiz-Pérez, C.; Filali, Y.; Chamoreau, L.-M.; Journaux, Y. Molecular-Programmed Self-Assembly of Homo- and Heterometallic Penta- and Hexanuclear Coordination Compounds: Synthesis, Crystal Structures, and Magnetic Properties of Ladder-Type CuII2MIIx (M = Cu, Ni; x = 3, 4) Oxamato Complexes with CuII2 Metallacyclophane Cores. Inorg. Chem. 2007, 46, 4504–4514. [Google Scholar] [PubMed]
- Fernandes, T.S.; Vilela, R.S.; Valdo, A.K.; Martins, F.T.; García-España, E.; Inclán, M.; Cano, J.; Lloret, F.; Julve, M.; Stumpf, H.O.; et al. Dicopper(II) Metallacyclophanes with N,N′-2,6-Pyridinebis(oxamate): Solution Study, Synthesis, Crystal Structures, and Magnetic Properties. Inorg. Chem. 2016, 5, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Carrasco, R.; Ruiz-García, R.; Julve, M.; Lloret, F.; Muñoz, M.C.; Journaux, Y.; Ruiz, E.; Cano, J. Structure and Magnetism of Dinuclear Copper(II) Metallacyclophanes with Oligoacenebis(oxamate) Bridging Ligands: Theoretical Predictions on Wirelike Magnetic Coupling. J. Am. Chem. Soc. 2008, 130, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Saiz, P.; Gil-García, R.; Maestro, M.A.; Pizarro, J.L.; Arriortua, M.I.; Lezama, L.; Rojo, T.; González-Álvarez, M.; Borrás, J.; García-Tojal, J. Structure, magnetic properties and nuclease activity of pyridine-2-carbaldehyde thiosemicarbazonecopper(II) complexes. J. Inorg. Biochem. 2008, 102, 1910–1920. [Google Scholar]
- Do Pim, W.D.; Oliveira, W.X.C.; Ribeiro, M.A.; de Faria, E.N.; Teixeira, I.F.; Stumpf, H.O.; Lago, R.M.; Pereira, C.L.M.; Pinheiro, C.B.; Figueiredo-Júnior, J.C.D.; et al. A pH-triggered bistable copper(II) metallacycle as a reversible emulsion switch for biphasic processes. Chem. Commun. 2013, 49, 10778–10780. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.K.; Aliaga-Alcalde, N.; Corbella, M.; Hundal, G. Synthesis, crystal structure, spectral and magnetic studies and catecholase activity of copper(II) complexes with di- and tri-podal ligands. Inorg. Chim. Acta 2010, 363, 97–106. [Google Scholar] [CrossRef]
- Fang, S.M.; Sañudo, E.C.; Hu, M.; Zhang, Q.; Zhou, L.M.; Liu, C.S. Copper(II) Complexes with cis-Epoxysuccinate Ligand: Syntheses, Crystal Structures, and Magnetic Properties. Aust. J. Chem. 2011, 64, 217–226. [Google Scholar] [CrossRef]
- Baggio, R.; Garland, M.T.; Manzur, J.; Peña, O.; Perec, M.; Spodine, E.; Vega, A. A dinuclear copper(II) complex involving monoatomic O-carboxylate bridging and Cu–S(thioether) bonds: [Cu(tda)(phen)]2·H2tda (tda = thiodiacetate, phen = phenanthroline). Inorg. Chim. Acta 1999, 286, 74–79. [Google Scholar] [CrossRef]
- Zhu, H.L.; Xu, W.; Lin, J.L.; Zhang, C.; Zheng, Y.-Q. Syntheses, crystal structures, and magnetism of two phenylacetate imidazolate copper(II) complexes. J. Coord. Chem. 2012, 65, 3983–3997. [Google Scholar] [CrossRef]
- Costes, J.P.; Dahan, F.; Laurent, J.P. A further example of a dinuclear copper(II) complex involving monoatomic acetate bridges. Synthesis, crystal structure, and spectroscopic and magnetic properties of bis(μ-acetato)bis(7-amino-4-methyl-5-aza-3-hepten-2-onato(1-))dicopper(II). Inorg. Chem. 1985, 24, 1018–1022. [Google Scholar] [CrossRef]
- Chiari, B.; Helms, J.H.; Piovesana, O.; Tarantelli, T.; Zanazzi, P.F. Exchange interaction in multinuclear transition-metal complexes. 8. Structural and magnetic studies on bis(acetato)bis(N-methyl-N′-(5-methoxysalicylidene)-1,3-propanediaminato)dicopper dihydrate, a novel “structural” ladderlike compound with “magnetic” alternating-chain behavior. Inorg. Chem. 1986, 25, 870–874. [Google Scholar]
- Mukherjee, P.; Sengupta, O.; Drew, M.G.B.; Ghosh, A. Anion directed template synthesis of Cu(II) complexes of a N,N,O donor mono-condensed Schiff base ligand: A molecular scaffold forming highly ordered H-bonded rectangular grids. Inorg. Chim. Acta 2009, 362, 3285–3291. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Cen, P.; Chen, X.; Zhou, H.; Song, W.; Hu, Q. Auxiliary ligand-triggered assembly of two dinuclear Cu(II) compounds with a pyridylhydrazone derivative: Synthesis, crystal structure and magnetic property. Inorg. Chim. Acta 2016, 447, 12–17. [Google Scholar] [CrossRef]
- Sarkar, B.; Drew, M.G.B.; Estrader, M.; Diaz, C.; Ghosh, A. CuII acetate complexes involving N,N,O donor Schiff base ligands: Mono-atomic oxygen bridged dimers and alternating chains of the dimers and Cu2(OAc)4. Polyhedron 2008, 27, 2625–2633. [Google Scholar] [CrossRef]
- Baggio, R.; Calvo, R.; Garland, M.T.; Peña, O.; Perec, M.; Slep, L.D. A new copper(II) di-μ2-carboxylato bridged dinuclear complex: [Cu(oda)phen]2·6H2O (oda = oxydiacetate, phen = phenanthroline). Inorg. Chem. Commun. 2007, 10, 1249–1252. [Google Scholar] [CrossRef]
- Greenaway, A.M.; O’Connor, C.J.; Overman, J.W.; Sinn, E. Magnetic properties and crystal structure of an acetate-bridged ferromagnetic copper(II) dimer. Inorg. Chem. 1981, 20, 1508–1513. [Google Scholar] [CrossRef]
- Bleaney, B.; Bowers, K.D. Anomalous Paramagnetism of Copper Acetate. Proc. R. Soc. London, Ser. A 1952, 214, 451–465. [Google Scholar] [CrossRef]


| Compound | 1 | 2 |
|---|---|---|
| Formula | C26H16N6O21.48Cu2Li3 | C44H52ClCu2KN12O25.38 |
| Fw/g mol−1 | 903.96 | 1344.65 |
| T/K | 150 | 150 |
| λ/Å | 0.71073 | 0.71073 |
| Crystal System | Triclinic | Monoclinic |
| Space group | ||
| a/Å | 9.3856 (5) | 14.0948(7) |
| b/Å | 10.3708 (6) | 15.6318(11) |
| c/Å | 19.3438(10) | 27.4889(15) |
| α/o | 92.925(4) | 90 |
| β/o | 96.846(4) | 102.879(5) |
| γ/o | 98.239(4) | 90 |
| V/Å3 | 1845.68(18) | 5904.2(6) |
| Z | 2 | 4 |
| ρ/mg m−3 | 1.627 | 1.513 |
| μ/mm−1 | 1.245 | 0.928 |
| F(000) | 906 | 2740 |
| Crystal size/mm3 | 0.40 × 0.17 × 0.10 | 0.38 × 0.13 × 0.11 |
| Reflections collected (Rint) | 15068 (0.069) | 13128 (0.113) |
| Unique Reflections | 15068 | 13128 |
| Reflections with I ≥ 2σ(I) | 10152 | 7712 |
| Goodness-of-fit on F2 | 1.426 | 1.173 |
| Ra, wRb | 0.1218, 0.3603 | 0.0815, 0.2378 |
| Ra, wRb(all data) | 0.1545, 0.3789 | 0.1219, 0.2539 |
| Larg. diff. peak and hole/e Å−3 | 3.268, −1.073 | 1.907, −0.952 |
| Bond Lengths/Å | Bond Angles/deg | ||
|---|---|---|---|
| Cu1—N1 | 1.975 (6) | N1—Cu1—N2 | 104.2 (3) |
| Cu1—N2 | 1.977 (7) | N1—Cu1—O1 | 83.2 (5) |
| Cu1—O1 | 1.984 (16) | N1—Cu1—O4 | 162.6 (4) |
| Cu1—O4 | 2.056 (12) | N1—Cu1—O13 | 94.9 (3) |
| Cu1—O13 | 2.299 (7) | N2—Cu1—O1 | 162.1 (5) |
| Cu2—N3 | 1.953 (6) | N2—Cu1—O4 | 82.7 (4) |
| Cu2—N4 | 1.951 (6) | N2—Cu1—O13 | 97.8 (3) |
| Cu2—O7 | 1.978 (5) | O1—Cu1—O4 | 85.9 (6) |
| Cu2—O10 | 1.985 (5) | O1—Cu1—O13 | 97.8 (5) |
| Cu2—O10i | 2.682 (6) | O4—Cu1—O13 | 100.0 (5) |
| Li1—O8 | 2.113 (15) | N3—Cu2—N4 | 102.9 (2) |
| Li1—O9 | 2.007 (15) | N3—Cu2—O7 | 84.0 (2) |
| Li1—O11ii | 2.083 (15) | N3—Cu2—O10 | 170.7 (2) |
| Li1—O12ii | 2.006 (16) | N3—Cu2—O10i | 100.4 (2) |
| Li1—O19 | 2.083 (18) | N4—Cu2—O7 | 167.4 (2) |
| Li2—O2 | 2.04 (3) | N4—Cu2—O10 | 84.1 (2) |
| Li2—O3 | 2.08 (3) | N4—Cu2—O10i | 105.2 (2) |
| Li2—O5ii | 2.08 (3) | O7—Cu2—O10 | 88.0 (2) |
| Li2—O6ii | 2.06 (3) | O7—Cu2—O10i | 85.3 (2) |
| Li2—O14 | 2.09 (3) | O10—Cu2—O10i | 83.3 (2) |
| Li3—O3 | 1.77 (3) | O8—Li1—O9 | 81.1 (5) |
| Li3—O6ii | 1.88 (3) | O8—Li1—O11ii | 100.9 (7) |
| Li3—O17 | 1.80 (3) | O8—Li1—O12ii | 157.9 (9) |
| Li3—O15 | 2.05 (3) | O8—Li1—O19 | 100.5 (8) |
| O9—Li1—O11ii | 154.6 (10) | ||
| O9—Li1—O12ii | 87.0 (6) | ||
| O9—Li1—O19 | 98.6 (7) | ||
| O11ii —Li1—O12ii | 82.1 (6) | ||
| O11ii —Li1—O19 | 105.9 (7) | ||
| O12ii —Li1—O19 | 99.7 (7) | ||
| O2—Li2—O3 | 81.1 (11) | ||
| O2—Li2—O5ii | 105.2 (13) | ||
| O2—Li2—O6ii | 156.3 (16) | ||
| O2—Li2—O14 | 97.1 (14) | ||
| O3—Li2—O14 | 103.7 (14) | ||
| O3—Li2—O6ii | 84.5 (12) | ||
| O3—Li2—O5ii | 157.6 (16) | ||
| O5ii—Li2—O6ii | 81.8 (11) | ||
| O5ii—Li2—O14 | 97.0 (13) | ||
| O6ii—Li2—O14 | 104.5 (14) | ||
| O3—Li3—O6ii | 85.9 (11) | ||
| O3—Li3—O15ii | 127.5 (15) | ||
| O17—Li3—O3 | 102.1 (13) | ||
| O17—Li3—O6ii | 118.5 (15) | ||
| O6ii—Li3—O17 | 97.8 (12) | ||
| O6ii—Li3—O15 | 135.5 (15) | ||
| O17—Li3—O15 | 101.6 (14) | ||
| Bond Lengths/Å | Bond Angles/deg | ||
|---|---|---|---|
| Cu1—N1 | 1.975 (6) | N1—Cu1—N2i | 106.9 (3) |
| Cu1—N2i | 1.977 (7) | N1—Cu1—O1 | 83.6 (3) |
| Cu1—O1 | 1.984 (16) | N1—Cu1—O4i | 83.0 (3) |
| Cu1—O4i | 2.056 (12) | N1—Cu1—O7 | 95.0 (3) |
| Cu1—O7 | 2.55 (1) | N2i—Cu1—O1 | 169.3 (2) |
| K1—O1 | 2.892 (6) | N2i —Cu1—O4i | 83.0 (3) |
| K1—O4 | 2.799 (6) | N2i —Cu1—O7 | 91.4 (3) |
| K1—O8 | 2.812 (9) | O1—Cu1—O4i | 86.4 (2) |
| K1—O9ii | 2.999 (7) | O1—Cu1—O7 | 89.4 (3) |
| O4i—Cu1—O7 | 86.0 (3) | ||
| O1—K1—O1vi | 127.1 (3) | ||
| O1—K1—O4 | 55.95 (16) | ||
| O1—K1—O4vi | 101.75 (18) | ||
| O1vi—K1—O4 | 101.75 (18) | ||
| O1vi—K1—O4vi | 55.95 (16) | ||
| O1—K1—O8 | 79.3 (2) | ||
| O1—K1—O8vi | 147.1 (2) | ||
| O1vi—K1—O8 | 147.1 (2) | ||
| O1vi—K1—O8vi | 79.3 (2) | ||
| O1vi—K1—O9ii | 67.76 (18) | ||
| O1—K1—O9ii | 131.30 (18) | ||
| O1vi—K1—O9iii | 131.30 (18) | ||
| O1—K1—O9iii | 67.75 (18) | ||
| O4vi—K1—O4 | 132.8 (3) | ||
| O4—K1—O8 | 75.8 (3) | ||
| O4—K1—O8vi | 146.4 (3) | ||
| O4vi—K1—O8 | 146.4 (3) | ||
| O4vi—K1—O8vi | 75.8 (3) | ||
| O4—K1—O9ii | 76.38 (19) | ||
| O4—K1—O9iii | 119.24 (17) | ||
| O4vi—K1—O9ii | 119.23 (17) | ||
| O4vi—K1—O9iii | 76.38 (19) | ||
| O8—K1—O8vi | 85.5 (4) | ||
| O8—K1—O9ii | 80.0 (3) | ||
| O8—K1—O9iii | 73.1 (3) | ||
| O8vi—K1—O9ii | 73.1 (3) | ||
| O8vi—K1—O9iii | 80.0 (3) | ||
| O9ii—K1—O9iii | 143.2 (3) | ||
| Compound a | J b/cm−1 | j c/cm−1 | daxial d/Å | Θ e/K | θCuOCu f/° | Reference |
|---|---|---|---|---|---|---|
| 1 | +10.6 | −0.68 | 2.682 | 96.7 | This work | |
| 2 | +8.22 | +0.27 | This Work | |||
| Na4[Cu2(μ-mpba)2]·10H2O | +16.8 | --- | --- | --- | [12] | |
| [Cu2(μ-mpba)2(H2O)F] [Cu(L1)]4 (PF6)3·5H2O | +17.0 | --- | --- | --- | [47] | |
| [Cu2(L2)2(H2O)2][Cu(L1)]4 (ClO4)4·12H2O | +9.0 | --- | --- | --- | ||
| [Ni(L3)][Cu2(μ-mpba)2] [Ni(L3)]3(ClO4)4·6H2O | +4.2 | --- | --- | --- | ||
| (Me4N)4[Cu2(L4)2(H2O)]2·H2O | +6.85 | --- | --- | --- | [48] | |
| (Me4N)2KNa [Cu(L4)2(H2O)6.8]·0.8H2O | +7.40 | --- | --- | --- | ||
| Na6[Cu2(L4)2Cl2(H2O)4]·7H2O | +7.90 | --- | --- | --- | [48] | |
| [Cu(L5)(μ-tfa)]2 | --- | −3.30 | 2.630 | 105.7 | [50] | |
| [Cu(H2L6)(μ-EtOH)]2 | --- | +2.93 | 2.365 | 102.5 | [51] | |
| [Cu(L7)(μ-OAc)]2 | --- | −0.37 | 2.399 | 102.2 | [52] | |
| [Cu(phen)(μ-L8)]2 | --- | +1.80 | 2.340 | 103.0 | [53] | |
| [Cu(bpy)-(μ-L9)]2 | --- | +1.50 | 2.369 | 104.9 | ||
| [Cu(phen)(μ-L10)]2·H2L4 | --- | +3.20 | 2.320 | 101.9 | [54] | |
| [Cu(im)(μ-L11)]2·2H2O | --- | +0.58 | 2.571 | 102.5 | [55] | |
| [Cu(L12)(μ-OAc)]2 | --- | −0.50 | 2.490 | 95.3 | [56] | |
| [Cu(L13)(μ-OAc)]2·2H2O | --- | −1.51 | 2.577 | 96.1 | [57] | |
| [Cu(L14)(μ-OAc)]2·2H2O | --- | −1.84 | 2.665 | 96.3 | ||
| [Cu(L15)(μ-OAc)]2 | --- | +2.85 | 2.440 | 98.1 | [58] | |
| [Cu(L16)(μ-HL11)]2 | --- | −2.03 | 2.299 | 103.3 | [59] | |
| Na2(C12H12N2)[Cu(L17)]2·4H2O | --- | −0.80 | 2.788 | 95.4 | [38] | |
| [Cu(L18)(μ-OAc)]2 | --- | −0.56 | 2.501 | 98.7 | [60] | |
| [Cu(phen)(μ-L19)]2 | --- | +3.30 | 2.332 | 104.6 | [61] | |
| [Cu(L20)(μ-OAc)]2·H2O·EtOH | --- | +0.63 | 2.650 | 102.6 | [62] | |
| (EDAP)2[Cu(L17)]2·4H2O | --- | −1.63 | 2.591 | 96.1 | [26] | |
| Na2(EDAP)[Cu(L17)]2·6H2O | --- | −2.29 | 2.616 | 93.7 | ||
| K2(EDAP)[Cu(L17)]2·5H2O | --- | −1.65 | 2.911 | 94.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
T. da Cunha, T.; X. C. Oliveira, W.; F. Pedroso, E.; Lloret, F.; Julve, M.; L. M. Pereira, C. Heterobimetallic One-Dimensional Coordination Polymers MICuII (M = Li and K) Based on Ferromagnetically Coupled Di- and Tetracopper(II) Metallacyclophanes. Magnetochemistry 2018, 4, 38. https://doi.org/10.3390/magnetochemistry4030038
T. da Cunha T, X. C. Oliveira W, F. Pedroso E, Lloret F, Julve M, L. M. Pereira C. Heterobimetallic One-Dimensional Coordination Polymers MICuII (M = Li and K) Based on Ferromagnetically Coupled Di- and Tetracopper(II) Metallacyclophanes. Magnetochemistry. 2018; 4(3):38. https://doi.org/10.3390/magnetochemistry4030038
Chicago/Turabian StyleT. da Cunha, Tamyris, Willian X. C. Oliveira, Emerson F. Pedroso, Francesc Lloret, Miguel Julve, and Cynthia L. M. Pereira. 2018. "Heterobimetallic One-Dimensional Coordination Polymers MICuII (M = Li and K) Based on Ferromagnetically Coupled Di- and Tetracopper(II) Metallacyclophanes" Magnetochemistry 4, no. 3: 38. https://doi.org/10.3390/magnetochemistry4030038