New Dmit-Based Organic Magnetic Conductors (PO-CONH-C2H4N(CH3)3)[M(dmit)2]2 (M = Ni, Pd) Including an Organic Cation Derived from a 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl (PO) Radical
Abstract
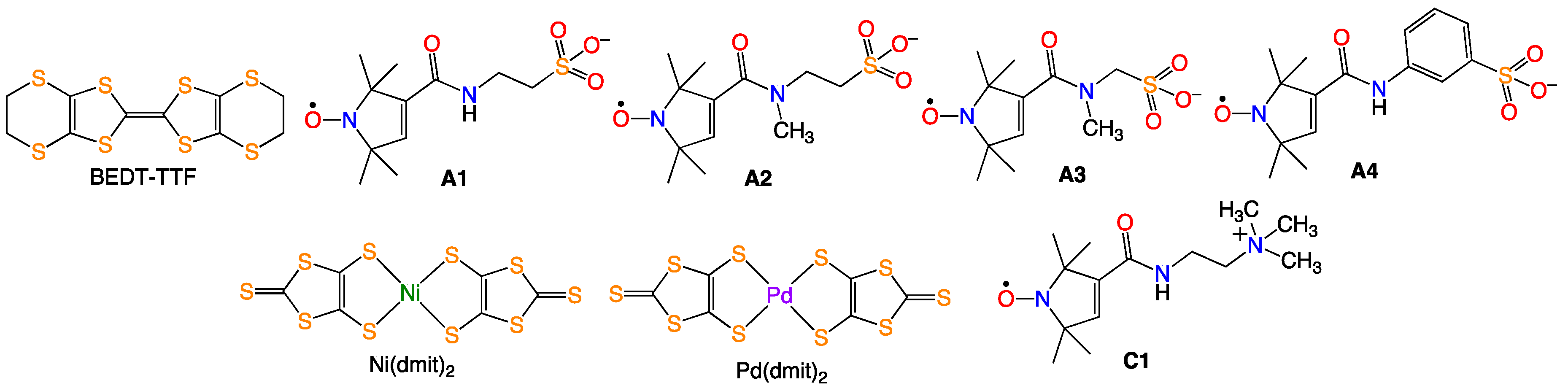
:1. Introduction
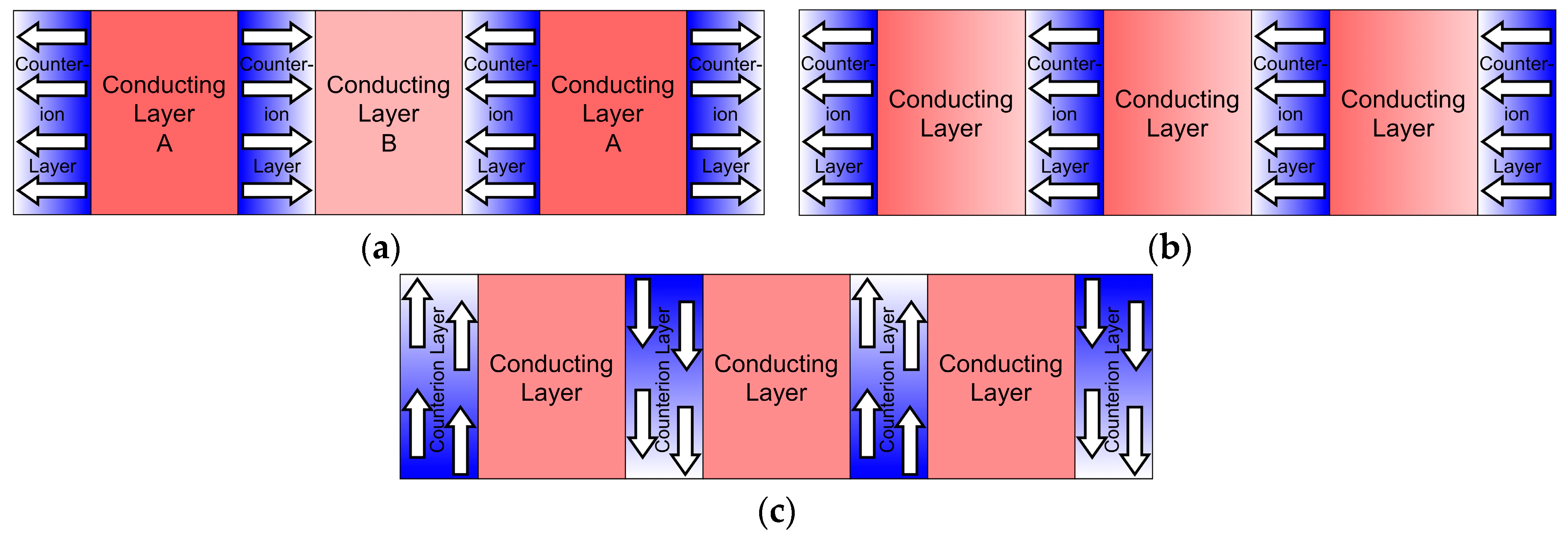
2. Results and Discussion
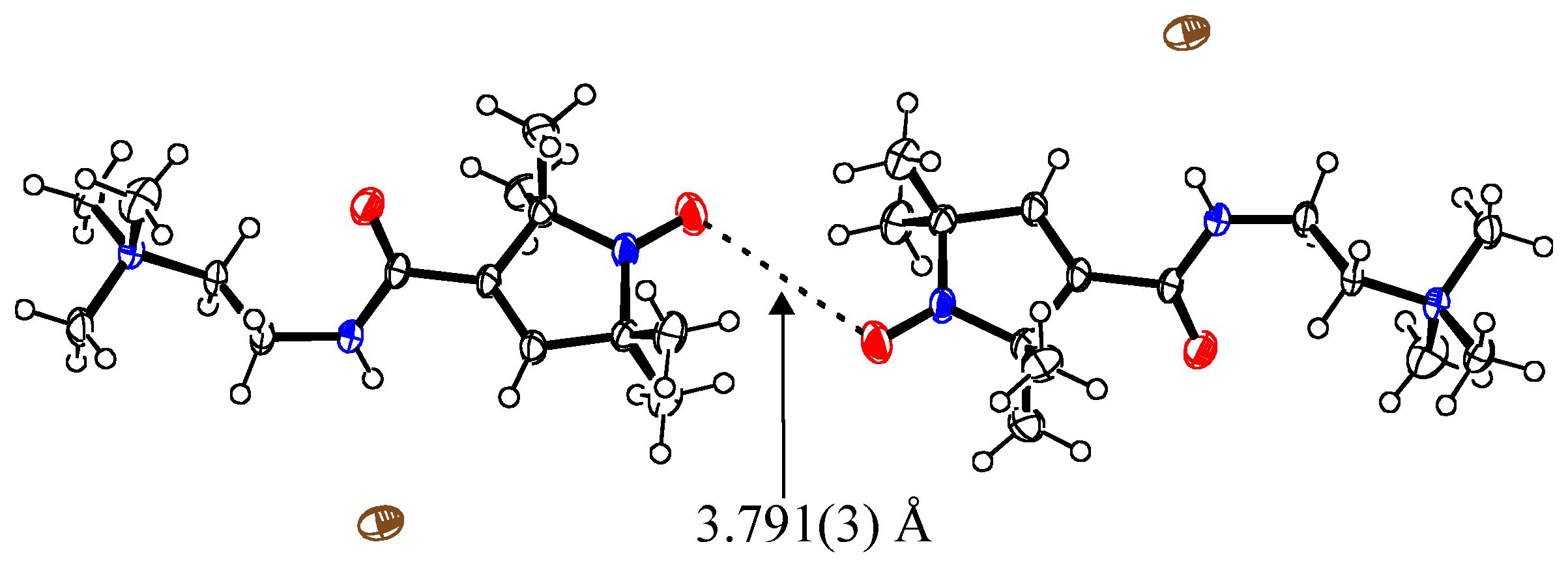
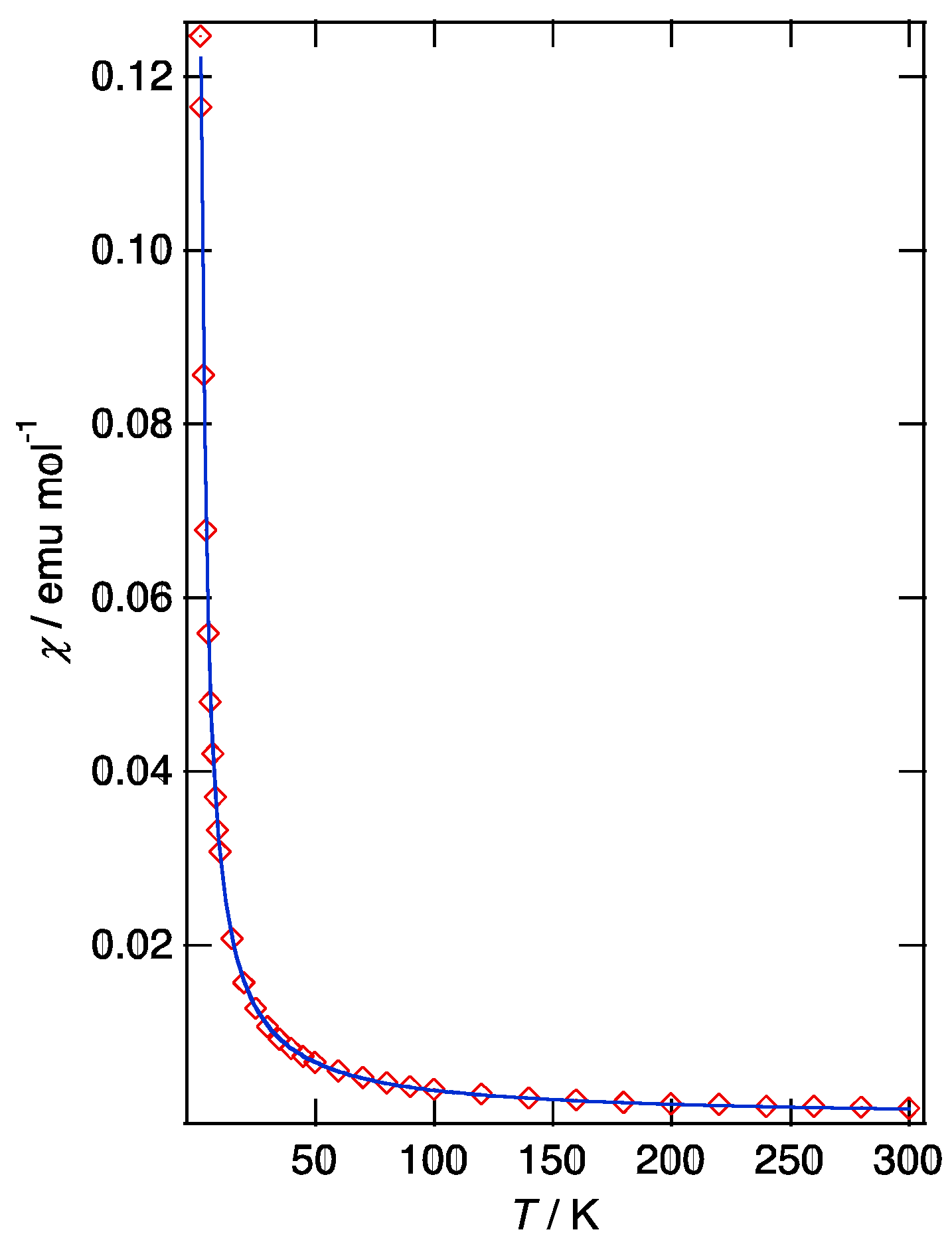
2.1. (PO-CONH-C2H4N(CH3)3)Cl (C1Cl, 1)
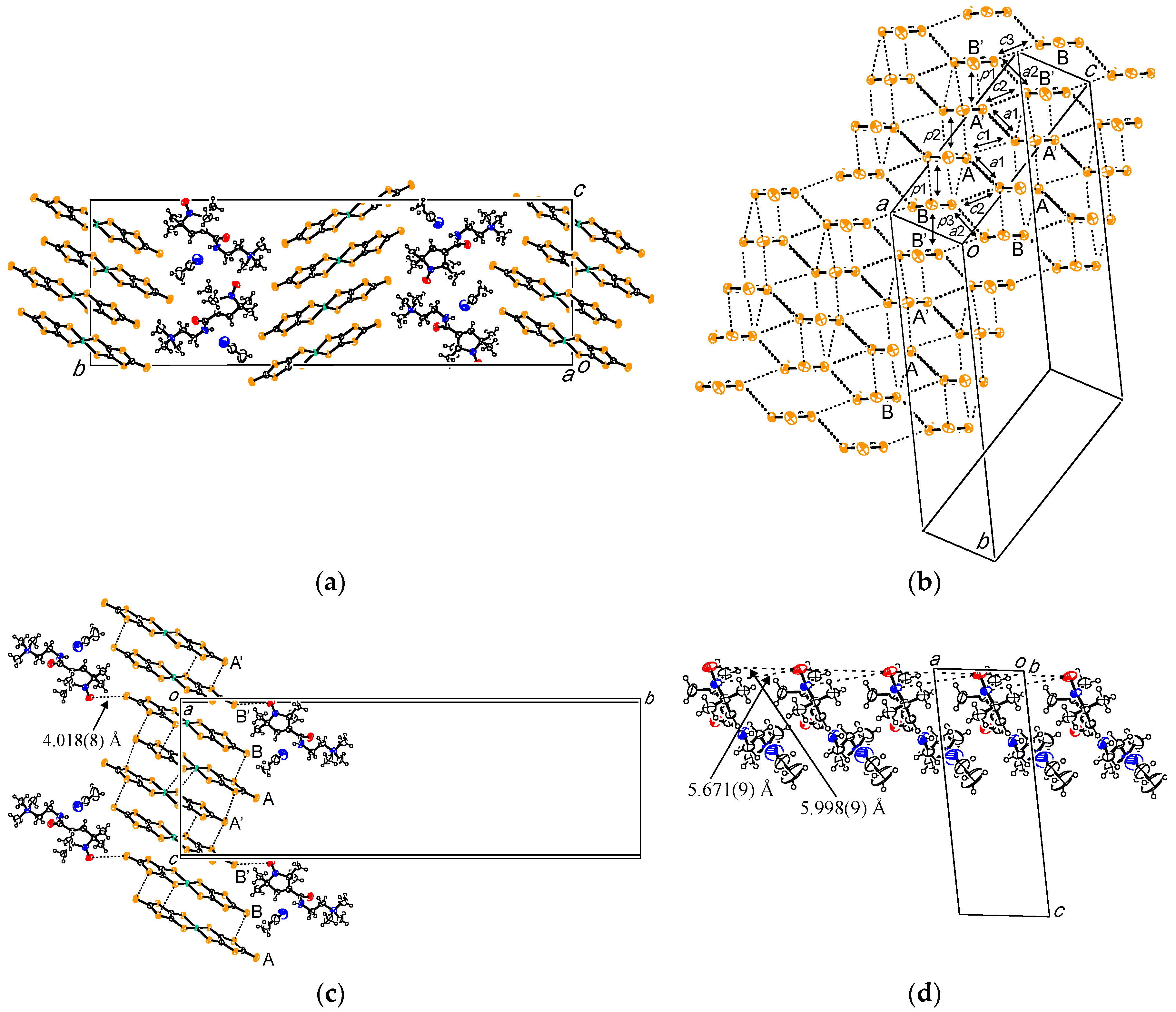
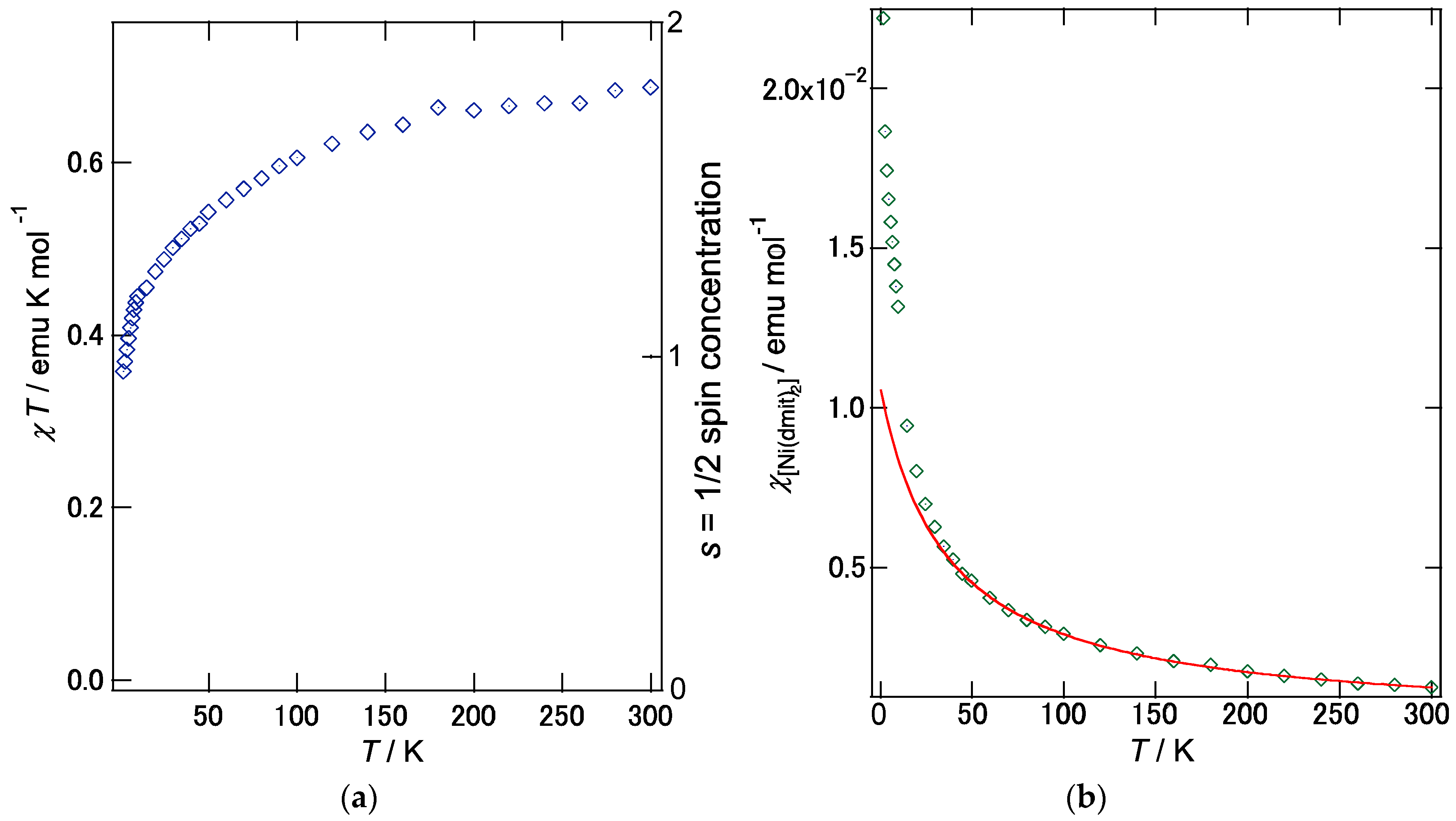
2.2. (PO-CONH-C2H4N(CH3)3)[Ni(dmit)2]2·CH3CN ((C1)[Ni(dmit)2]2·CH3CN, 2)
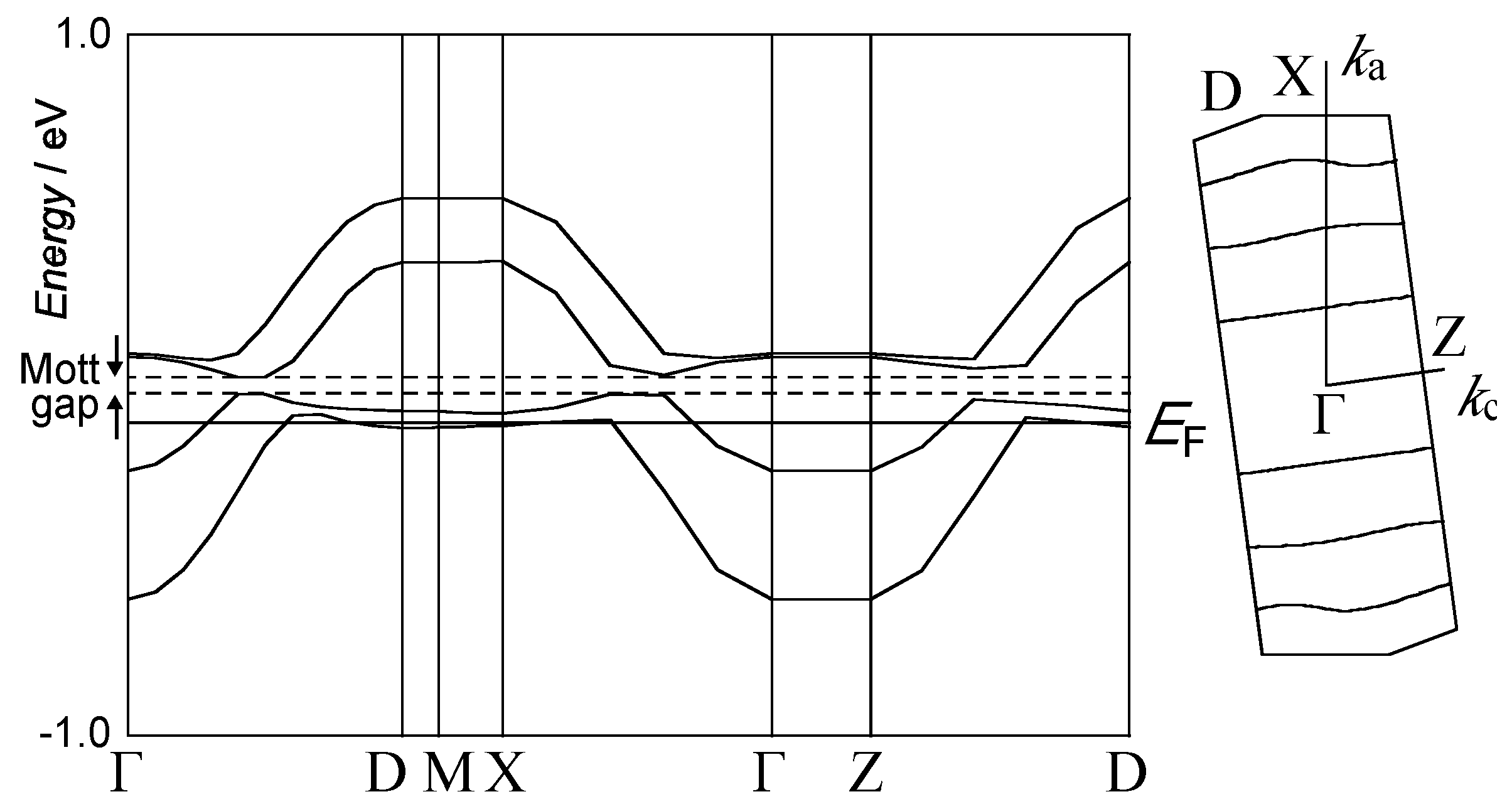
2.3. (PO-CONH-C2H4N(CH3)3)[Pd(dmit)2]2 ((C1)[Pd(dmit)2]2, 3)
3. Materials and Methods
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kobayashi, H.; Cui, H.; Kobayashi, A. Organic metals and superconductors based on BETS (BETS = bis(ethylenedithio)tetraselenafulvalene. Chem. Rev. 2004, 104, 5265–5288. [Google Scholar] [CrossRef] [PubMed]
- Day, P.; Coronado, E. Magnetic molecular conductors. Chem. Rev. 2004, 104, 5419–5448. [Google Scholar]
- Vyaselev, O.M.; Kartsovnik, M.V.; Biberacher, W.; Zorina, L.V.; Kushch, N.D.; Yagubskii, E.B. Magnetic transformations in the organic conductor κ-(BETS)2Mn[N(CN)2]3[N(CN)2]3 at the metal-insulator transition. Phys. Rev. B 2011, 83, 094425:1–094425:6. [Google Scholar] [CrossRef]
- Nakatsuji, S. Preparations, Reactions, and Properties of Functional Nitroxide Radicals. In Nitroxide: Application in Chemistry, Biomedicine, and Materials Chemistry; Likhtenshtein, G.I., Yamauchi, J., Nakatsuji, S., Smirnov, A.I., Tamura, R., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 161–204. [Google Scholar]
- Matsushita, M.M.; Kawakami, H.; Kawada, Y.; Sugawara, T. Negative Magneto-resistance Observed on an Ion-radical Salt of a TTF-based Spin-polarized Donor. Chem. Lett. 2007, 36, 110–111. [Google Scholar] [CrossRef]
- Sugawara, T.; Komatsu, H.; Suzuki, K. Interplay between magnetism and conductivity derived from spin-polarized donor radicals. Chem. Soc. Rev. 2011, 40, 3105–3118. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, H.; Yamada, J.; Nakatsuji, S. Preparation and characterization of novel organic radical anions for organic conductors: TEMPO–NHSO3− and TEMPO-OSO3−. Synth. Met. 2001, 120, 871–872. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S. A New Organic Anion Consisting of the TEMPO Radical for Organic Charge-Transfer Salts: 2,2,6,6-Tetramethylpiperidinyloxy-4-sulfamate (TEMPO–NHSO3−). Chem. Lett. 2001, 30, 208–209. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S. New BEDT-TTF-based Organic Conductor Including an Organic Anion Derived from the TEMPO Radical, α-(BEDT-TTF)3(TEMPO–NHCOCH2SO3)2·6H2O. Chem. Lett. 2003, 32, 1118–1119. [Google Scholar] [CrossRef]
- Akutsu, H.; Masaki, K.; Mori, K.; Yamada, J.; Nakatsuji, S. New organic free radical anions TEMPO-A-CO-(o-, m-, p-)C6H4SO3− (A = NH, NCH3, O) and their TTF and/or BEDT-TTF salts. Polyhedron 2005, 24, 2126–2132. [Google Scholar] [CrossRef]
- Yamashita, A.; Akutsu, H.; Yamada, J.; Nakatsuji, S. New organic magnetic anions TEMPO–CONA(CH2)nSO3− (n = 0–3 for A = H, n = 2 for A = CH3) and their TTF, TMTSF and/or BEDT-TTF salts. Polyhedron 2005, 16, 2796–2802. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S. A novel BEDT-TTF-based purely organic magnetic conductor, α-(BEDT-TTF)2(TEMPO–N(CH3)COCH2SO3)·3H2O. Solid State Commun. 2006, 140, 256–260. [Google Scholar] [CrossRef]
- Akutsu, H.; Sato, K.; Yamashita, S.; Yamada, J.; Nakatsuji, S.; Turner, S.S. The first organic paramagnetic metal containing the aminoxyl radical. J. Mater. Chem. 2008, 18, 3313–3315. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamashita, S.; Yamada, J.; Nakatsuji, S.; Turner, S.S. Novel Purely Organic Conductor with an Aminoxyl Radical, α-(BEDT-TTF)2(PO–CONHCH2SO3)·2H2O (PO = 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl Free Radical). Chem. Lett. 2008, 37, 882–883. [Google Scholar] [CrossRef]
- Akutsu, H.; Yamashita, S.; Yamada, J.; Nakatsuji, S.; Hosokoshi, Y.; Turner, S.S. A Purely Organic Paramagnetic Metal, κ-β″-(BEDT-TTF)2(PO–CONHC2H4SO3), Where PO = 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl Free Radical. Chem. Mater. 2011, 23, 762–764. [Google Scholar] [CrossRef]
- Akutsu, H.; Kawamura, A.; Yamada, J.; Nakatsuji, S.; Turner, S.S. Anion polarity-induced dual oxidation states in a dual-layered purely organic paramagnetic charge-transfer salt, (TTF)3(PO–CON(CH3)C2H4SO3)2, where PO = 2,2,5,5-tetramethyl-3-pyrrolin-1-oxyl free radical. CrystEngComm 2011, 13, 5281–5284. [Google Scholar] [CrossRef] [Green Version]
- Akutsu, H.; Ishihara, K.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Nakazawa, Y. A strongly polarized organic conductor. CrystEngComm 2016, 18, 8151–8154. [Google Scholar] [CrossRef]
- Akutsu, H.; Ishihara, K.; Ito, S.; Nishiyama, F.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Nakazawa, Y. Anion Polarity-Induced Self-doping in Purely Organic Paramagnetic Conductor, α′-α′-(BEDT-TTF)2(PO-CONH-m-C6H4SO3)·H2O where BEDT-TTF is Bis(ethylenedithio)tetrathiafulvalene and PO is 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl Free Radical. Polyhedron 2017, in press. [Google Scholar] [CrossRef]
- Kato, R. Conducting Metal Dithiolene Complexes: Structural and Electronic Properties. Chem. Rev. 2004, 104, 5319–5346. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kobayashi, A.; Tajima, H. Studies on Molecular Conductors: From Organic Semiconductors to Molecular Metals and Superconductors. Chem. Asian J. 2011, 6, 1688–1704. [Google Scholar] [CrossRef] [PubMed]
- Cassoux, P.; Valade, L.; Kobayashi, H.; Kobayashi, A.; Clark, R.A.; Underhill, A.E. Molecular metals and superconductors derived from metal complexes of 1,3-dithiol-2-thione-4,5-dithiolate (dmit). Coord. Chem. Rev. 1991, 110, 115–160. [Google Scholar] [CrossRef]
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The Intermolecular Interaction of Tetrathiafulvalene and Bis(ethylenedithio)tetrathiafulvalene in Organic Metals. Calculation of Orbital Overlaps and Models of Energy-band Structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC7, Stewart Computational Chemistry: Colorado Springs, CO, USA, 1993.
- Rozantsev, E.G. Free Nitroxide Radicals; Plenum Press: New York, NY, USA; London, UK, 1970. [Google Scholar]
- Stewart, J.J.P. MOPAC2016, Stewart Computational Chemistry: Colorado Springs, CO, USA, 2016.
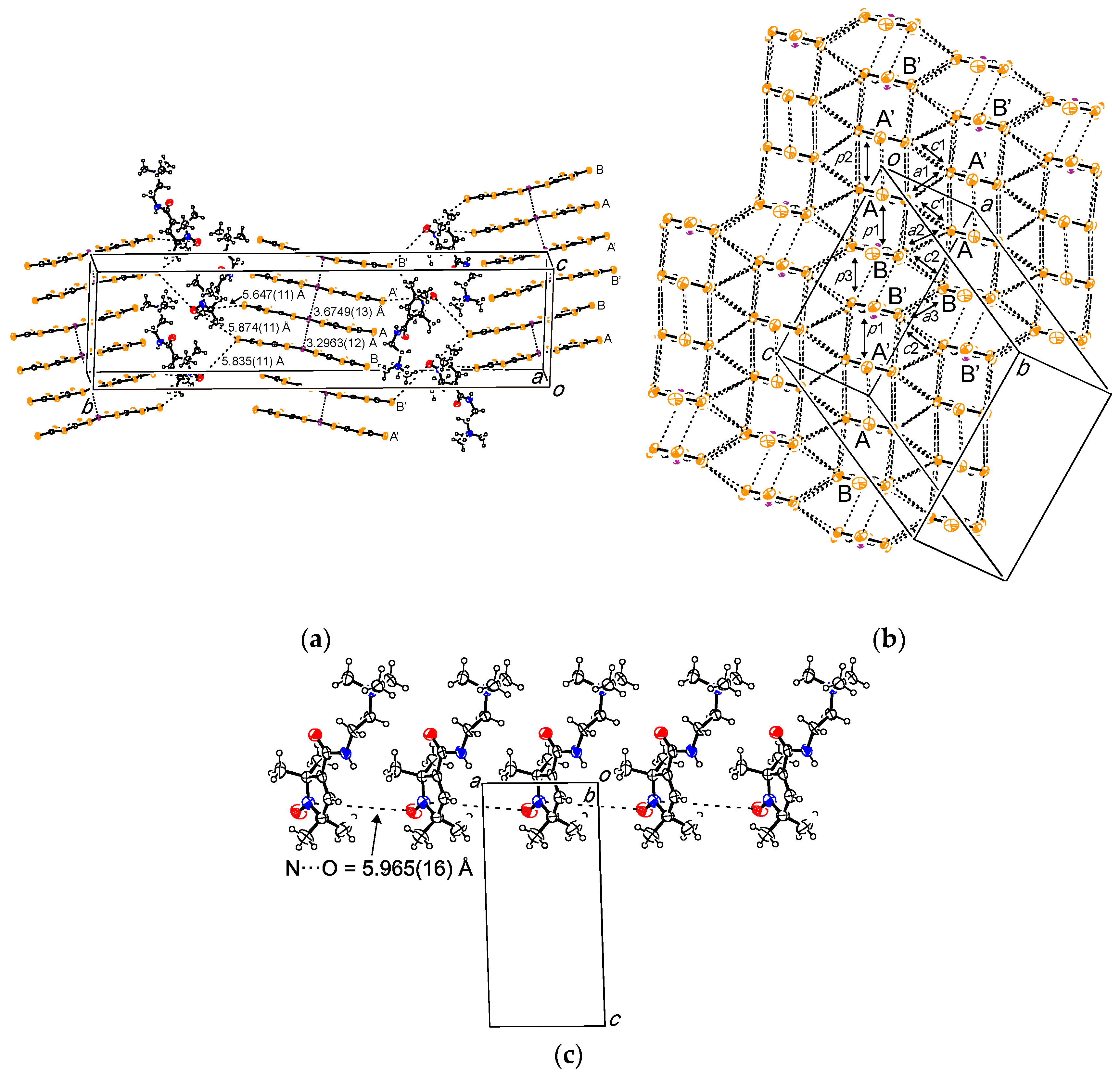

| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Composition | C1Cl | (C1)[Ni(dmit)2]2·CH3CN | (C1)[Pd(dmit)2]2 |
| Formula | C14H27N3O2Cl1 | C28H30O2N4S20Ni2 | C26H27O2N3S20Pd2 |
| Fw | 304.84 | 1213.17 | 1267.52 |
| Space Group | P21/n | P21/c | P21/n |
| a (Å) | 6.2618(3) | 5.9977(3) | 6.4052(3) |
| b (Å) | 11.5017(7) | 47.6504(19) | 50.0326(18) |
| c (Å) | 23.6111(14) | 16.4464(7) | 13.5041(5) |
| β (°) | 92.693(7) | 97.507(7) | 91.407(6) |
| V (Å3) | 1698.62(16) | 4660.0(4) | 4326.3(3) |
| Z | 4 | 4 | 4 |
| T (K) | 200 | 290 | 250 |
| dcalc (g·cm−1) | 1.192 | 1.729 | 1.946 |
| μ (cm−1) | 2.304 | 17.392 | 18.309 |
| F (000) | 660 | 2472 | 2528 |
| 2θ range (°) | 6–55 | 6–55 | 6–55 |
| Total ref. | 15621 | 43525 | 39491 |
| Unique ref. | 3844 | 10638 | 9854 |
| Rint | 0.0337 | 0.0605 | 0.0607 |
| Parameters | 188 | 505 | 478 |
| R1 (I > 2σ(I)) | 0.066 | 0.071 | 0.079 |
| wR2 (all data) | 0.178 | 0.227 | 0.217 |
| S | 0.981 | 0.915 | 1.115 |
| Δρmax (e Å−3) | 0.61 | 0.81 | 1.71 |
| Δρmin (e Å−3) | −0.32 | −0.71 | −1.30 |
| CCDC reference | 1532085 | 1532069 | 1532070 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akutsu, H.; Turner, S.S.; Nakazawa, Y. New Dmit-Based Organic Magnetic Conductors (PO-CONH-C2H4N(CH3)3)[M(dmit)2]2 (M = Ni, Pd) Including an Organic Cation Derived from a 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl (PO) Radical. Magnetochemistry 2017, 3, 11. https://doi.org/10.3390/magnetochemistry3010011
Akutsu H, Turner SS, Nakazawa Y. New Dmit-Based Organic Magnetic Conductors (PO-CONH-C2H4N(CH3)3)[M(dmit)2]2 (M = Ni, Pd) Including an Organic Cation Derived from a 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl (PO) Radical. Magnetochemistry. 2017; 3(1):11. https://doi.org/10.3390/magnetochemistry3010011
Chicago/Turabian StyleAkutsu, Hiroki, Scott S. Turner, and Yasuhiro Nakazawa. 2017. "New Dmit-Based Organic Magnetic Conductors (PO-CONH-C2H4N(CH3)3)[M(dmit)2]2 (M = Ni, Pd) Including an Organic Cation Derived from a 2,2,5,5-Tetramethyl-3-pyrrolin-1-oxyl (PO) Radical" Magnetochemistry 3, no. 1: 11. https://doi.org/10.3390/magnetochemistry3010011








