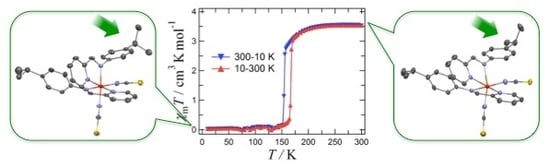
Spin-Crossover Hysteresis of [FeII(LHiPr)2(NCS)2] (LHiPr = N-2-Pyridylmethylene-4-Isopropylaniline) Accompanied by Isopropyl Conformation Isomerism
Abstract
:1. Introduction

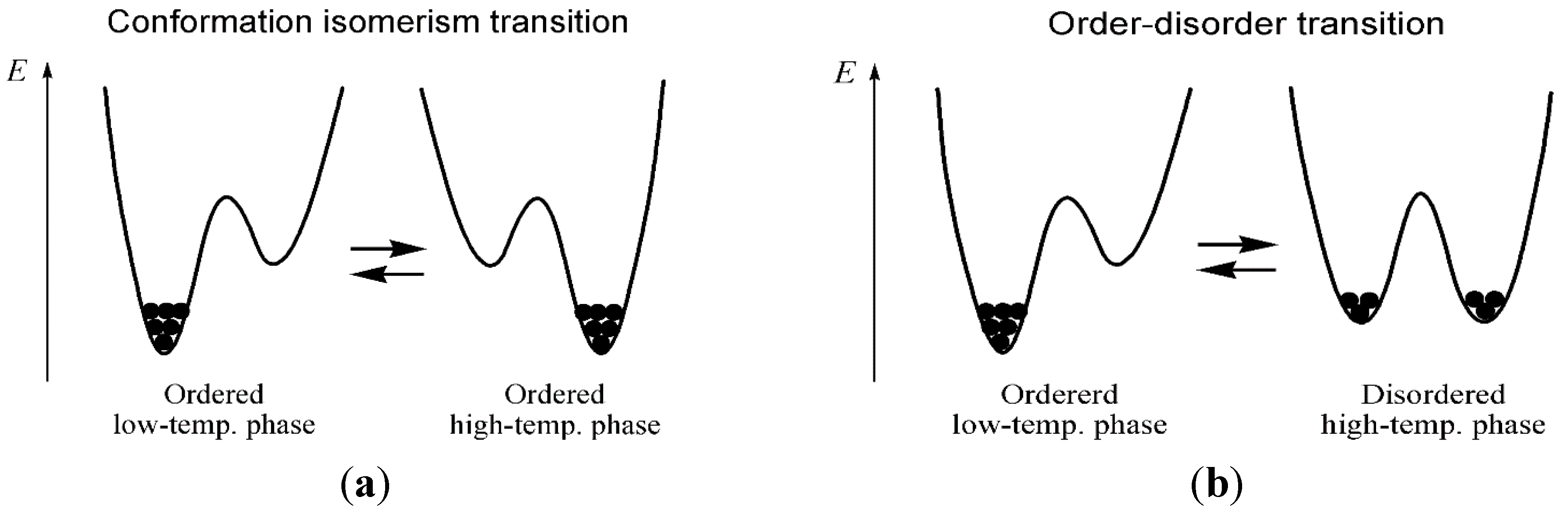
2. Results and Discussion
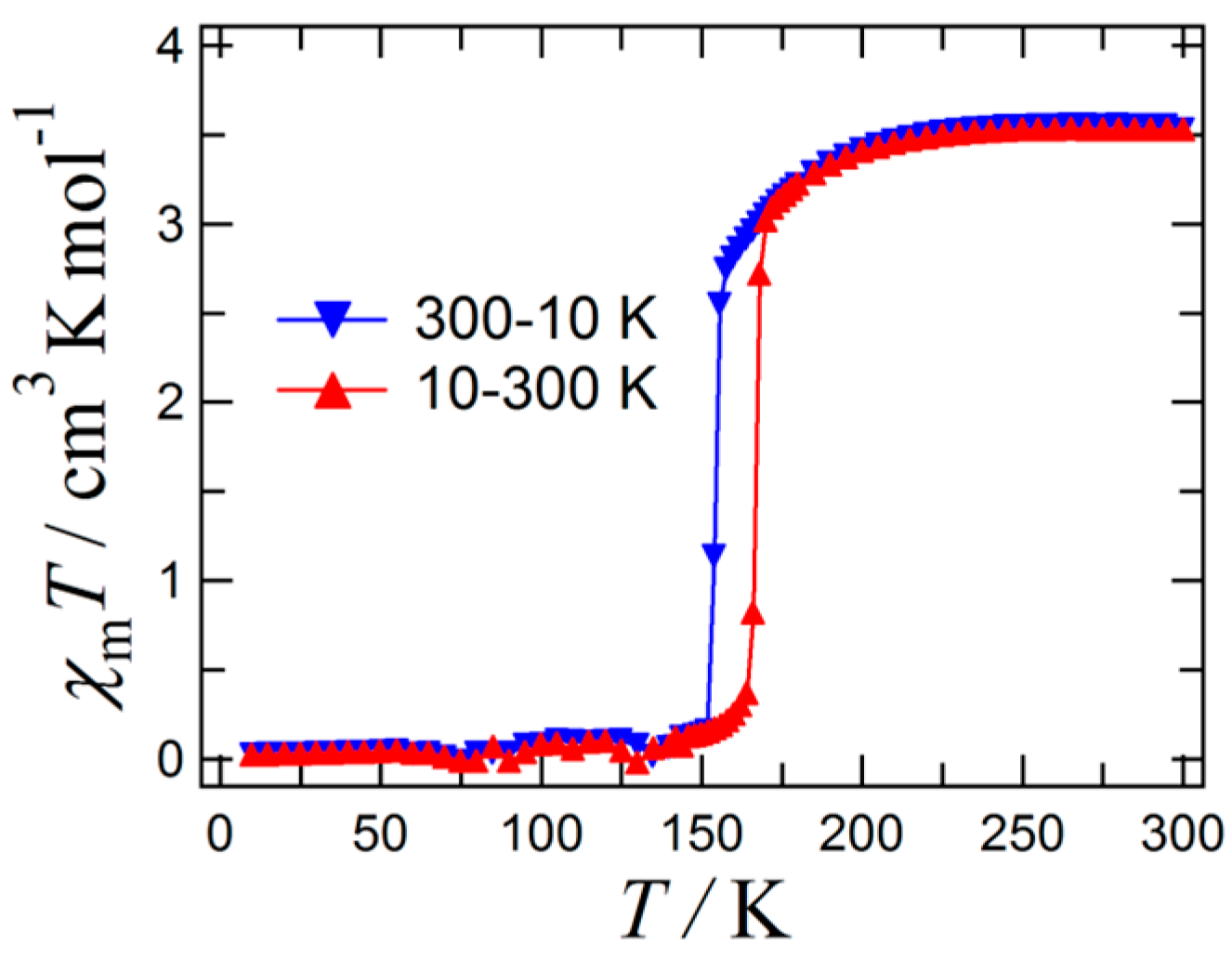

| Formula | C32H32FeN6S2 | C32H32FeN6S2 |
|---|---|---|
| formula weight | 620.61 | 620.61 |
| T/K | 130 | 180 |
| crystal system | monoclinic | monoclinic |
| space group | P21/n | P21/n |
| a/Å | 21.381(5) | 21.521(5) |
| b/Å | 12.668(3) | 12.363(3) |
| c/Å | 23.151(5) | 24.605(4) |
| β/° | 102.153(9) | 105.247(9) |
| V/Å3 | 6130(3) | 6316(3) |
| Z | 8 | 8 |
| dcalcd/g·cm−3 | 1.345 | 1.305 |
| μ(MoKα)/mm−1 | 0.660 | 0.640 |
| number of total reflections | 12913 | 14268 |
| R(F) (I > 2σ(I)) a | 0.0983 | 0.0644 |
| wR (F2) (all reflections) b | 0.1112 | 0.0690 |
| goodness-of-fit parameter | 1.157 | 1.185 |
| d | 130 K | 180 K | d | 130 K | 180 K |
|---|---|---|---|---|---|
| Fe1–N1 | 1.953(6) | 2.156(3) | Fe2–N7 | 1.971(7) | 2.136(3) |
| Fe1–N2 | 1.946(6) | 2.241(3) | Fe2–N8 | 1.968(6) | 2.239(3) |
| Fe1–N3 | 1.956(6) | 2.168(3) | Fe2–N9 | 1.958(7) | 2.116(4) |
| Fe1–N4 | 1.969(7) | 2.274(3) | Fe2–N10 | 1.964(6) | 2.179(3) |
| Fe1–N5 | 1.943(6) | 2.088(3) | Fe2–N11 | 1.934(7) | 2.026(4) |
| Fe1–N6 | 1.942(7) | 2.069(4) | Fe2–N12 | 1.956(6) | 2.066(4) |
| Average | 1.952 | 2.166 | Average | 1.959 | 2.127 |
| θ | 130 K | 180 K | θ | 130 K | 180 K |
|---|---|---|---|---|---|
| N2–Fe1–N5 | 173.3(3) | 164.52(11) | N8–Fe2–N11 | 173.2(3) | 165.18(13) |
| N4–Fe1–N6 | 174.5(3) | 165.95(11) | N10–Fe2–N12 | 168.5(3) | 159.38(13) |
| Average | 173.9 | 165.2 | Average | 170.9 | 162.3 |

3. Experimental Section
3.1. Materials
3.2. Crystallographic Analysis
3.3. Magnetic Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds I, II, and III; Springer-Verlag: Berlin, Germany, 2004.
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and optical switching of iron(II) complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Halcrow, M.A. Spin-Crossover Materials: Properties and Applications; John Wiley & Sons, Ltd.: Oxford, UK, 2013. [Google Scholar]
- Craig, G.A.; Roubeau, O.; Aromi, G. Spin state switching in 2,6-bis(pyrazol-3-yl)pyridine (3-bpp) based Fe(II) complexes. Coord. Chem. Rev. 2014, 269, 13–31. [Google Scholar] [CrossRef]
- Munoz, M.C.; Real, J.A. Thermo-, piezo-, photo- and chemo-switchable spin crossover iron(II)-metallocyanate based coordination polymers. Coord. Chem. Rev. 2011, 255, 2068–2093. [Google Scholar] [CrossRef]
- Hayami, S.; Holmes, S.M.; Halcrow, M.A. Themed Issue: Spin-State Switches in Molecular Materials Chemistry. J. Mater. Chem. C 2015, 3, 7767–7977. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Munoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalton Trans. 2005. [Google Scholar] [CrossRef] [PubMed]
- Real, J.A.; Gaspar, A.B.; Niel, V.; Münoz, M.C. Communication between iron(II) building blocks in cooperative spin transition phenomena. Coord. Chem. Rev. 2003, 236, 121–141. [Google Scholar] [CrossRef]
- Aromi, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Gamez, P.; Costa, J.S.; Quesada, M.; Aromi, G. Iron spin-crossover compounds: From fundamental studies to practical applications. Dalton Trans. 2009. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. The spin-states and spin-transitions of mononuclear iron(ii) complexes of nitrogen-donor ligands. Polyhedron 2007, 26, 3523–3576. [Google Scholar] [CrossRef]
- Halcrow, M.A. Structure: Function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. Spin-crossover compounds with wide thermal hysteresis. Chem. Lett. 2014, 43, 1178–1188. [Google Scholar] [CrossRef]
- Hirosawa, N.; Oso, Y.; Ishida, T. Spin-crossover and light-induced excited spin-state trapping observed for an iron(II) complex chelated with tripodal tetrakis(2-pyridyl)methane. Chem. Lett. 2012, 41, 716–718. [Google Scholar] [CrossRef]
- Yamasaki, M.; Ishida, T. Spin-crossover thermal hysteresis and light-induced effect on iron(II) complexes with tripodal tris(2-pyridyl)methanol. Polyhedron 2015, 85, 795–799. [Google Scholar] [CrossRef]
- Chernyshov, D.; Hostettler, M.; Tornroos, K.W.; Burgi, H.-B. Ordering phenomena and phase transitions in a spin-crossover compound- uncovering the nature of the intermediate phase of [Fe(2-pic)3]Cl2·EtOH. Angew. Chem. Int. Ed. 2003, 42, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Nakamoto, T.; Ikeuchi, S.; Saito, K.; Inaba, A.; Sorai, M.; Tojo, T.; Atake, T.; Matouzenko, G.S.; Zein, S.; et al. Spin crossover phenomenon accompanying order-disorder phase transition in the ligand of [FeII(DAPP)(abpt)](ClO4)2 compound (DAPP = bis(3-aminopropyl)(2-pyridylmethyl)amine, abpt = 4-amino-3,5-bis(pyridin-2-yl)-1,2,4-triazole) and its successive self-grinding effect. J. Phys. Chem. B 2007, 111, 12508–12517. [Google Scholar] [PubMed]
- Guionneau, P.; Letard, J.-F.; Yufit, D.S.; Chasseau, D.; Bravic, G.; Goeta, A.E.; Howard, J.A.K.; Kahn, O. Structural approach of the features of the spin crossover transition in iron(II) compounds. J. Mater. Chem. 1999, 9, 985–994. [Google Scholar] [CrossRef]
- Hayami, S.; Shigeyoshi, Y.; Akita, M.; Inoue, K.; Kato, K.; Osaka, K.; Takata, M.; Kawajiri, R.; Mitani, T.; Maeda, Y. Reverse spin transition triggered by a structural phase transition. Angew. Chem. Int. Ed. 2005, 117, 4977–4981. [Google Scholar] [CrossRef]
- Yamasaki, M.; Ishida, T. Heating-rate dependence of spin-crossover hysteresis observed in an iron(II) complex having tris(2-pyridyl)methanol. J. Mater. Chem. C 2015, 3, 7784–7787. [Google Scholar] [CrossRef]
- Létard, J.-F.; Guionneau, P.; Codjovi, E.; Lavastre, O.; Bravic, G.; Chasseau, D.; Kahn, O. Wide thermal hysteresis for the mononuclear spin-crossover compound cis-bis(thiocyanato)bis[N-(2′-pyridylmethylene)-4-(phenylethynyl)anilino]iron(II). J. Am. Chem. Soc. 1997, 119, 10861–10862. [Google Scholar] [CrossRef]
- Letard, J.-F.; Guionneau, P.; Nguyen, O.; Costa, J.S.; Marcen, S.; Chastanet, G.; Marchivie, M.; Goux-Capes, L. A guideline to the design of molecular-based materials with long-lived photomagnetic lifetimes. Chem. Eur. J. 2005, 11, 4582–4589. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, Y.; Ooidemizu, M.; Yamahata, Y.; Yamada, M.; Osa, S.; Matsumoto, N.; Iijima, S.; Sunatsuki, Y.; Kojima, M.; Dahan, F.; et al. A new family of spin crossover complexes with a tripod ligand containing three imidazoles: Synthesis, characterization, and magnetic properties of [FeIIH3LMe](NO3)2·1.5H2O, [FeIIILMe]·3.5H2O, [FeIIH3LMe][FeIILMe]NO3, and [FeIIH3LMe][FeIIILMe](NO3)2 (H3LMe = tris[2-(((2-methylimidazol-4-yl)methylidene)amino)-ethyl]amine). Inorg. Chem. 2003, 42, 7001–7017. [Google Scholar] [PubMed]
- Oso, Y.; Ishida, T. Spin-crossover transition in a mesophase iron(II) thiocyanate complex chelated with 4-hexadecyl-N-(2-pyridylmethylene)aniline. Chem. Lett. 2009, 38, 604–605. [Google Scholar] [CrossRef]
- Oso, Y.; Kanatsuki, D.; Saito, S.; Nogami, T.; Ishida, T. Spin-crossover transition coupled with another solid-solid phase transition for iron(II) thiocyanate complexes chelated with alkylated N-(di-2-pyridylmethylene)anilines. Chem. Lett. 2008, 37, 760–761. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Broeker, J.L.; Hoffmann, R.W.; Houk, K.N. Conformational analysis of chiral alkenes and oxonium ions: Ab initio molecular orbital calculations and an improved MM2 force field. J. Am. Chem. Soc. 1991, 113, 5006–5017. [Google Scholar] [CrossRef]
- Schaefer, T.; Sebastian, R.; Penner, G.H. Theoretical and experimental data on the internal rotatioinal potential in isopropylbenzene. Can. J. Chem. 1988, 66, 1495–1499. [Google Scholar] [CrossRef]
- Schaefer, T.; Parr, W.J.E.; Danchura, W. Concerning the barrier to internal rotation in isopropylbenzene in solution. J. Mag. Reson. 1977, 25, 167–170. [Google Scholar] [CrossRef]
- True, N.S.; Farag, M.S.; Bohn, R.K.; Madregor, M.A.; Radhakrishnan, J. Low-resolution microwave studies of substituted ethyl- and isopropylbenzenes. J. Phys. Chem. 1983, 87, 4622–4627. [Google Scholar] [CrossRef]
- Seredyuk, M.; Muñoz, M.C.; Castro, M.; Morcillo, T.R.; Gaspar, A.B.; Real, J.A. Unprecedented multi-stable spin crossover molecular material with two thermal memory channels. Chem. Eur. J. 2013, 19, 6591–6596. [Google Scholar] [CrossRef] [PubMed]
- Lakhloufi, S.; Guionneau, P.; Lemee-Cailleau, M.H.; Rosa, P.; Letard, J.-F. Structural phase transition in the spin-crossover complex [Fe(ptz)6](BF4)2 studied by X-ray diffraction. Phys. Rev. B 2010. [Google Scholar] [CrossRef]
- Craig, G.A.; Costa, J.S.; Roubeau, O.; Teat, S.J.; Aromí, G. Coupled crystallographic order-disorder and spin state in a bistable molecule: Multiple transition dynamics. Chem. Eur. J. 2011, 17, 3120–3127. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Marcus, M.A.; Smeigh, A.L.; McCusker, J.K.; Chong, H.H.W.; Schoenlein, R.W. Picosecond X-ray absorption spectroscopy of a photoinduced iron(II) spin crossover reaction in solution. J. Phys. Chem. A 2006, 110, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Villar, N.; Thompson, A.L.; Munoz, M.C.; Ugalde-Saldivar, V.M.; Goeta, A.E.; Moreno-Esparza, R.; Real, J.A. Solid- and solution-state studies of the novel µ-dicyanamide-bridged dinuclear spin-crossover system {[(Fe(bztpen))2[µ-N(CN)2]}(PF6)3·nH2O. Chem. Eur. J. 2005, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Walker, F.A. Solution NMR studies of iron(II) spin-crossover complexes. Inorg. Chem. 2007, 46, 6794–6803. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.; Schmauss, G.; Specker, H. Complexes of iron(II) with substituted 2-pyridinalphenylimines. Z. Naturforsch. B 1972, 27b, 1149–1154. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: Weinhein, Germany, 1993. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochida, N.; Kimura, A.; Ishida, T. Spin-Crossover Hysteresis of [FeII(LHiPr)2(NCS)2] (LHiPr = N-2-Pyridylmethylene-4-Isopropylaniline) Accompanied by Isopropyl Conformation Isomerism. Magnetochemistry 2015, 1, 17-27. https://doi.org/10.3390/magnetochemistry1010017
Mochida N, Kimura A, Ishida T. Spin-Crossover Hysteresis of [FeII(LHiPr)2(NCS)2] (LHiPr = N-2-Pyridylmethylene-4-Isopropylaniline) Accompanied by Isopropyl Conformation Isomerism. Magnetochemistry. 2015; 1(1):17-27. https://doi.org/10.3390/magnetochemistry1010017
Chicago/Turabian StyleMochida, Naotaka, Akifumi Kimura, and Takayuki Ishida. 2015. "Spin-Crossover Hysteresis of [FeII(LHiPr)2(NCS)2] (LHiPr = N-2-Pyridylmethylene-4-Isopropylaniline) Accompanied by Isopropyl Conformation Isomerism" Magnetochemistry 1, no. 1: 17-27. https://doi.org/10.3390/magnetochemistry1010017