1. Introduction
The grafting technique for horticultural crops is widely considered a valid tool due to its effectiveness for controlling pests and disease that are otherwise difficult to manage. This approach is particularly valuable when faced with the issues of the high cost of the phytochemicals, their environmental impact, risks associated with their application and, in some instances, limitations on their usage [
1,
2]. Consequently, the grafted plant represents a promising eco-friendly means for the control of adverse biotic factors, enhancing the cultivation of
Solanaceae and
Cucurbitaceae cultivars of high value in terms of product quality but sensitive to several root system pathogens [
3,
4,
5,
6]. Furthermore, the presence of eustressors in the soil, such as high salt concentrations, can induce abiotic stress in plants and cause significant damage for the crops [
7,
8]. The exploitation of grafting techniques extends to the tomato landraces as well, driven by the growing demand for these varieties. These landraces are highly valued for their superior organoleptic properties in contrast to commercial F1 hybrids [
9].
In the last several decades, several alternative techniques have emerged as potential solutions for managing soil-borne pests. These techniques encompass the soil solarization, the trap plants, repellents and bio fumigation, but their individual efficacy has often been limited [
10,
11]. Despite the fact that recently, the use of herbaceous grafting has gained popularity in horticultural production, critical issues remain. These pertain to the selection of suitable rootstocks, the interactions between rootstock and scion, the development of protocols managing the grafted plant cultivation from the seedling stage in nurseries (topping and breeding with two or more branches) as well as the determination of the optimal plant density in relation to the increased vigour expressed during production phases. Moreover, the optimization of fertigation and water uptake represents an important concern, particularly within the context where water resource management assumes paramount importance for the future of agriculture [
12,
13,
14,
15,
16,
17]. The use of the grafting technique contributes to the development of sustainable agricultural practices. This contribution allows for the accumulation of knowledge useful for organic farming, thereby ensuring food production and safety, while preserving invaluable natural resources [
18,
19,
20,
21,
22]. Combined with the grafting technique, the adoption of a soilless cultivation system represents a sustainable approach due to the significant reduction in arable land, urbanization, water scarcity, and climate changes [
23]. Soilless cultivation presents several advantages to eggplant greenhouse cultivation, including the notably enhanced regulation of nutrients within the solution medium, thus improving plant nutrient uptake [
24]. As it is widely recognized, beyond the influence of the solution medium, the air temperature and substrate conditions also play crucial roles in a soilless eggplant cultivation system [
25].
The selection of suitable rootstocks for commercial use presents an ongoing challenge due to the uncertain performance of the genetically characterized materials in terms of their tolerance or resistance to adverse factors. Therefore, the prediction of the agronomic outcomes of the chosen plant materials becomes uncertain, encompassing factors such as the plant precocity, the production capacity, cycle length, the structure reproductive organs and the organoleptic quality [
26]. Molecular markers can sometimes aid in the detection of the aforementioned traits [
27,
28,
29,
30,
31]. Although grafting is widely used in horticultural production, the effect of this technique has received limited consideration. The attention has mainly been focused on gauging the successes or failures in terms of engraftment in relation to the ability of the operator and the conditions in which the grafting operation is carried out. Therefore, an important challenge is determined by the manipulation of the plant tissues that can either mask or enhance the characteristics of the rootstock and its interactions with the scion. Concerning the mechanisms that control the agronomic and functional performance of the grafted plants, it is important to identify and acquire an in-depth understanding on how environmental conditions impact these mechanisms during the cultivation phase. Additionally, the environmental conditions inside the greenhouse and the agronomic practices adopted can directly influence plant cultivation [
32,
33,
34,
35].
Although grafting offers several benefits for the horticultural production, it also poses technical, biological and economic implications of the production process of the grafted plants and their use. In addition to the previously cited issues regarding the rootstock development, the critical points which were most frequently reported concerns the following: a lack of graft rooting; reduced or excessive vigour; the cost of seedlings; more difficult and burdensome production protocols than those related to the production of seedlings from seeds in nurseries; excessive resistance or tolerance expressed by rootstocks; the incomplete knowledge of the behaviour and characteristics of rootstocks and the possible low affinity of rootstocks with subsequent functional imbalances. The selection of the growing substrate is a crucial point for promoting the plant growth of grafted plants. As reported by several authors, the appropriate substrate can stimulate the root system to grow deeply, which is essential for the establishment of a healthy and robust grafted plant [
36,
37]. Furthermore, in the case of eggplant, using interspecific rootstock or wild relatives can enhance plant vigour and promote growth [
38].
In this framework, the aim of the present work is the evaluation of different environmental conditions, represented by the greenhouse and the soil substrate temperatures, in different grafting combinations of the eggplant cultivar, Black Bell, used as scion. This cultivar was grafted with the interspecific S. lycopersicum F1 hybrid Beaufort, and with the well-known S. torvum. The environmental conditions tested included the cold and heated greenhouse and the application of refrigerated or heated soil. The investigation performed was supported by a morphometric evaluation of the vegetative plant traits followed by a physiological analysis related to water uptake. Furthermore, a histological analysis of the xylem vessels was also performed. The study aims to lay the basis for the development of new sustainable protocols of cultivation in the perspective of the organic farming for the future of agriculture.
2. Materials and Methods
2.1. Plant Material and Experimental Design
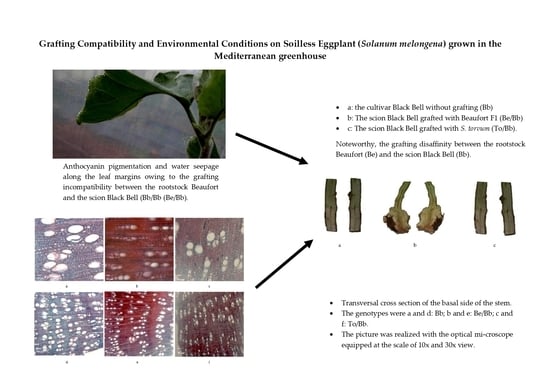
The experimental trial was carried out during the winter season in the experimental greenhouse belonging to the Department of Agriculture, Food and Environment and (Di3A) of the University of Catania (UNICT). The greenhouse was situated 50 m above the sea level, in Catania (37°24′33″ N, 15°03′32″ E). The plant material consisted of the eggplant cv Black Bell (Bb), which was used as scion. It was grafted with the interspecific tomato hybrid F1 Beaufort (Be), and
Solanum torvum (To), which were used as rootstock (
Figure 1).
The non-grafted counterpart of the Black Bell cultivar was employed as the control for the trial. The first experimental factor was the genotype (GE), represented by the different plant combinations tested, which were Bb, Be/Bb and To/Bb. The second factor was the greenhouse temperature (GT), while the third one was the substrate temperature (ST) used, which was heated or not. The experimental design employed was split plot arrangement with randomized blocks of each of the two plants, with four repetitions.
For the soilless cultivation, growing containers with a dimension of 30 cm depth and 40 cm width were used, spaced 1.0 m apart. Within each container, plants were positioned 45 cm apart, resulting in a plant density of 2.3 plants m−2.
The sowing was performed in cellular trays in December 2020 while the grafting was carried out in the seedlings, 30 days after the sowing. The transplanting was carried out in the middle of February 2021 when the plantlets reached the phenological stage of 3–4 true leaves. The trial was stopped on May 25, at the beginning of the fruiting phase, after 90 days from transplantation. Throughout the growth cycle, essential cultivation practices were implemented in accordance with organic nutrition protocols. Specifically, each plant received 36 L of water and nutrients, excluding microelements. Over the growth period, a total of approximately 6 g of nitrogen (N), 2 g of phosphorus (P) and 9 g of potassium (K) were supplied per plant.
2.2. Greenhouse Temperature Set Up
The second experimental factor under examination was the greenhouse temperature (GT), categorized into two conditions: cold (CG) and heated greenhouse (WG). The latter maintained a minimum air temperature above 15 °C. Inside the cold greenhouse (CG), the mean temperature varied from 19 °C to 26 °C for the third week of February and the first one of May, respectively (
Figure 1a). On the other hand, the mean temperature recorded in the heated greenhouse (WG) varied from 21 °C for the third week of February and the first one of March to 26 °C, which were registered in the first week of May (
Figure 1b). The thermal air and substrate conditions resulted in average air/substrate temperatures of 18/22 °C (CG/CS), 15/20 °C (CG/WG), 20/20 °C (WG/CS) and 20/24 °C (WG/WS). The minimum, maximum and mean temperatures of the thermal air inside the cold and heated greenhouse were recorded using the USB data logger (Testo, 174-T, Lenzkirch, Germany) as shown in
Figure 1.
2.3. Substrate Temperature Set Up
The third experimental factor was the substrate temperature (ST). In both greenhouses, plants were cultivated using soilless culture techniques, utilizing specially designed growing containers. These containers were equipped with a pipe system that circulated water at room temperature (CS) or heated to 40 °C (WS). The growing medium consisted of a combination of peat and sand, both authorized for the organic farming, mixed in a 1:1 volume ratio. To ensure the water and nutritional elements saturation in the substrate, a localized fertigation system was employed, delivering the nutrient solution of 4 L h−1 per plant.
2.4. Plant Morphometric Characterization
The growth and development processes of the plants were assessed using the international IBPGR descriptors for eggplant. Special emphasis was given to traits that are directly influenced by the grafting such as the ratio between the diameter of the graft and that of the rootstock, which serves as an index of affinity. The descriptors adopted are listed in
Table 1.
2.5. Physiological Analysis
During the final phase of the trial, a physiological analysis was conducted. This analysis involved the calculation of key parameters, specifically the foliar water potential (WP), which is expressed in -MPa, and the relative water content (RWC), expressed as a percentage. The determination of the first parameter (WP) was carried out throughout the Scholander pressure chamber (PMS Instrument Company, PMS-600, Albany, OR, USA). A transversal leaf slice was collected and subsequently introduced into the pressure chamber. The end of the petiole was left outside the chamber. Nitrogen gas was then blown inside the pressure chamber for stimulating the movement of the free water present in the leaf tissue along the ribs. Once the pressure (bar) built up, it determined the appearance of water drop in the cutting surface of the petiole. Conversely, the relative water content was detected on a leaf sample at the complete growth stage (10°–12° leaf starting from the bottom). For each leaf, the fresh weight (Fw) was registered at the time of sampling, while the weight at the maximum turgor (Mt) achieved via the immersion of the petiole in water was also detected. The hydrated leaf was then placed to dry in the oven at a temperature of 70 °C in order to determine the dry weight (Dw). The data were used according to the following formula developed by Turner [
39], for calculating the relative water content (RWC):
The index represents the water content in the leaf at the time of sampling as a percentage of the water contained in the fully hydrated tissues. Both the WP and RWC measurements were registered at 12.00 a.m. and in three moments corresponding to the time in which irrigation finished (T0), in addition to 20 and 40 h after the irrigation (T20 and T40, respectively). During the whole growing cycle (from transplanting to harvesting the first fruit), the thermal values of the air and the substrate were continuously registered.
2.6. Histological Analysis
The sections of the samples for the histological analysis were obtained using a manual microtome (Leica, RM2125RTS, Wetzlar, Germany) and they were adjusted at a thickness of about 20 μm. The colouring phase was conducted through the immersion of the tissue samples in the safranine dye for two seconds. Afterwards, the tissues were immerged in 100% ethanol and then in the fast green dye for 5 s. The fast green staining was repeated two times. The use of the safranine dye was to highlight the xylem vessels, while the fast green dye targeted the parenchymatic tissues. All the grafting combinations were observed through a compound optical microscope (Nikon DM500, Japan) equipped with a camera at a scale of 30 and 10 μm, respectively, to assess the distribution of the xylem vessels. The area and the diameter of the xylem vessels were registered using the software ImageJ (National Institute of Health, Bethesda, MD, USA).
2.7. Data Analysis
Data were statistically processed by calculating the analysis of variance (ANOVA) with the stepwise multiple comparison procedures of Student–Newman–Keuls, with different replicates. Three-way ANOVA was performed to assess the variance of the morphometric data, while the four-way ANOVA was applied for the physiological data. ANOVA was followed by Tukey’s multiple comparisons test. The software adopted for the previously mentioned statistical tests was CoStat version 6.4 (CoHort software, Birmingham, UK). The statistical significance was assigned for p-values < 0.05. Subsequently, the means of the different rootstock–scion combinations with different greenhouse and substrate temperatures were used for the Pearson’s correlation and for the Principal Component Analysis (PCA) to assess the sample distribution in relation to the GT and ST adopted. The software used for the Pearson’s correlation and for the PCA was IBM SPSS version 27 (IBM, Armonk, NY, USA).
4. Discussion
Our findings provide an extended comprehension of the performance of grafted eggplants under different growing conditions. The analysed traits were chosen to highlight the variations in vegetative growth among the different rootstock–scion combinations. In alignment with this context, Johnson et al. [
40] assessed the growth performance of
S. melongena scion grafted onto Beaufort F1 rootstock, which is one of the rootstocks employed in our study. They measured stem diameter and plant height after approximately 85 days from transplanting and compared the results between grafted and non-grafted plants. In this framework, our results were consistent with those achieved by Johnson et al. [
40]. Moreover, similar to the previously cited study, we adopted a shorter growing cycle to facilitate a direct comparison of various environmental conditions, aiming to mitigate the effects of excessive Beaufort F1 rootstock growth. Furthermore, our study builds upon their findings by examining additional aspects of plant performance under different growing conditions.
Regarding the affinity index, it serves as a gauge of the compatibility between the scion and rootstock in a grafted plant. This index reflects the degree to which the two plant parts can establish a functional vascular connection, which is essential for the transport of water and for the nutrients uptake in the plant vascular system [
41]. In this context, our manuscript delved into the analysis of the affinity index (AI) of grafted eggplants under varying greenhouse and soil temperatures. In contrast to the outcomes of Goto et al. [
42], who tested the behaviour of Beaufort F1 and
S. torvum rootstocks grafted with tomato scions, our observations regarding the AI significantly diverged from their findings. Specifically, they reported AI values close to 1.00 only for the interspecific grafting combination with the rootstock Beaufort F1, while they ascertained intermediate AI values for the grafting with
S. torvum. On the other hand, we registered AI values close to 1.00 for the combination between
S. torvum and Black Bell (To/Bb). This discrepancy in results can be attributed to the robust affinity between the
S. torvum rootstock and the Black Bell scion, in contrast to the imbalance observed in the Be/Bb combination. As it shown in
Figure 2, the incompatibility between Be/Bb is evidenced by the discrepant sizes of the rootstock and scion at the grafting point. The incompatibility detected at the grafting point was probably a result of the rootstock’s robust root system, which had a high capacity for absorbing water and nutrients from the deeper soil layers.
Concerning the vegetative parameters that we registered after 90 days, we achieved higher values for the number of plant leaves than those obtained in the work of Poudel and Lee [
43]. They reported around 13 leaves in 62 days for the ungrafted and self-grafted eggplant, along with approximatively 12 leaves for various rootstock–scion combinations. Additionally, the results that we achieved are consistent to the ones in the study of Gökseven and Akbudak [
44], who studied different rootstock–scion combinations in
S. melongena. However, our study showed lower values of plant stem diameters, likely due to our 90-day evaluation period, contrasting with their 110-day analysis.
The different behaviour of Beaufort F1 and S. torvum rootstocks grafted with the Black Bell eggplant cultivar affects the physiological water relations of the grafted eggplant during the 40 h after irrigation. In this framework, the physiological analysis serves as a valuable tool for understanding the reasons behind the distinct responses in terms of vigour among the various grafting combinations. In this context, combinations involving Beaufort F1 (Be) rootstock showed lower water potential values and higher relative water content in leaves. This phenomenon could be attributed to the rootstock’s excessive water absorption, leading to a vegetative imbalance characterized by hydropic leaf tissue and observed seepage from the leaf margins.
In our study, all the genotypes showed a significant increase in the water potential (WP) values from the time when irrigation occurred (T0) to 40 h post-irrigation (T40). RWC values were about 17 % higher on average compared to the work of Semida et al. [
45], who examined S. melongena growth under deficit irrigation with nanoparticle foliar application. In reference to the work of Sanwal et al. [
46], who evaluated salinity effects on tomato grafted with eggplant rootstocks, we ascertained that the RWC values were significantly higher when the plants were well irrigated (at T0 moments) but the values were similar at T40 in relation to the ungrafted control that they used. Furthermore, we registered RWC values at T40, showing insignificant variations from the ones obtained in the manuscript of Plazas et al. [
47], who tested eggplant relatives under drought stress. Concerning the water potential (WP), our results aligned with those achieved by
Oda et al. [
48], which evaluated the WP after 8 weeks from the grafting with different combinations using tomato as scion in
S. torvum and eggplant rootstocks. Particularly, we obtained overlapping WP values at the T0 for the
S. torvum grafting. However, notably higher values were evident at T20 and T40 conditions due to the extended duration without irrigation in comparison to the previously mentioned study. Notably, in our experiment, the 40 h period without irrigation had a notable effect on the water potential in leaves, resulting in an increase in its value. Consequently, the amount of water within the leaves decreased, as confirmed by the relative water content. The increase in WP values from T0 to T40 can be attributed to the reduced water content in the plant’s tissues after 40 h without irrigation. This resulted in a higher pressure requirement for water to seep out from the leaf tissue during the WP analysis. Consequently, this shift led to a notable decrease in the relative water content in the leaf tissue from T0 to T40, as indicated by the physiological analysis. In this study, histological analysis was conducted with the intention of gaining a deeper understanding of the complex relationship between the rootstock and the scion, particularly within the context of grafting incompatibility observed in the Be/Bb combination. In this framework, histological analysis confirmed that the combination of Black Bell cultivar as the scion grafted onto the Beaufort F1 rootstock exhibited a higher vigour and water uptake compared to the grafting combination with
S. torvum and the non-grafted Black Bell. The results of our study showed that the areas and diameters of the xylem vessels (AXVs and DXVs) in the Black Bell (Bb) and To/Bb grafting combinations were consistent with those reported by Magdaleno-Garcia et al. [
49], who measured the area of xylem vessels in response to the application of zinc oxide nanoparticles. However, the Be/Bb genotype showed higher values for AXVs and DXVs, likely due to its higher vigour and the disequilibrium between the two bionts. In particular, the DXV values for Be/Bb were higher than those reported by Wu et al. [
50], who analysed histological parameters of wild eggplants in response to
Verticillium wilt. The notable increase in both the area and diameter of the xylem vessels in Be/Bb is probably associated with the greater water uptake capacity of the Beaufort F1 rootstock compared to
S. torvum and the non-grafted plant. Increased water absorption applies pressure to the xylem vessels, resulting in their dilation. The increased water adsorption, as previously mentioned, can be attributed to the strong root system of Beaufort F1, as corroborated by the high fresh and dry weights reported by Johnson et al. [
40].
5. Conclusions
The data acquired enables us to evaluate the impact of the affinity between the rootstock and the scion utilized on plant growth and development, and the water physiology in response to the tested environmental conditions.
Significant variations emerged in our study regarding the impact of greenhouse and soil temperatures on leaf count and fruit weight. Particularly, the Be/Bb combination exhibited a significant vegetative biomass increase under cold greenhouse conditions, while displaying the highest affinity index and resulting in the greatest fruit weight in the heated greenhouse. Our physiological and histological analyses further corroborated these variations. Specifically, the interspecific combination Be/Bb showcased the highest absolute water potential (WP) values, coupled with elevated relative water content, validating its excessive vegetative growth.
The main outcome of the present work is the mitigation of the huge plant vigour of the interspecific combination Be/Bb as a consequence of the reduction in the temperature of both thermal air and substrate. In this context, the grafting technique proved to be an effective response to the various environmental conditions examined. This work allows us to establish a foundation for the development of innovative, sustainable cultivation protocols within the framework of organic farming, with a forward-looking perspective on the future of agriculture. Additionally, the utilization of suitable rootstock–scion combinations can promote better vegetative growth in plants, leading to improved water resource management.