Temporal Changes in Flavonoid Components, Free Radical Scavenging Activities and Metabolism-Related Gene Expressions during Fruit Development in Chinese Dwarf Cherry (Prunus humilis)
Abstract
:1. Introduction
2. Materials and Methods
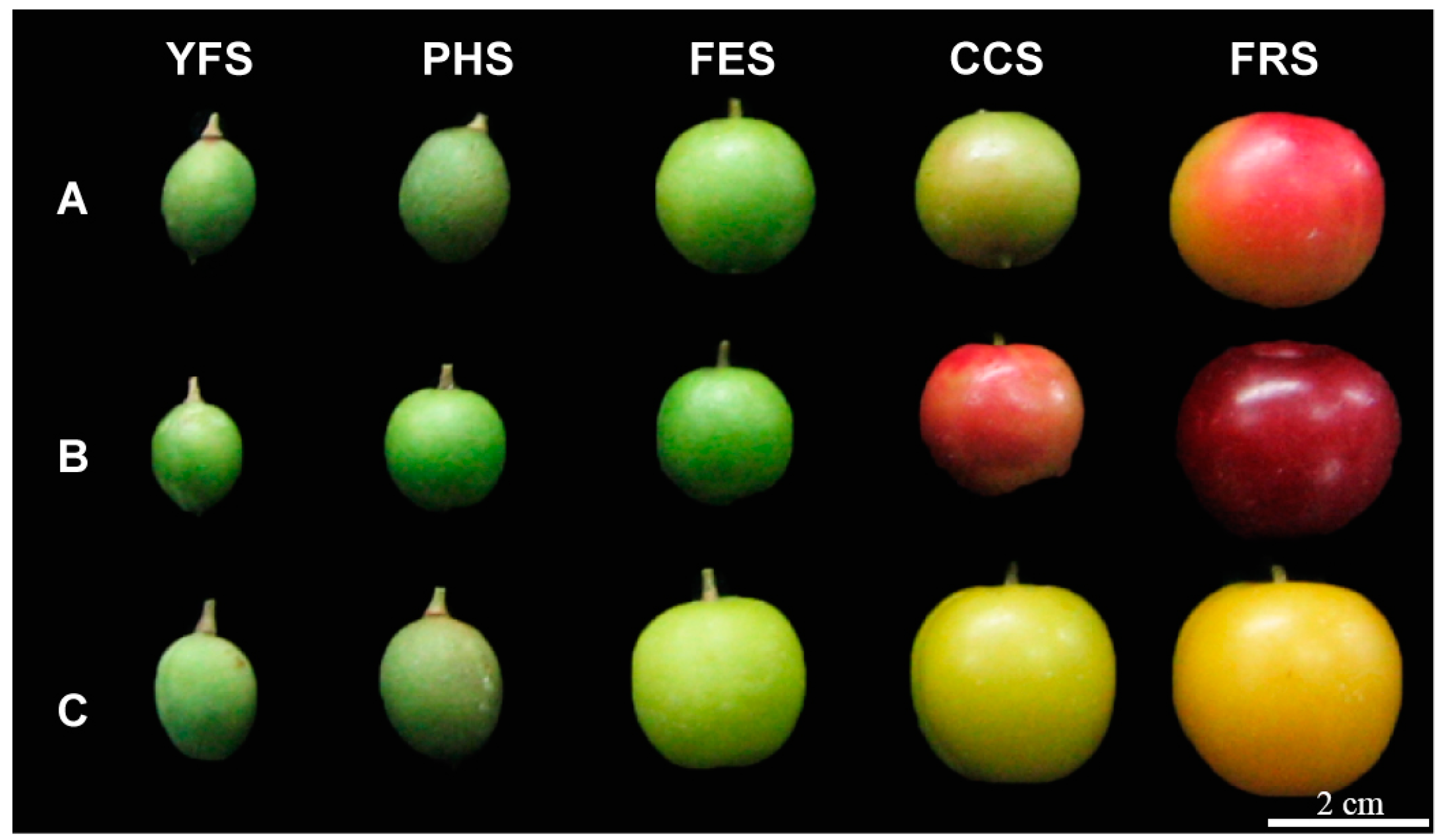
2.1. Plant Materials and Chemicals
2.2. Extraction and Determination of Flavonoids
2.3. Flavonoid Composition Determination
2.4. Antioxidant Determination
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. Changes in Total Flavonoid Content during Fruit Development
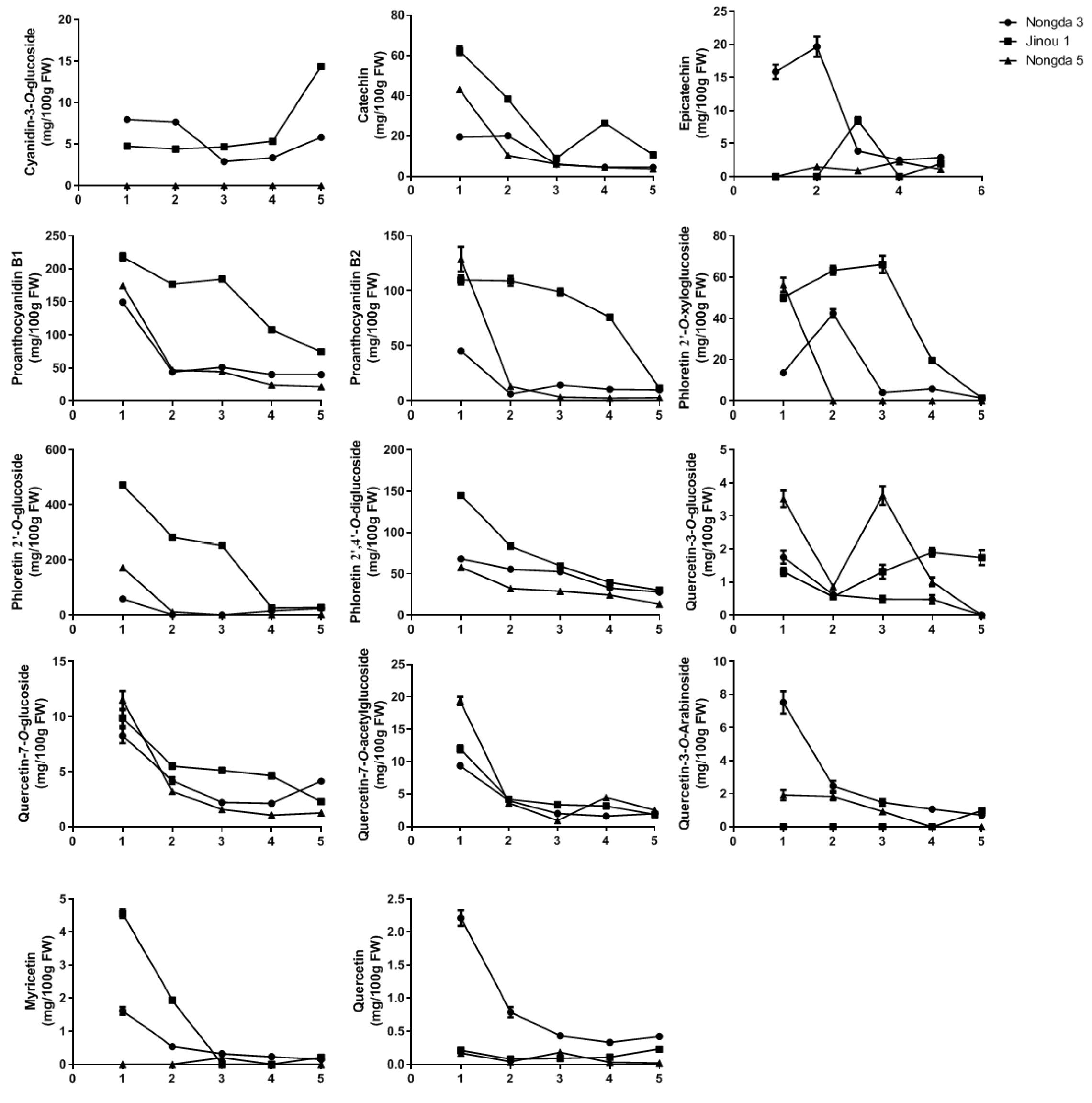
3.2. Flavonoid Composition Variation during Fruit Development
3.3. Antioxidant Activity Variation during Fruit Development
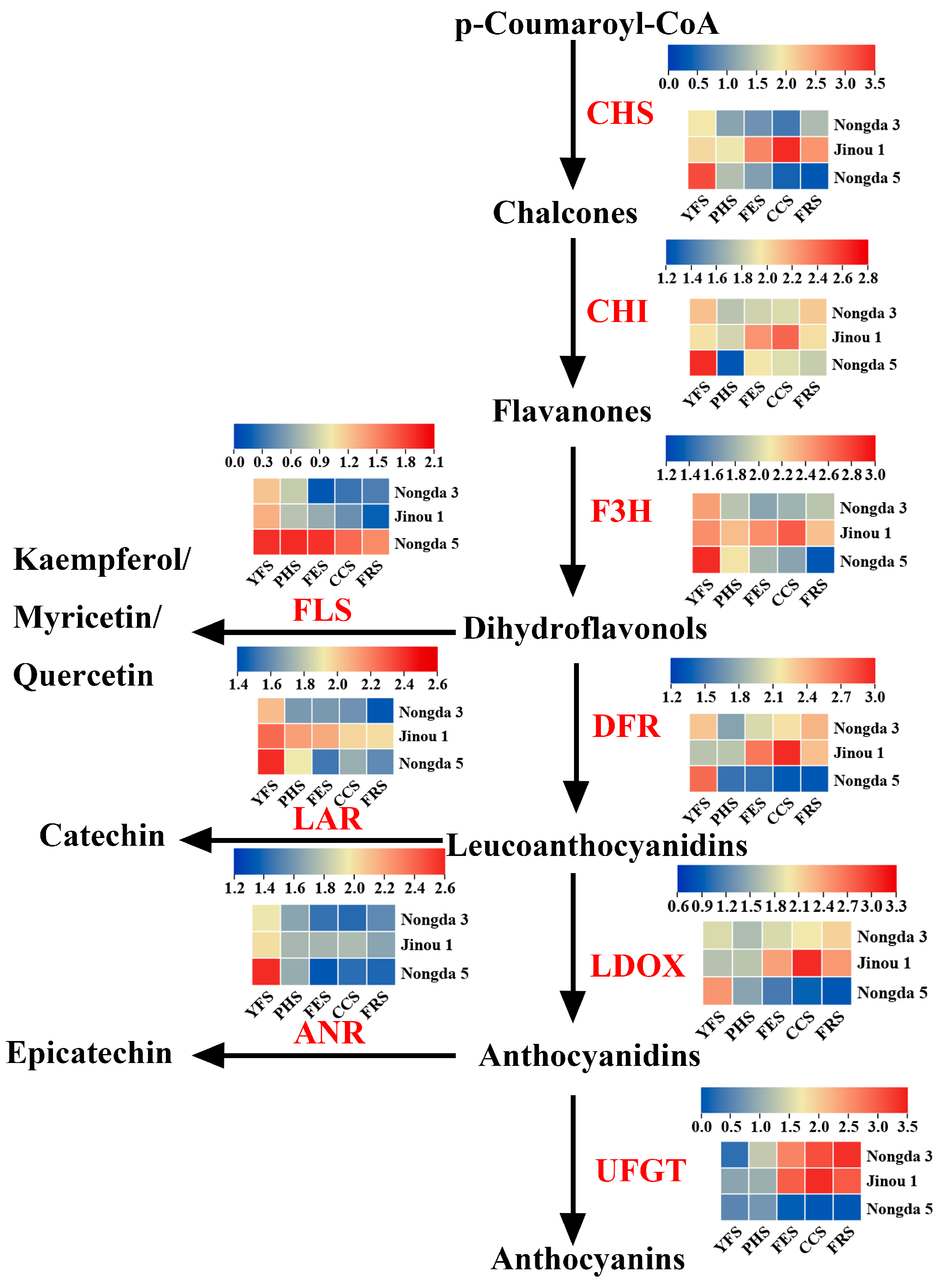
3.4. Expression Analysis of Flavonoid Biosynthetic Genes during Fruit Development
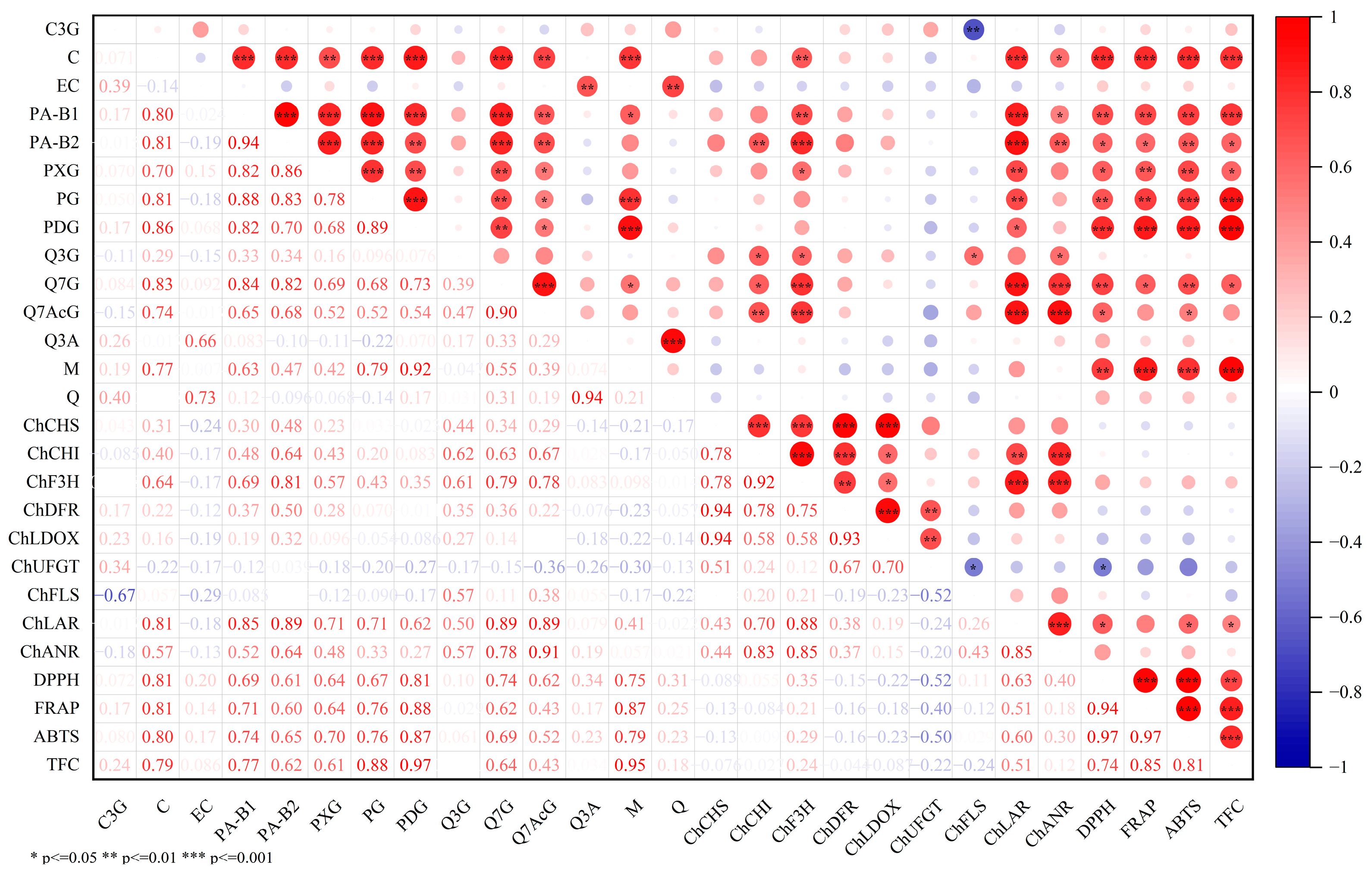
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.A.; Rahman, S.M.M. Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple. Food Res. Int. 2011, 44, 672–676. [Google Scholar] [CrossRef]
- Makita, C.; Chimuka, L.; Steenkamp, P.; Cukrowska, E.; Madala, E. Comparative analyses of flavonoid content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. South Afr. J. Bot. 2016, 105, 116–122. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104, S40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Haskell-Ramsay, C.; Gallegos, J.L.; Lodge, J.K. Effects of chronic consumption of specific fruit (berries, cherries and citrus) on cognitive health: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Clin. Nutr. 2022, 77, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef]
- Wu, E.; Ni, J.; Zhou, W.; You, L.; Tao, L.; Xie, T. Consumption of fruits, vegetables, and legumes are associated with overweight/obesity in the middle- and old-aged Chongqing residents: A case-control study. Medicine 2022, 101, e29749. [Google Scholar] [CrossRef]
- Hassanpour, H.; Yousef, H.; Jafar, H.; Mohammad, A. Antioxidant capacity and phytochemical properties of cornelian cherry (Cornus mas L.) genotypes in Iran. Sci. Hortic. 2011, 129, 459–463. [Google Scholar] [CrossRef]
- Wang, P.; Mu, X.; Gao, Y.G.; Zhang, J.; Du, J. Successful induction and the systematic characterization of tetraploids in Cerasus humilis for subsequent breeding. Sci Hortic. 2020, 256, 109216. [Google Scholar] [CrossRef]
- Mu, X.; Wang, P.; Du, J.; Gao, Y.G.; Zhang, J. Comparison of fruit organic acids and metabolism-related gene expression between Cerasus humilis (Bge.) Sok and Cerasus glandulosa (Thunb.) Lois. PLoS ONE 2018, 13, e0196537. [Google Scholar] [CrossRef]
- Song, X.S.; Shang, Z.W.; Yin, Z.P.; Ren, J.; Sun, M.C.; Ma, X.L. Mechanism of xanthophyll-cycle-mediated photoprotection in Cerasus humilis seedlings under water stress and subsequent recovery. Photosynthetica 2011, 49, 523–530. [Google Scholar] [CrossRef]
- Dong, X.; Liu, L.; Li, J.; Du, J.; Wang, P.; Zhang, J. Soil and Water Conservation Function of Cerasus humilis in Hilly-gully Region of Loess Plateau. Bull. Soil Water Conserv. 2016, 36, 242–247. [Google Scholar]
- Guo, C.; Wang, P.; Zhang, J.; Guo, X.; Mu, X.; Du, J. Organic acid metabolism in Chinese dwarf cherry [Cerasus humilis (Bge.) Sok.] is controlled by a complex gene regulatory network. Front. Plant Sci. 2022, 13, 982112. [Google Scholar] [CrossRef] [PubMed]
- Li, W.D.; Li, O.; Mo, C.; Jiang, Y.S.; He, Y.; Zhang, A.R.; Chen, L.M.; Jin, J.S. Mineral element composition of 27 Chinese dwarf cherry (Cerasus humilis (Bge.) Sok.) genotypes collected in China. J. Hortic. Sci. Biotechnol. 2014, 89, 674–678. [Google Scholar] [CrossRef]
- Wang, P.; Mu, X.; Du, J.; Gao, Y.G.; Bai, D.; Jia, L.T.; Zhang, J.; Ren, H.; Xue, X. Flavonoid content and radical scavenging activity in fruits of Chinese dwarf cherry (Cerasus humilis) genotypes. J. For. Res. 2018, 29, 55–63. [Google Scholar] [CrossRef]
- Fu, H.; Qiao, Y.; Wang, P.; Mu, X.; Zhang, J.; Fu, B.; Du, J. Changes of bioactive components and antioxidant potential during fruit development of Prunus humilis Bunge. PLoS ONE 2021, 16, e0251300. [Google Scholar] [CrossRef]
- Fu, H.; Mu, X.; Wang, P.; Zhang, J.; Fu, B.; Du, J. Fruit quality and antioxidant potential of Prunus humilis Bunge accessions. PLoS ONE 2020, 15, e0244445. [Google Scholar] [CrossRef]
- Li, W.D.; Li, O.; Zhang, A.; Li, L.; Hao, J.H.; Jin, S.J.; Yin, S.J. Genotypic diversity of phenolic compounds and antioxidant capacity of Chinese dwarf cherry (Cerasus humilis (Bge.) Sok.) in China. Sci. Hortic. 2014, 175, 208–213. [Google Scholar] [CrossRef]
- Qiao, F.; Zhang, K.M.; Zhou, L.Y.; Qiu, Q.S.; Chen, Z.N.; Lu, Y.H.; Wang, L.H.; Geng, G.G.; Xie, H.C. Analysis of flavonoid metabolism during fruit development of Lycium chinense. J. Plant Physiol. 2022, 279, 153856. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef]
- Van Acker, S.A.; Van Den, D.J.; Tromp, M.N.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 2011, 35, 331–342. [Google Scholar] [CrossRef]
- Sabli, F.; Mohamed, M.; Rahmat, A.; Ibrahim, H.A.; Bakar, M.F.A. Antioxidant properties of selected Etlingera and Zingiber species (Zingiberaceae) from Borneo Island. Int. J. Biochem. 2012, 6, 1–9. [Google Scholar] [CrossRef]
- Li, J.E.; Fan, S.T.; Qiu, Z.H.; Li, C.; Nie, S.P. Total flavonoids content, antioxidant and antimicrobial activities of extracts from Mosla chinensis Maxim. cv. Jiangxiangru. LWT Food Sci. Technol. 2015, 64, 1022–1027. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Kim, Y.I.; Chung, S.K. A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chem. 2012, 131, 106–110. [Google Scholar] [CrossRef]
- Pereira, C.; López-Corrales, M.; Serradilla, M.J.; Villalobos, M.G.; Ruiz-Moyano, S.; Martín, A. Influence of ripening stage on bioactive compounds and antioxidant activity in nine fig (Ficus carica L.) varieties grown in Extremadura, Spain. J. Food Compos. Anal. 2017, 64, 203–212. [Google Scholar] [CrossRef]
- Han, H.; Mu, X.; Wang, P.; Wang, Z.; Fu, H.; Gao, Y.G.; Du, J. Identification of LecRLK gene family in Cerasus humilis through genomic-transcriptomic data mining and expression analyses. PLoS ONE 2021, 16, e0254535. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S.; Lister, C. Flavonoid function in plants. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 397–441. [Google Scholar]
- Ferdinando, M.D.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as Antioxidants in Plants Under Abiotic Stresses. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Parvaiz, A., Prasad, M.N.V., Eds.; Springer Press: New York, NY, USA, 2012; pp. 159–179. [Google Scholar]
- Dragović-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The Content of Polyphenols and Carotenoids in Three Apricot Cultivars Depending on Stage of Maturity and Geographical Region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, J.B.; Kim, H.J.; Im, N.K.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in Free Amino Acid, Protein, and Flavonoid Content in Jujube (Ziziphus jujube) Fruit during Eight Stages of Growth and Antioxidative and Cancer Cell Inhibitory Effects by Extracts. J. Agric. Food Chem. 2012, 60, 10245–10255. [Google Scholar] [CrossRef] [PubMed]
- Elmastas, M.; Demir, A.; Gen, N.; Dlek, Ü.; Güne, M. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 2017, 235, 154–159. [Google Scholar] [CrossRef]
- Simmonds, M.S.J. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Oxford University Press: Oxford, UK, 2014; pp. 625–664. [Google Scholar]
- Liu, Y.; Liu, X.Y.; Zhong, F.; Tian, R.; Zhang, K.C.; Zhang, X.M.; Li, T.H. Comparative Study of Phenolic Compounds and Antioxidant Activity in Different Species of Cherries. J. Food Sci. 2011, 76, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Harzallah, A.; Bhouri, A.M.; Amri, Z.; Soltana, H.; Hammami, M. Phytochemical Content and Antioxidant Activity of Different Fruit Parts Juices of Three Figs (Ficus carica L.) varieties grown in Tunisia. Ind. Crops Prod. 2016, 83, 255–267. [Google Scholar] [CrossRef]
- Prvulovic, D.; Popovic, M.; Malencic, D.; Ljubojevic, M.; Barac, G.; Ognjanov, V. Phenolic content and antioxidant capacity of sweet and sour cherries. Stud. Univ. Babes-Bolyai Chem. 2012, 57, 175–181. [Google Scholar]
- Celli, G.B.; Pereira-Netto, A.B.; Beta, T. Comparative analysis of total phenolic content, antioxidant activity, and flavonoids profile of fruits from two varieties of Brazilian cherry (Eugenia uniflora L.) throughout the fruit developmental stages. Food Res. Int. 2011, 44, 2442–2451. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, J.; Qin, M. Protective effect of cyanidin 3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats. Neurosci. Lett. 2013, 534, 285–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Q.; Guo, C.; Zhang, X.; Liu, S.; Wang, X.; Zhao, J.; Zhao, Z.; Li, W. A rapid chemometrics model for antioxidant substance mining of Chinese dwarf cherry [Cerasus humilis (Bge.) Sok.] based on polyphenol profile and antioxidant capacity of 30 germplasms. Food Biosci. 2023, 53, 102795. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, Distribution and Physiological Relevance in Plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, L.; Xu, Y.; Zheng, W.; Zhang, W. Transcriptomic Analysis of Anthocyanin and Carotenoid Biosynthesis in Red and Yellow Fruits of Sweet Cherry (Prunus avium L.) during Ripening. Horticulturae 2023, 9, 516. [Google Scholar] [CrossRef]
- Yang, R.; Yang, Y.; Hu, Y.; Yin, L.; Qu, P.; Wang, P.; Mu, X.; Zhang, S.; Xie, P.; Cheng, C.; et al. Comparison of Bioactive Compounds and Antioxidant Activities in Differentially Pigmented Cerasus humilis Fruits. Molecules 2023, 28, 6272. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xia, R. Expression of chalcone synthase and chalcone isomerase genes and accumulation of corresponding flavonoids during fruit maturation of Guoqing No. 4 satsuma mandarin (Citrus unshiu Marcow). Sci. Hortic. 2010, 125, 110–116. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Jia, K.; Liao, K.; Liu, L.; Fan, G.; Zhang, S.; Wang, Y. Metabolomic and transcriptomice analyses of flavonoid biosynthesis in apricot fruits. Front. Plant Sci. 2023, 14, 1210309. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed]
| P. humilis Gene ID | P. humilis Gene Name | Forward Primer | Reverse Primer |
|---|---|---|---|
| CL2555.Contig1 | ChCHS | TACCAACAAGGGTGTTTCGC | GTGATCTCCGAGCACACAAC |
| Unigene14928 | ChCHI | GAGGAGGAAGCCTTGGAGAA | TCCTCCTTCCCTTCAGTGTG |
| Unigene3562 | ChF3H | TACAGGGAGAAGCTGTGCAA | TCACCTCTCTCCATCCCTCA |
| Unigene6640 | ChDFR | TGTCGAAGAGCACCAGAAGT | GGCCAATCACAAGAGTTGGG |
| Unigene14860 | ChLDOX | GGAAGGCTGGAGAAGGAAGT | TGAGCTTCAACACCAAGTGC |
| Unigene6233 | ChUFGT | TGTTTGATGTGGCTGATGGC | CGTCGGTAATCAAGCAGGTG |
| Unigene5990 | ChLAR | TGGCATCTCTGTGGGAGAAG | TTTCCGGTATGCGGTTCTCT |
| Unigene7152 | ChANR | GAGGACCCTGAGAACGACAT | TCGTTCTCGTCTGTGACCAA |
| Unigene17057 | ChFLS | GAGTTGAGGTCGTCATTGCC | TCAAGGACCCTCCCATGAAC |
| - | ChActin | GCAGCGACTGAAGACATACA | GTGGCATTAGCAAGTTCCTC |
| Developmental Stages | Nongda 3 (mg/g RE·FW) | Jinou 1 (mg/g RE·FW) | Nongda 5 (mg/g RE·FW) |
|---|---|---|---|
| YFS | 22.33 ± 0.88 a | 47.21 ± 1.01 a | 12.33 ± 0.92 a |
| PHS | 16.98 ± 0.99 b | 24.33 ± 1.56 b | 9.97 ± 1.05 b |
| FES | 12.56 ± 0.67 c | 20.65 ± 0.93 c | 9.56 ± 0.98 b |
| CCS | 11.43 ± 0.33 cd | 13.23 ± 1.23 d | 9.21 ± 0.65 b |
| FRS | 10.00 ± 0.42 e | 11.94 ± 0.26 de | 7.23 ± 0.32 c |
| Cultivar | Developmental Stages | DPPH (mg TE/g) | FRAP (mg TE/g) | ABTS (mg TE/g) |
|---|---|---|---|---|
| Nongda 3 | YFS | 28.25 ± 0.39 a | 53.54 ± 0.77 a | 78.03 ± 1.01 a |
| PHS | 23.22 ± 0.41 b | 45.02 ± 0.59 b | 68.41 ± 0.74 b | |
| FES | 6.13 ± 0.20 c | 14.73 ± 0.34 c | 31.23 ± 0.57 c | |
| CCS | 4.10 ± 0.16 d | 10.88 ± 0.44 d | 9.82 ± 0.46 d | |
| FRS | 3.33 ± 0.18 e | 9.07 ± 0.50 e | 8.18 ± 0.37 e | |
| Jinou 1 | YFS | 36.21 ± 0.53 a | 88.14 ± 1.28 a | 121.02 ± 2.04 a |
| PHS | 35.88 ± 0.62 a | 87.92 ± 1.52 a | 120.41 ± 1.98 a | |
| FES | 7.23 ± 0.14 b | 18.31 ± 0.55 b | 43.23 ± 0.63 b | |
| CCS | 5.12 ± 0.11 c | 16.80 ± 0.47 c | 14.82 ± 0.58 c | |
| FRS | 4.04 ± 0.22 d | 11.23 ± 0.53 d | 12.18 ± 0.49 d | |
| Nongda 5 | YFS | 23.72 ± 0.60 a | 27.21 ± 0.57 a | 60.01 ± 1.04 a |
| PHS | 19.65 ± 0.59 b | 25.76 ± 0.55 b | 57.65 ± 0.89 b | |
| FES | 5.72 ± 0.23 c | 12.23 ± 0.45 c | 23.31 ± 0.58 c | |
| CCS | 3.53 ± 0.14 d | 7.92 ± 0.43 d | 7.44 ± 0.45 d | |
| FRS | 2.84 ± 0.12 e | 6.88 ± 0.46 e | 6.57 ± 0.46 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Zhang, L.; Liu, S.; Li, N.; Huo, J.; Mu, X. Temporal Changes in Flavonoid Components, Free Radical Scavenging Activities and Metabolism-Related Gene Expressions during Fruit Development in Chinese Dwarf Cherry (Prunus humilis). Horticulturae 2023, 9, 1040. https://doi.org/10.3390/horticulturae9091040
Han H, Zhang L, Liu S, Li N, Huo J, Mu X. Temporal Changes in Flavonoid Components, Free Radical Scavenging Activities and Metabolism-Related Gene Expressions during Fruit Development in Chinese Dwarf Cherry (Prunus humilis). Horticulturae. 2023; 9(9):1040. https://doi.org/10.3390/horticulturae9091040
Chicago/Turabian StyleHan, Hongyan, Lingjuan Zhang, Shan Liu, Na Li, Jianxin Huo, and Xiaopeng Mu. 2023. "Temporal Changes in Flavonoid Components, Free Radical Scavenging Activities and Metabolism-Related Gene Expressions during Fruit Development in Chinese Dwarf Cherry (Prunus humilis)" Horticulturae 9, no. 9: 1040. https://doi.org/10.3390/horticulturae9091040





