1. Introduction
Photosynthesis is a process that has a significant impact on all life on the Earth, both through the production of large amounts of biomass and atmospheric oxygen reserves [
1,
2,
3,
4,
5]. Despite the fact that photosynthetic organisms in the process of evolution have developed a highly efficient photosynthetic apparatus, the efficiency of converting light energy reaching the surface of plants is low [
6,
7,
8,
9].
Currently, there are several approaches that help increase the efficiency of light use by photosynthetic organisms: genetic engineering approaches [
10], increasing the effective area of photosynthetic antenna complexes by including exogenous light absorbers (organic dyes, nanoparticles, etc.) capable of transmitting excitons on photosynthetic-light-harvesting complexes [
11,
12,
13], and methods of directional changes of the intensity and the spectrum of light [
14]. The first two approaches still have numerous limitations due to the high cost, labor costs, and instability of modified organisms [
15,
16]. Methods for the quantitative and qualitative conversion of light have long been effective in farms due to their ease of application and relatively low price. In turn, they can be combined into several groups: illumination, shading, and the application of photoconversion covers (PCCs) [
14,
17,
18]. Currently, PCCs have become more and more popular. While the main reference to PCCs at present is for research projects, some farms are already using PCCs for profit [
19,
20].
Several types of phosphors are used in such covers. The most widely used phosphors now are based on organic dyes [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36]. Despite all their advantages, such phosphors have a significant drawback: they lose their effectiveness very quickly due to light burnout [
37]. Another type of phosphor that is gaining more popularity is nanosized metal-containing phosphors [
33,
38,
39], among which luminescent nanoparticles based on rare earth metals occupy a special place [
34,
40,
41,
42,
43,
44,
45]. A relatively widely used rare earth metal in the production of phosphors is europium (Eu). Basically, europium is used as an oxide (Eu
2O
3): in optical devices, in the production of banknotes, televisions, and monitors [
46]. Europium(III) oxide is of interest in the production of covers for greenhouses due to the fact that the main maximum of its fluorescence is in the red region (612 nm) [
47,
48,
49]. As is known, red light (λ = 600 nm–700 nm) is most effectively used by plants, and the addition of red light most strongly activates photosynthetic processes in plants under low light conditions [
50]. In recent years, many studies have been devoted to the synthesis, properties, and application of complex nanocrystals, which consist of an inorganic matrix that enhances the stability of nanoparticles and europium ions [
51,
52]. The structure of these nanoparticles contributes to an increase in the lifetime of the excited state of europium ions and increases the fluorescence intensity. Such structures include nanocrystals based on yttrium vanadate, lutetium oxide compounds, zinc oxide or sulfide, lanthanum fluoride, etc., doped with Eu
3+ ions [
53,
54,
55,
56]. Due to their spectral properties, Eu-based nanoparticles can be used as nanofluorophores (NFs) for PCCs, converting part of the ultraviolet light that is little used by plants into red light. In several studies, the effectiveness of nanocrystals as elements of the photoconversion covers of greenhouses has been tested [
34,
43]. The results of these studies demonstrate a positive effect of PCCs on the growth of plant biomass. Note that europium nanocrystals deposited on greenhouse covers had only one fluorescence maximum (λ = 612 nm or 630 nm) [
34,
43], while three pronounced maxima were observed outside the covers (λ = 590 nm, 615 nm, 700 nm) [
53,
54,
55,
56].
In this work, we studied the optical properties of two PCCs containing Eu
2O
3 and Eu
3+:LaF
3 nanoluminophores and their effect on the growth and development of tomato (
Solanum lycopersicum) and cucumber (
Cucumis sativus) plants grown in laboratory conditions and greenhouses. A very important difference between the Eu
3+:LaF
3 PCC used in this study and earlier works is the presence of a pronounced luminescence maximum at 591 nm, which was provided by using lanthanum fluoride as a matrix [
57,
58,
59].
2. Materials and Methods
2.1. Preparation of Nanoparticles and Study of Their Properties
Stable aqueous colloidal solutions of NPs x at.% Eu3+:LaF3, where x = 0.1, 10, 30, 50, were synthesized by hydrothermal-microwave treatment (HTMW) of freshly precipitated gels with HTMW laboratory device speedwave XPERT (Berghof Products+ Instruments GmbH, Eningen unter Achalm, Germany) with two magnetrons (2.45 GHz, 2 kW maximum output power).
The initial reagents used in the synthesis without any further purification include Eu(NO3)3 5H2O (Aldrich, 99.999% purity), La(NO3)3 6H2O (99.999%), NH4F (>98%) (Sigma-Aldrich, Stockholm, Sweden AB). For the synthesis of aqueous colloidal solutions of Eu3+:LaF3 NPs doped with (0.1–50 mol.%) Nd3+ ions, La(NO3)3 6H2O (0.4995–0.25 mmol) and Eu(NO3)3 5H2O (0.0005–0.234 mmol) were dissolved in deionized water (15 mL). The solution of rare earth salts was added dropwise to the NH4F solution (5 mmol) in deionized water (25 mL) under vigorous stirring. The freshly precipitated gels were diluted with deionized water (10 mL) and left stirring for 15 min. The resulting solutions were transferred into a 100 mL Teflon autoclave and placed under microwave irradiation for 2 h at 200 °C using HTMW laboratory device. After they were cooled, they were centrifuged using a Hermle Z326 device and washed several times with deionized water. The resulting precipitates were redispersed in deionized water using ultrasonication.
Eu2O3 nanoparticles were obtained by laser fragmentation in an aqueous solution of europium oxide (Sigma-Aldrich, Stockholm, Sweden, purity 99.99%) on a laser facility with second-harmonic generation (Ekspla, Vilnius, Lithuania) at a wavelength of 532 nm with a laser pulse frequency of 1 kHz, individual pulse duration 3.6 ns, and pulse energy 2 mJ for 1 h.
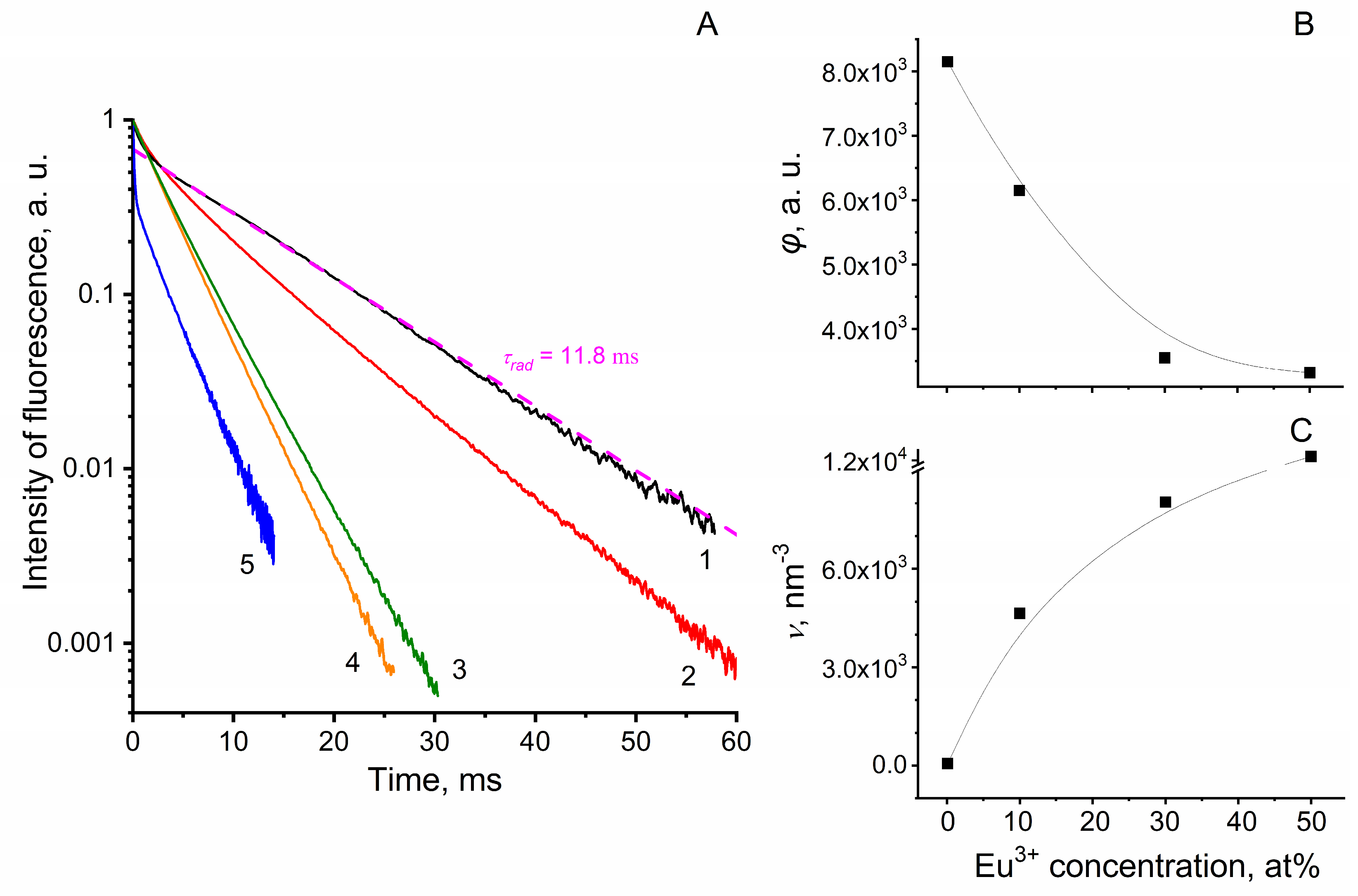
The luminescence decay kinetics of Eu3+ ions was measured upon excitation of luminescence using a pulsed tunable Al2O3-Ti laser (LOTIS-TII, Minsk, Belarus) at a wavelength of 395 nm and its registration at a wavelength of 591 nm (electronic transition 5D0 → 7F1) using a monochromator MDR-23 (LOMO, Russia), a photomultiplier Hamamatsu R13456P, and a multichannel counter Timeharp 260 (PicoQuant GmbH, Berlin, Germany) operating in the gated photon counting mode.
A Malvern Zetasizer ULTRA RED LABEL setup (Malvern panalytical LTD, Worcestershire, UK) was used to determine the hydrodynamic radius of NF in solution by dynamic light scattering. An amount of 1.5 mL of the solution was poured into a DTS0012 polystyrene cuvette with an optical path length of 10 mm. Scattering was observed at an angle of 174.7° at a temperature of 25 °C. In each experiment, 10 measurements were carried out.
2.2. The Glass Surface Application of NF
A solution of the obtained nanoparticles in acetone (107 ppm) was used for the manufacture of PCCs. They were mixed with the liquid component of the fluoroplastic polymer in a ratio of 1/100. Fluoroplast-32L (St. Petersburg Paint and Varnish Plant, KRASKI SPB LLC, Russia) served as the basis for obtaining a polymer varnish. The mixture was stirred for 10 min until a homogeneous mass was obtained. The glasses were washed with ionic and nonionic detergents and then degreased with a solvent before applying the NF integrated into the polymer. NF was applied to glass using an airbrush with No. 4 nozzles. The pressure in the compressor was raised to 2.5–3.0 atm, when applying photoconversion nanoparticles integrated into a fluoropolymer matrix. The distance of the spray gun nozzle from the surface was 15–25 cm. The covers applied to the glass were tested for adhesion strength: they were not washed off with deionized water or detergent solutions, and were extremely difficult to mechanically clean. Thus, a PCC containing photoconversion nanoparticles was formed on the glass surface.
2.3. Fluorescence Spectroscopy
A Jasco FP-8300 spectrofluorimeter (JASCO Applied Sciences, Victoria, BC, Canada) was used to analyze the absorption and fluorescence properties of the obtained PCC. A glass slide with a photoconversion cover was placed in a special holder. The experiments were carried out at room temperature.
2.4. Planting and Growing Conditions
Two types of plants were selected for the experiment: tomato (Solanum lycopersicum) and cucumber (Cucumis sativus). Three seeds were planted in an organomineral plug (45 mm × 45 mm) in a fertilizer solution containing 0.05 g/L KNO3; 0.17 g/L Mg(NO3)2 6H2O; 1.06 g/L Ca(NO3)2 4H2O; 0.38 g/L K2SO4; 0.135 g/L KH2PO4; 0.49 g/L MgSO4 7H2O. One week after germination, one plant was left in each plug, and the concentration of nutrients in the solution was doubled.
After planting, the trays were immediately placed under the glass with (experimental) or without (control) NF. Plants were grown under a 16 h photoperiod at 25–26 °C. The intensity of photosynthetically active radiation (PAR, λ = 350–800 nm) was ≈130 µmol photons s−1 m−2. The UV-A component (λ = 370 nm, photon flux density (PPF) ≤ 10 µmol photons s−1 m−2) was added to the illumination spectrum. The PG200N spectrometer (UPRtek, Zhunan, Miaoli, Taiwan) was used to estimate the light flux density of the PAR spectrum.
For growing plants in protected ground, 4 experimental boxes (2 m × 0.5 m × 0.7 m, length, width, height, respectively) of a similar design were assembled using two covers: glass without any cover and glass with PCCs based on Eu2O3 nanoparticles. On the north side, the boxes were upholstered with a single spunbond layer for natural ventilation. Boxes were planted with seven 35-day-old S. lycopersicum bushes (16 June 2022). Temperature and humidity sensors were placed in the boxes to control the growing conditions. The plants were watered every three days with tap water. The collection of fruits, as well as accounting for their number and weight, was carried out from 30 July until 14 August.
2.5. Measurement of Leaf Chlorophyll Content
In order to control the concentration of chlorophyll in the leaves of plants without damaging them, during the entire experiment, we carried out measurements using a CL-01 chlorophyll meter. To convert the obtained values into generally accepted units of measurement (mg chl g
−1 of fresh weight), we investigated the dependence of the values obtained using CL-01 on the actual chlorophyll content in the leaves of plants grown under the same conditions as an experimental plant. The actual chlorophyll content was measured in fresh leaf samples (0.3 g). Leaf samples were homogenized in ethanol (95%
v/
v), left in the dark for 10 min, then filtered and centrifuged for 5 min at 15,000 rpm. The chlorophyll concentration was calculated from the absorbance of the extract at 664 nm and 648 nm using the formula:
where
A(664) is the absorption at λ = 664 nm,
A(648) is the absorption at λ = 648 nm [
60]. Based on the obtained data, a calibration curve was built, and a linear dependence was calculated to calculate the chlorophyll content in the leaf “mg chl g
−1 of fresh weight”, as previously described in [
61].
2.6. Measurement of Plant Morphological Parameters
The number of leaves was determined manually. The stem length was measured with a graduated ruler accurate to the millimeter. The GreenImage software was used to determine the leaf area [
33]. Dry weight was determined using a moisture analyzer OHAUS MB23 (Ohaus corporation, NJ, USA). Plant leaves were dried at 150 °C for 15 min.
2.7. Measurement of the Kinetics of Photoinduced Changes in Chlorophyll a Fluorescence (FChl) and the Intensity of Carbon Dioxide Assimilation and Transpiration
To measure FChl and the intensity of carbon dioxide assimilation and transpiration in plant leaves, a GFS-3000 gas analyzer integrated with a DUAL-PAM-100 was used (Waltz, Eichenring, Effeltrich, Germany). The measurements were carried out in a measuring cuvette on untouched leaves at room temperature (25 °C) and 65% humidity in a laminar CO2 flow 400 with concentration 200 ppm. The measurements were preceded by a 1 h incubation of plants in the dark at room temperature to ensure complete relaxation of all photoinduced processes and thirty-minute adaptation in a cuvette.
To measure the maximal photochemical quantum yield of photosystem II (PSII) in dark-adapted leaves (Fv/Fm) and FChl parameters after light adaptation (1–21 min, λ = 625 nm, 200 µmol photons s
−1 m
−2), the leaves were illuminated with a 300 ms saturating pulse (λ = 625 nm, 12,000 µmol photons s
−1 m
−2). The FChl parameters were calculated using the DUAL-PAM software according to Equations (2)–(7) [
62,
63]:
where F
0—the intensity of FChl caused by measured light, Fm—the maximal intensity of FChl, Fv—the photoinduced change in the yield of FChl, Y(II)—the effective quantum yield of PSII photochemistry, Y(NPQ)—the quantum yield of light-induced non-photochemical fluorescence quenching, Fm′—the induced maximal level of FChl in light-adapted leaves, F—the intensity of FChl measured immediately before a saturated pulse of light, Pm—the difference signal between the fully reduced and oxidized states of P700, P—the P700 signal recorded just before a saturated pulse, Pm′—the P700 signal recorded briefly after onset of a saturated pulse, ETR(II)—rate of linear electron transport through photosystem II, ETR(I)—rate of linear electron transport through photosystem I, Y(I)—effective quantum yield of PSI photochemistry, absI—absorbed irradiance taken as 0.84 of incident irradiance, 0.5—the fraction of absorbed light reaching PSI or PSII.
The calculation of the intensity of assimilation and transpiration was carried out using the GFS-win software according to Equations (8) and (9) [
64]:
where E—transpiration rate, A—assimilation rate, Ue—molar flow rate at the inlet of the cuvette, Wo—H
2O mole fraction at the outlet of the cuvette, We—H
2O mole fraction at the inlet of the cuvette, LA—leaf area (4 cm
2), Co—CO
2 mole fraction at the outlet of the cuvette, Ce—CO
2 mole fraction at the inlet of the cuvette.
2.8. Accounting for the Development of Late Blight on Tomato Leaves during Natural and Artificial Infection
Accounting for the development of late blight during spontaneous infection of plants in boxes (as described in
Section 2.4.) was carried out according to the scale of the British Mycological Society [
65].
To calculate the number and diameter of colonies during artificial infection of detached leaves with
Phytophthora infestans, two independent experiments were carried out according to Filippov’s method [
66]. For each experiment, 12 leaves were selected randomly. In the first experiment, the leaves were sprayed with a suspension of zoosporangia (300,000 pieces m
−2) using a spray gun and kept in the dark at 20 °C and 80% humidity for 3 days. The number of necroses on the leaf surface was counted. In the second experiment, leaves were infected by spot application of 10 µL of zoosporangia suspension. After that, the leaves were kept in the dark at 20 °C and 80% humidity. After 18 h, the remains of the suspension were removed with filter paper and incubation continued for another 3 days.
2.9. Statistical Analysis
To determine statistically significant differences between plant groups, one-way analysis of variance (ANOVA) was performed, followed by post hoc comparison using Student’s t-test for independent means. The difference was considered statistically significant if p ≤ 0.05.
4. Discussion
Nowadays, a wide variety of europium compounds with particle sizes from 4 nm to 500 nm are used [
47,
48,
49,
53,
54,
55,
56,
69]. It is known that the use of europium nanoparticles compounds is based on their luminescent properties, which, in turn, are determined by factors such as particle size, morphology, and symmetry, environmental coordination, the presence of defects and impurities, and particle temperature [
70]. One of the main qualities of europium compounds, which allows them to be used in the creation of PCCs for greenhouses, is the ability to emit light in the photosynthetically active range due to the absorption of near-ultraviolet radiation [
47,
48,
49]. The main absorption of europium is observed at 380 nm and 395 nm (
7F
0 →
5G
2 and
7F
0 →
5L
6), and the main luminescence is observed at 580 nm and 613 nm (the position and relative intensity of which very strongly depend on the environment of europium ions and particle size) [
49]. The remaining luminescence maxima make an insignificant contribution to the total emission and change only slightly with changes in the environment or particle size. In this work, two types of compounds were used to prepare nanophosphors: Eu
2O
3 and Eu
3+:LaF
3. Europium oxide nanoparticles are relatively widely used in various branches of science and technology [
46], as they are an example of primary nanoparticles, that is, obtained from europium compounds as such, which significantly speeds up and reduces the cost of their production. Eu
3+:LaF
3 nanocrystals are representative of nanoluminophores based on a lanthanide substrate doped with Eu
3+ ions. The advantage of Eu
3+:LaF
3 nanoparticles is their biocompatibility [
70,
71], which can be crucial when dealing with food products. However, the production of such particles is more difficult. Eu
2O
3 nanoparticles have less biocompatibility [
72,
73] in comparison to Eu
3+:LaF
3 nanoparticles. However, there are approaches to increase the biocompatibility of Eu
2O
3 nanoparticles. For example, a decrease in the concentration of nanoparticles [
72,
74], particle size [
75], and environment [
72,
75] affects the biocompatibility of Eu
2O
3 nanoparticles. Currently, biological (green) methods for the synthesis of nanoparticles have gained popularity. This method is more eco-friendly and allows you to receive ultrasmall nanoparticles, which are more biocompatible in comparison to physical and chemical methods of synthesis [
76]. The PCC developed by us can be safely used in greenhouses since nanoparticles created in our work do not interact with plants or soil, due to their inclusion in a fluoroplastic matrix, which has a very high adhesion to greenhouse glass.
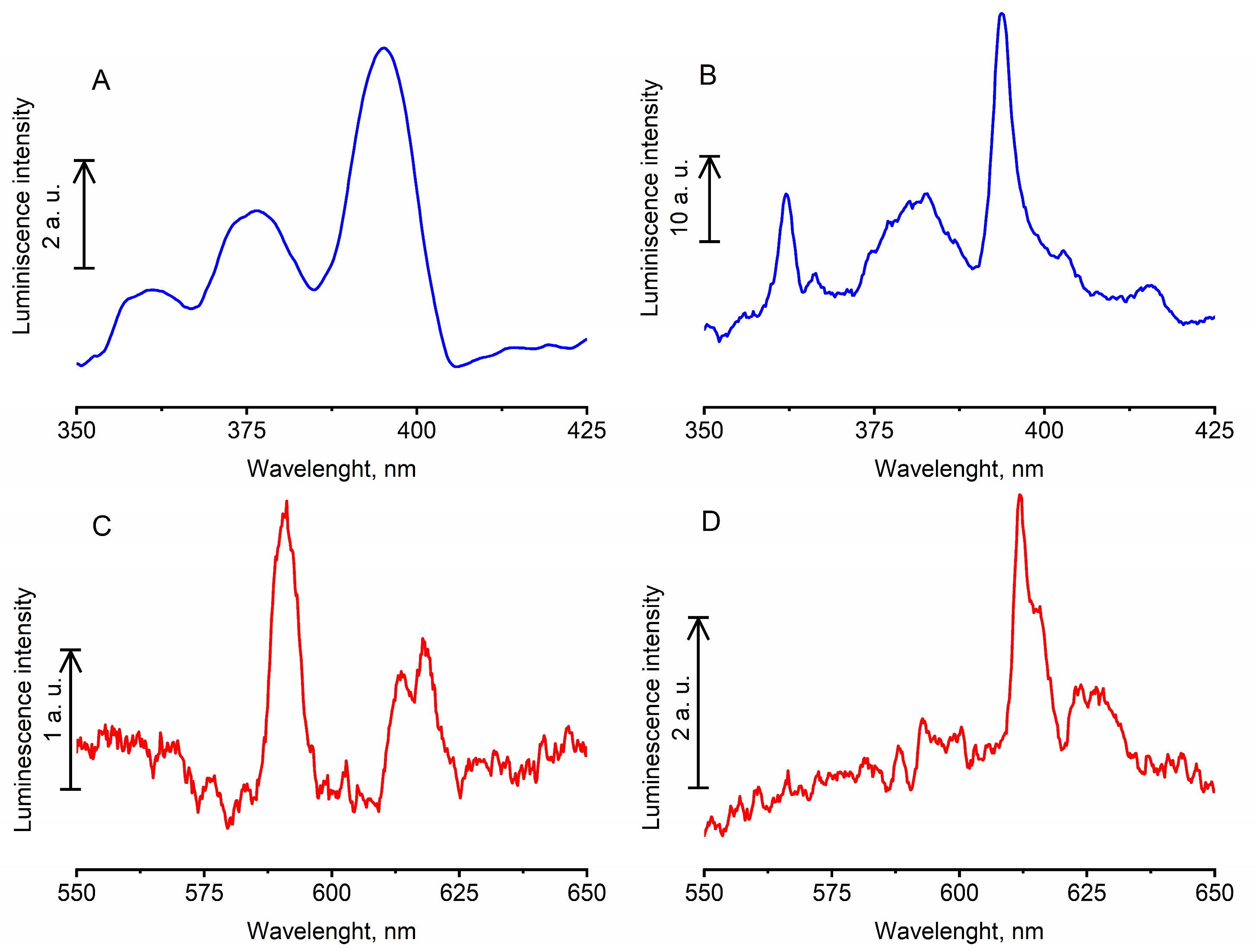
In earlier published works, it was shown that the incorporation of europium nanocrystals into a PCC reduces the ability of Eu
3+ to luminesce in the region of
5D
0 →
7F
1 (λ = 591 nm) and
5D
0 →
7F
4 (λ = 690 nm) transitions and retains the ability to emit in the region of the
5D
0 →
7F
2 transition (615 nm) [
34,
43]. Phosphors in the PCC-Eu
3+:LaF
3 obtained by us retained the ability to effectively luminesce in the region of the
5D
0 →
7F
1 transition, and the luminescence band at 591 nm was much more intensive than the band at 615 nm (
Figure 4C). The presence of an intensive band at 591 nm is associated with a feature of the crystal field of lanthanum fluoride [
57,
58,
59]. The luminescence spectrum of PCC-Eu
2O
3 differed from the luminescence spectrum of PCC-Eu
3+:LaF
3 and had a pronounced luminescence maximum corresponding to the
5D
0 →
7F
2 transition (
Figure 4D).
It is known that light of different wavelengths has different effects on plants [
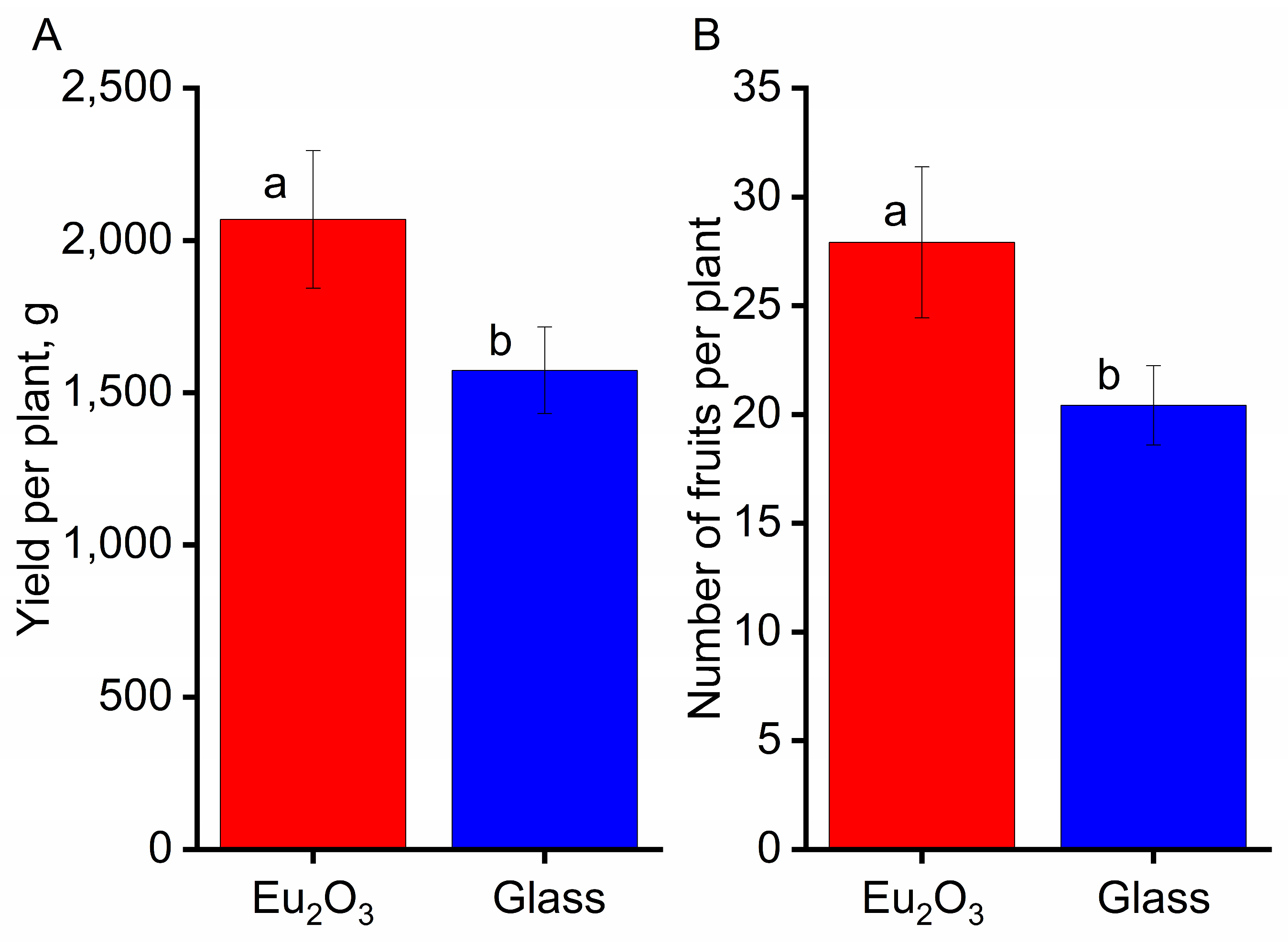
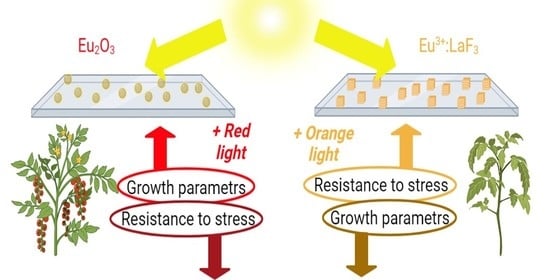
77]. In the present work, we observe stimulation (up to 40%) of the growth and development of tomato plants, as well as an increase in yield under PCC-Eu
2O
3, which converts ultraviolet light to red (with a maximum of 612 nm) (
Figure 5). A statistically significant increase in biomass was not observed in cucumber plants (
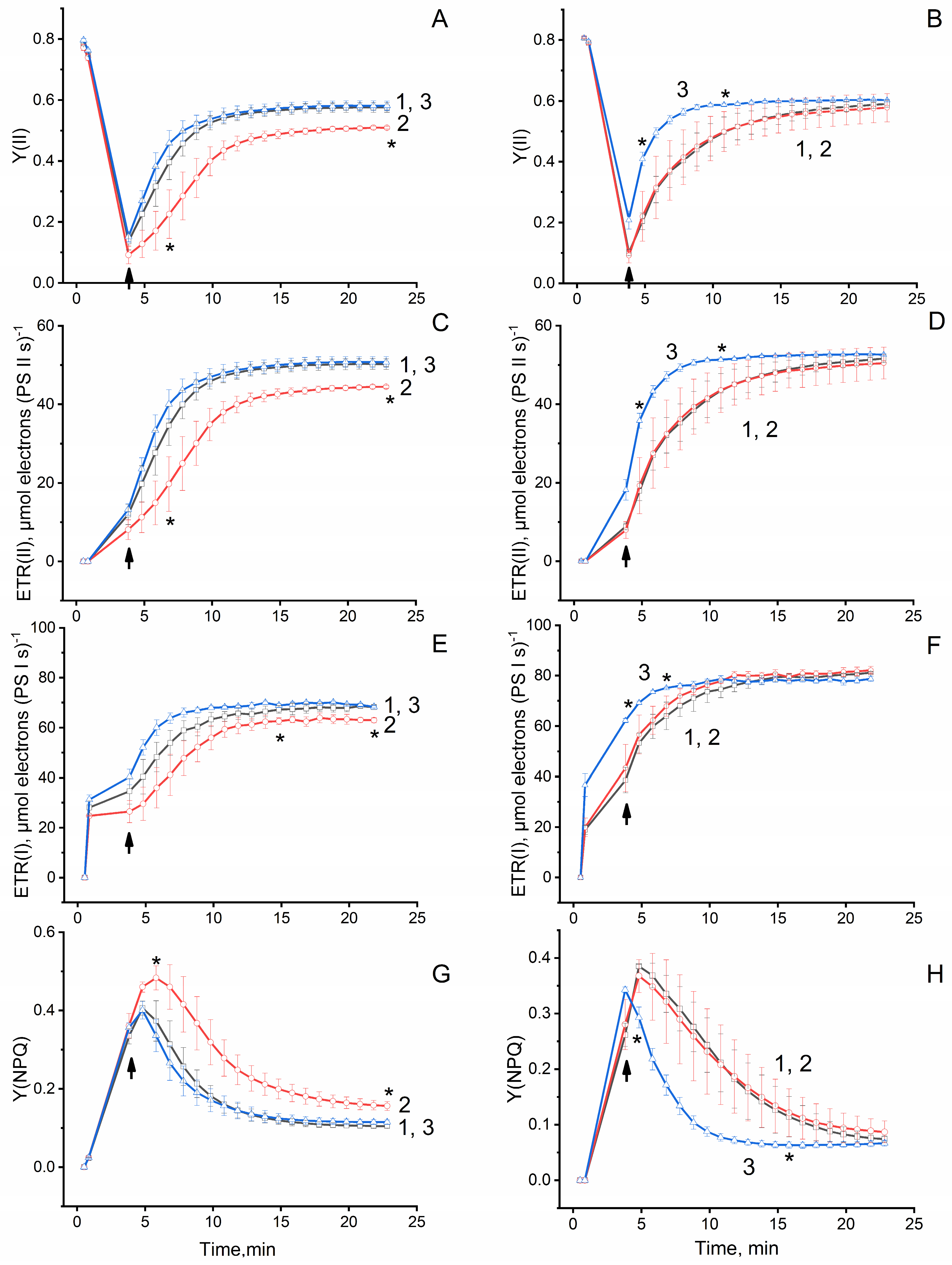
Table 1). In the leaves of plants grown under PCC-Eu
2O
3, an intensification of gas exchange processes, especially CO
2 assimilation, correlating with a redistribution of the energy of absorbed light in favor of the dark stages of photosynthesis, confirmed by a change in the parameters of chlorophyll
a fluorescence, was observed (
Figure 6C,D and
Figure 7B,D,F,H). PCC-Eu
3+:LaF
3, the main luminescence maximum of which is observed at λ = 591 nm, did not stimulate the growth and development of all the studied plant species. In contrast, cucumber plants grew 30% slower than control plants, and tomato plants grew on average lower than control plants, although without a statistically significant difference (
Table 1). These data correlate with data on the development of gas exchange processes and chlorophyll fluorescence parameters (
Figure 6 and
Figure 7). It is known that the induction of carbon dioxide assimilation (photosynthesis) includes at least two phases [
78]. It is assumed that the first fast phase (with a time constant of 1–2 min) is where the stock of ribulose-1,5-bisphosphate or other Calvin cycle intermediates available in the dark is consumed, while the second slow phase (with a time constant of 4–5 min) is where the photoactivation of Rubisco by Rubisco activase occurs. Differences in the kinetics of Rubisco activation may be due to differences in the total concentration of Rubisco activase or the ratio of its isoforms (α- and β-Rubisco activase), in the binding affinity of Rubisco activase to Rubisco, and/or in the localization of Rubisco activase relative to Rubisco. Arabidopsis mutants expressing only the β-Rubisco activase isoform, which is less sensitive to the redox status of chloroplasts than α-Rubisco activase, had a faster induction of photosynthesis [
79]. In rice, Rubisco activase overexpressing mutants maintain higher Rubisco activation states in the dark and respond faster to changes in illumination than wild-type plants [
80]. Differences in the development of the first phase of CO
2 assimilation induction were found in our study among groups of plants grown under different covers, likely due to different contents of Calvin cycle intermediates in plants previously adapted in the dark, which may be the reason for the different plant productivity. The kinetics of changes in the parameters of chlorophyll fluorescence induction also have two phases and correlate well with gas exchange curves in plants. Some features in the fluorescence kinetics of chlorophyll
a may be due to the lower activity of the Calvin cycle, and delayed activation of ferredoxin-NADP+ reductase, which is responsible for the transfer of electrons to NADP+ at the end of the electron transport chain of chloroplasts [
81,
82,
83,
84]. It was previously shown that the addition of even a small amount of red light leads to the intensification of photosynthesis [
50] and an increase in plant productivity [
34,
43]. Moreover, additional red light strongly affects the parameters of the so-called “variable” fluorescence of chlorophyll
a [
85,
86,
87], having no effect on the intensity of gas exchange, due to a weak effect on the opening of stomata [
88]. It was shown that the use of PCCs with europium compounds as a luminophore (with a luminescence maximum at 612 nm) has a positive effect on plant development [
34,
43]. It is believed that even a small increase in the proportion of red light (in particular, a change in the ratio of red light to far red) under PCCs can affect the functioning of the phytochrome system, which can regulate plant growth and increase resistance to adverse environmental conditions [
40,
45,
89,
90]. An increase in the proportion of orange light, in contrast to red, somewhat inhibits the growth and development of plants, and also leads to the appearance of signs of stress [
91,
92]. For example, in the work of Brazaityte et al., it was shown that the addition of 15 µmol photon s
−1 m
−2 of orange light (λ = 590 nm) slows down the development of cucumber seedlings [
92]. It is known that orange light causes an increase in lipid peroxidation, an increase in antioxidant activity, and an increase in the content of osmolytes in plant leaves [
86,
93]. Thus, plants grown under light with an increased proportion of orange light, on the one hand, gain biomass more slowly, and, on the other hand, have increased resistance to oxidative stress due to an increased level of activity of the antioxidant system. It is likely that we observe a similar effect of orange light in the present study (
Table 6). Red light can have the opposite effect: inhibit the synthesis of protective antioxidant enzymes, but stimulate photosynthesis [
87,
94,
95].
Light is perceived by plants and microorganisms, which react even to small changes in intensity in a narrow wavelength range, as a signal, activates specific internal reactions, and affects the interaction of plants and microorganisms [
96,
97,
98,
99,
100,
101]. Plant light responses include changes in hormonal levels, the production of secondary metabolites, and the release of volatile compounds, which ultimately affect the interactions between plants and the phyllosphere, laimosphere, and rhizosphere. The reactions of microorganisms to light may include the release of various substances, including plant growth regulators. It is known that light can have a great influence on the development of both beneficial and neutral and pathogenic microflora [
41,
97,
102,
103]. On the one hand, light can have a direct effect on the microflora [
104,
105,
106,
107,
108,
109,
110]. On the other hand, light has a plant-mediated effect on the microflora [
111,
112,
113,
114,
115,
116,
117,
118,
119]. Light can both increase plant resistance to phytopathogens [
116,
120,
121,
122,
123,
124] and decrease it [
122,
125]. Many studies have shown that microflora is affected not only by white or sunlight per se, but each component of the solar spectrum is important: ultraviolet radiation, blue, far red, etc. Red light has a great influence on the microflora [
106,
126] and the interaction of plants with microorganisms [
114,
123,
124,
125,
126,
127,
128,
129,
130,
131]. As a rule, red light inhibits the development of phytopathogens and increases plant resistance. The increase in the resistance of tomato plants to the development of
P. infestans on leaves observed in our experiments may be associated with an increase in the proportion of red light (
Table 6) due to the use of PCC-Eu
2O
3. At the same time, the effect of PCC-Eu
2O
3 was manifested both in the light (
Figure 9A) and in the dark (
Figure 9B,C), which may indicate a plant-mediated effect of the changed light spectrum. Also, an important factor can be the ratio of the light intensities of different parts of the spectrum, for example, the ratio of red light to far red. On the one hand, an increase in the proportion of far red light leads to an increase in plant susceptibility to phytopathogens [
115,
125,
132,
133], and on the other hand, plant morphology altered by an increased proportion of far red light can prevent the spread of diseases [
134,
135]. However, some studies highlight the crucial role of biological rhythms rather than climate factors in exploring seasonal carbon dynamics and global carbon balance. For example, terrestrial ecosystems in the Northern Hemisphere in the peak of the growing season were dominated by the shifts in plant phenology [
136]. Therefore, under our conditions, changes in both the light spectrum and biological rhythms can influence the growth and development of plants.
Thus, we developed two types of europium-based PCC with different luminescence spectra. The covers had various effects on the growth and development, including biochemical and photochemical processes, of agricultural plants. It was assumed that changes in the illumination spectrum by the PCC cause both the activation of plant growth in the case of using Eu2O3 and the increase in plant resistance to the action of high and low temperatures in the case of using Eu3+:LaF3. Moreover, PCC-Eu2O3 can be used to increase the resistance of tomato plants to P. infestans.