Effects of Light Intensity on Endogenous Hormones and Key Enzyme Activities of Anthocyanin Synthesis in Blueberry Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Site
2.2. Experimental Materials
2.3. Experimental Design
2.4. Sample Collection
2.5. Method of Index Determination
2.5.1. Methods for Determination of Endogenous Hormone Contents and Enzyme Activities
2.5.2. Method for Determination of Anthocyanin Content
2.6. Data Analysis
3. Results
3.1. Effects of Light Intensity on Endogenous Hormone Contents in Blueberry Leaves
3.2. Effects of Light Intensity on Key Enzyme Activities in the Anthocyanin Synthesis Pathway of Blueberry Leaves
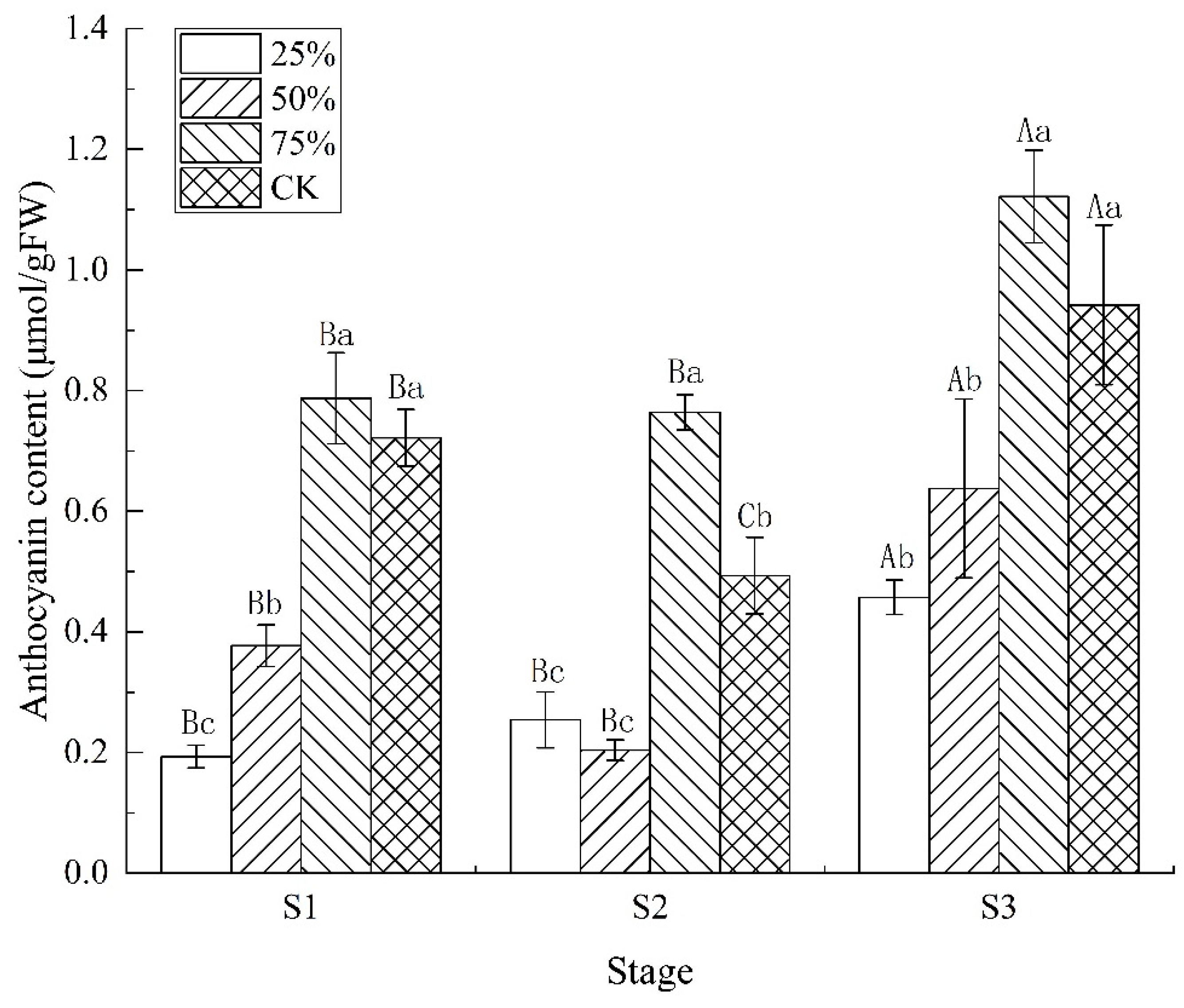
3.3. Effect of Light Intensity on Anthocyanin Content in Blueberry Leaves
3.4. Correlation Analysis
4. Discussion
4.1. Light Intensity Promotes Anthocyanin Synthesis by Regulating the Content of Endogenous Hormones
4.2. Light Intensity Regulates Anthocyanin Synthesis by Inducing the Expression of Enzyme Activities
4.3. Regulation of Light Intensity in Anthocyanin Biosynthesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Merzlyak, M.N.; Chivkunova, O.B.; Solovchenko, A.E.; Naqvi, K.R. Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J. Exp. Bot. 2008, 59, 3903–3911. [Google Scholar] [CrossRef]
- Ntefidou, M.; Manetas, Y. Optical Properties of Hairs During the Early Stages of Leaf Development in Platanus orientalis. Funct. Plant Biol. 1996, 23, 535–538. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J. Biomed. Biotechnol. 2004, 2004, 314–320. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Zhang, H.-N.; Li, W.-C.; Wang, H.-C.; Shi, S.-Y.; Shu, B.; Liu, L.-Q.; Wei, Y.-Z.; Xie, J.-H. Transcriptome Profiling of Light-Regulated Anthocyanin Biosynthesis in the Pericarp of Litchi. Front. Plant Sci. 2016, 7, 963. [Google Scholar] [CrossRef]
- Guo, X.; Shakeel, M.; Wang, D.; Qu, C.; Yang, S.; Ahmad, S.; Song, Z. Metabolome and transcriptome profiling unveil the mechanisms of light-induced anthocyanin synthesis in rabbiteye blueberry (vaccinium ashei: Reade). BMC Plant Biol. 2022, 22, 223. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, Y.; Peng, W.; Wang, Z.; Xie, D. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 2009, 60, 3849–3860. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, S.; Yin, H.; Zhang, S.; Tuo, X.; Tran, L.-S.P. Transcriptome Analysis Reveals Roles of Anthocyanin- and Jasmonic Acid-Biosynthetic Pathways in Rapeseed in Response to High Light Stress. Int. J. Mol. Sci. 2021, 22, 13027. [Google Scholar] [CrossRef] [PubMed]
- Araguirang, G.E.; Richter, A.S. Activation of anthocyanin biosynthesis in high light—What is the initial signal? New Phytol. 2022, 236, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Sudha, G.; Ravishankar, G.A. Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta Physiol. Plant. 2003, 25, 249–256. [Google Scholar] [CrossRef]
- Jeong, S.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- Qi, T.; Song, S.; Ren, Q.; Wu, D.; Huang, H.; Chen, Y.; Fan, M.; Peng, W.; Ren, C.; Xie, D. The Jasmonate-ZIM-Domain Proteins Interact with the WD-Repeat/bHLH/MYB Complexes to Regulate Jasmonate-Mediated Anthocyanin Accumulation and Trichome Initiation in Arabidopsis thaliana. Plant Cell 2011, 23, 1795–1814. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, G.; Hu, Z.; Gao, Q.; Cui, B.; Tian, S.; Wang, B.; Chen, G. Genetically engineered anthocyanin pathway for high health-promoting pigment production in eggplant. Mol. Breed. 2016, 36, 54. [Google Scholar] [CrossRef]
- Zorenc, Z.; Veberic, R.; Koron, D.; Miosic, S.; Hutabarat, O.S.; Halbwirth, H.; Mikulic-Petkovsek, M. Polyphenol metabolism in differently colored cultivars of red currant (Ribes rubrum L.) through fruit ripening. Planta 2017, 246, 217–226. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef]
- Albert, N.; Lewis, D.H.; Zhang, H.; Irving, L.; Jameson, P.; Davies, K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef]
- Nguyen, P.; Cin, V.D. The role of light on foliage colour development in coleus (Solenostemon scutellarioides (L.) Codd). Plant Physiol. Biochem. 2009, 47, 934–945. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Zhai, W.; Liu, Y.; Gao, Q.; Liu, J.; Ren, L.; Chen, H.; Zhu, Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino). PLoS ONE 2017, 12, e0179305. [Google Scholar] [CrossRef]
- Guo, X.; Wang, D.; Shakeel, M. Transcriptome analysis reveals light-induced anthocyanin synthesis candidate genes in rabbiteye blueberry (Vaccinium ashei: Reade). Biotechnol. Biotechnol. Equip. 2021, 35, 746–757. [Google Scholar] [CrossRef]
- Schraer, S.M.; Shaw, D.R.; Boyette, M.; Coupe, R.H.; Thurman, E.M. Comparison of Enzyme-Linked Immunosorbent Assay and Gas Chromatography Procedures for the Detection of Cyanazine and Metolachlor in Surface Water Samples. J. Agric. Food Chem. 2000, 48, 5881–5886. [Google Scholar] [CrossRef]
- LaFountain, A.M.; Yuan, Y. Repressors of anthocyanin biosynthesis. New Phytol. 2021, 231, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-J.; Wang, Q.; Zhang, H.-B.; Wang, S.-H.; Song, H.-D.; Hao, J.-M.; Dong, H.-Z. Light shading improves the yield and quality of seed in oil-seed peony (Paeonia ostii Feng Dan). J. Integr. Agric. 2018, 17, 1631–1640. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, F.; Zhang, H.; Zhu, Z. Responses of the growth, photosynthetic characteristics, endogenous hormones and antioxidant activity of Carpinus betulus L. seedlings to different light intensities. Front. Plant Sci. 2022, 13, 1055984. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Cai, X.; Wan, Y.-T.; Fu, Y.-F.; Yang, X.-Y.; Zhang, Z.-W.; Yuan, S. Relatively Low Light Intensity Promotes Phosphorus Absorption and Enhances the Ethylene Signaling Component EIN3 in Maize, Wheat, and Oilseed Rape. Agronomy 2022, 12, 427. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, Y.; Yang, X.; Wei, C.; Guan, J. Ethylene Signal Is Involved in the Regulation of Anthocyanin Accumulation in Flesh of Postharvest Plums (Prunus salicina Lindl.). Plants 2023, 12, 893. [Google Scholar] [CrossRef]
- An, J.; Xu, R.; Liu, X.; Zhang, J.; Wang, X.; You, C.; Hao, Y. Jasmonate induces biosynthesis of anthocyanin and proanthocyanidin in apple by mediating the JAZ1–TRB1–MYB9 complex. Plant J. 2021, 106, 1414–1430. [Google Scholar] [CrossRef]
- Boo, H.O.; Chon, S.U.; Lee, S.Y. Effects of temperature and plant growth regulators on anthocyanin synthesis and phenylalanine ammonia-lyase activity in chicory (Cichorium intybus L.). J. Hortic. Sci. Biotechnol. 2006, 81, 478–482. [Google Scholar] [CrossRef]
- Li, Y.; Nie, P.; Zhang, H.; Wang, L.; Wang, H.; Zhang, L. Dynamic changes of anthocyanin accumulation and endogenous hormone contents in blueberry. J. Beijing For. Univ. 2017, 39, 64–71. [Google Scholar] [CrossRef]
- Teribia, N.; Tijero, V.; Munné-Bosch, S. Linking hormonal profiles with variations in sugar and anthocyanin contents during the natural development and ripening of sweet cherries. New Biotechnol. 2016, 33, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, J.; Qin, B.; Yin, Y.; An, W.; Mu, Z.; Cao, Y. ABA mediates development-dependent anthocyanin biosynthesis and fruit coloration in Lycium plants. BMC Plant Biol. 2019, 19, 317. [Google Scholar] [CrossRef]
- Gao, C.; Sun, Y.; Li, J.; Zhou, Z.; Deng, X.; Wang, Z.; Wu, S.; Lin, L.; Huang, Y.; Zeng, W.; et al. High Light Intensity Triggered Abscisic Acid Biosynthesis Mediates Anthocyanin Accumulation in Young Leaves of Tea Plant (Camellia sinensis). Antioxidants 2023, 12, 392. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Z.; Chen, W.; Xu, H.; Feng, H. Anthocyanin Degrading and Chlorophyll Accumulation Lead to the Formation of Bicolor Leaf in Ornamental Kale. Int. J. Mol. Sci. 2019, 20, 603. [Google Scholar] [CrossRef]
- Cotado, A.; Müller, M.; Morales, M.; Munné-Bosch, S. Linking jasmonates with pigment accumulation and photoprotection in a high-mountain endemic plant, Saxifraga longifolia. Environ. Exp. Bot. 2018, 154, 56–65. [Google Scholar] [CrossRef]
- Bai, S.; Sun, Y.; Qian, M.; Yang, F.; Ni, J.; Tao, R.; Li, L.; Shu, Q.; Zhang, D.; Teng, Y. Transcriptome analysis of bagging-treated red Chinese sand pear peels reveals light-responsive pathway functions in anthocyanin accumulation. Sci. Rep. 2017, 7, 63. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Miki, S.; Wada, K.C.; Takeno, K. A possible role of an anthocyanin filter in low-intensity light stress-induced flowering in Perilla frutescens var. crispa. J. Plant Physiol. 2015, 175, 157–162. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, M.; Zhang, G.; Li, P.; Ma, F. Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities. Int. J. Mol. Sci. 2019, 20, 6060. [Google Scholar] [CrossRef]
- Heller, W.; Forkmann, G.; Britsch, L.; Grisebach, H. Enzymatic reduction of (+)-dihydroflavonols to flavan-3,4-cis-diols with flower extracts from Matthiola incana and its role in anthocyanin biosynthesis. Planta 1985, 165, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.R.; Hackett, W.P. Dihydroflavonol Reductase Activity in Relation to Differential Anthocyanin Accumulation in Juvenile and Mature Phase Hedera helix L. Plant Physiol. 1991, 97, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Boss, P.K.; Davies, C.; Robinson, S.P. Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Mol. Biol. 1996, 32, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, Y.; Ren, L.; Lian, H.; Chen, H. Molecular cloning and characterization of anthocyanin biosynthesis genes in eggplant (Solanum melongena L.). Acta Physiol. Plant. 2016, 38, 163. [Google Scholar] [CrossRef]
- Bizjak, J.; Weber, N.; Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Alam, Z.; Stich, K.; Halbwirth, H.; Veberic, R. Influence of Phostrade Ca on Color Development and Anthocyanin Content of ‘Braeburn’ Apple (Malus domestica Borkh.). Hortscience 2013, 48, 193–199. [Google Scholar] [CrossRef]
- Sharma, H.; Chawla, N.; Dhatt, A.S. Role of phenylalanine/tyrosine ammonia lyase and anthocyanidin synthase enzymes for anthocyanin biosynthesis in developing Solanum melongena L. genotypes. Physiol. Plant. 2022, 174, e13756. [Google Scholar] [CrossRef]
- Manetas, Y. Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora Morphol. Distrib. Funct. Ecol. Plants 2005, 201, 163–177. [Google Scholar] [CrossRef]
- Ibañez, S.; Rosa, M.; Hilal, M.; González, J.A.; Prado, F.E. Leaves of Citrus aurantifolia exhibit a different sensibility to solar UV-B radiation according to development stage in relation to photosynthetic pigments and UV-B absorbing compounds production. J. Photochem. Photobiol. B Biol. 2008, 90, 163–169. [Google Scholar] [CrossRef]
- Ma, Z.-H.; Li, W.-F.; Mao, J.; Li, W.; Zuo, C.-W.; Zhao, X.; Dawuda, M.M.; Shi, X.-Y.; Chen, B.-H. Synthesis of light-inducible and light-independent anthocyanins regulated by specific genes in grape ‘Marselan’ (V. vinifera L.). PeerJ 2019, 7, e6521. [Google Scholar] [CrossRef]
- Weiss, D.; Halevy, A.H. The role of light reactions in the regulation of anthocyanin synthesis in Petunia corollas. Physiol. Plant. 1991, 81, 127–133. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, P.; Lin, W.; Zheng, X.; Cai, M.; Peng, C. Sequencing of anthocyanin synthesis-related enzyme genes and screening of reference genes in leaves of four dominant subtropical forest tree species. Gene 2019, 716, 144024. [Google Scholar] [CrossRef] [PubMed]



| Light Intensity | S1/μmol·m−2s−1 | S2/μmol·m−2s−1 | S3/μmol·m−2s−1 |
|---|---|---|---|
| 25% | 372 ± 34.06 Ad | 369 ± 29.44 Ad | 379 ± 28.29 Ad |
| 50% | 750 ± 31.18 Ac | 699 ± 24.83 Ac | 778 ± 30.02 Ac |
| 75% | 1123 ± 40.99 Ab | 1094 ± 48.50 Ab | 1143 ± 36.37 Ab |
| CK | 1498 ± 39.26 Aa | 1456 ± 44.46 Aa | 1587 ± 37.53 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, X.; Tan, T.; Zhang, X.; Guo, X.; Zhu, Y.; Song, Z.; Wang, D. Effects of Light Intensity on Endogenous Hormones and Key Enzyme Activities of Anthocyanin Synthesis in Blueberry Leaves. Horticulturae 2023, 9, 618. https://doi.org/10.3390/horticulturae9060618
An X, Tan T, Zhang X, Guo X, Zhu Y, Song Z, Wang D. Effects of Light Intensity on Endogenous Hormones and Key Enzyme Activities of Anthocyanin Synthesis in Blueberry Leaves. Horticulturae. 2023; 9(6):618. https://doi.org/10.3390/horticulturae9060618
Chicago/Turabian StyleAn, Xiaoli, Tianyu Tan, Xinyu Zhang, Xiaolan Guo, Yunzheng Zhu, Zejun Song, and Delu Wang. 2023. "Effects of Light Intensity on Endogenous Hormones and Key Enzyme Activities of Anthocyanin Synthesis in Blueberry Leaves" Horticulturae 9, no. 6: 618. https://doi.org/10.3390/horticulturae9060618




