Research on Chilling Requirements and Physiological Mechanisms of Prunus mume
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Method
2.2.1. Method for Measuring Chilling Requirements
2.2.2. Method for Measuring Physiological Indicators in FB Dormancy Period
2.3. Statistical Analysis
3. Results
3.1. Chilling Requirement for Dormancy Release
3.2. Determination of Physiological Indicators during Low-Temperature Storage and Dormancy
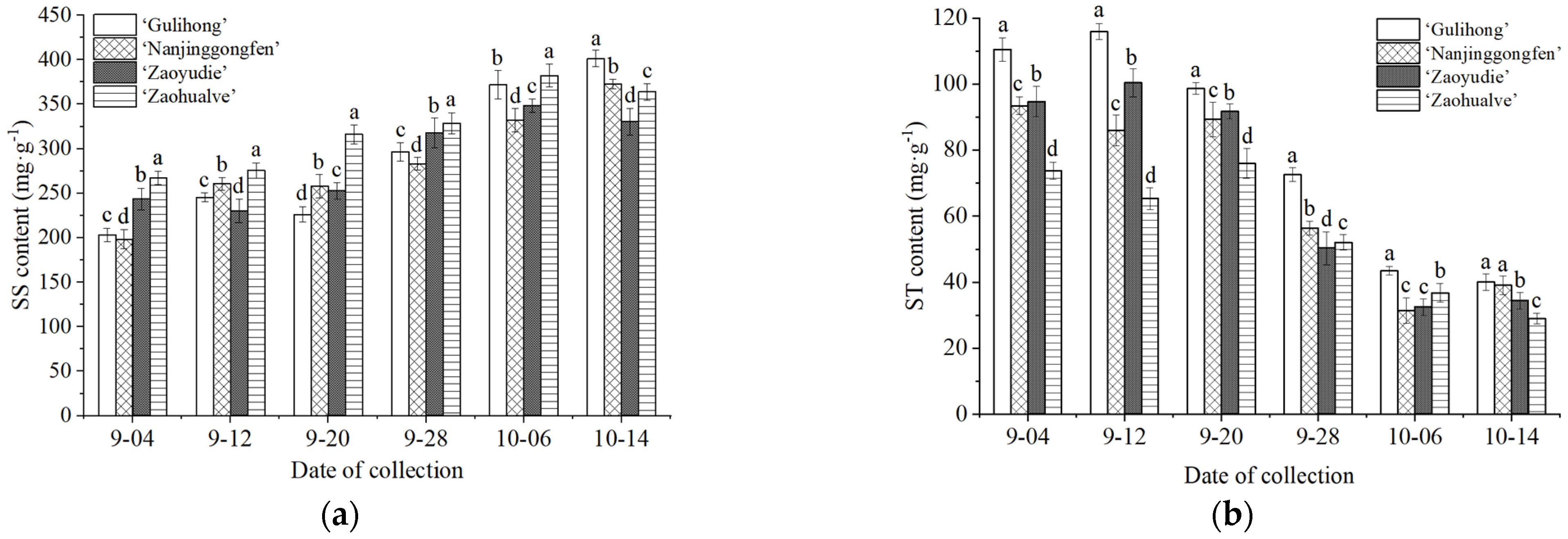
3.2.1. Changes of SS and ST Contents in FBs during Low-Temperature Release Dormancy
3.2.2. Effects of Low Temperatures on the Antioxidant Enzyme System in FBs during the Release of Dormancy
3.2.3. The Effect of Low Temperatures on the Content Changes of Endogenous Hormones in FBs during the Release of Dormancy
4. Discussion
4.1. Chilling Requirement
4.2. Carbohydrates
4.3. Antioxidant Enzyme Activities
4.4. Endogenous Hormone
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, R.; Du, D.; Wang, T.; Yang, W.; Wang, J.; Zhang, Q. A comparative analysis of characteristic floral scent compounds in Prunus mume and related species. Biosci. Biotechnol. Biochem. 2014, 78, 1640–1647. [Google Scholar] [CrossRef]
- Bergstrand, K.J.I. Methods for growth regulation of greenhouse produced ornamental pot-and bedding plants—A current review. Folia Hortic. 2017, 29, 63–74. [Google Scholar] [CrossRef]
- Faust, M.; Erez, A.; Rowland, L.J.; Wang, S.Y.; Norman, H.A. Bud dormancy in perennial fruit trees: Physiological basis for dormancy induction, mainte-nance, and release. HortScience 1997, 32, 623–629. [Google Scholar] [CrossRef]
- Angevine, M.W.; Chabot, B.F. Seed germination syndromes in higher plants. In Topics in Plant Population Biology; Palgrave: London, UK, 1979; pp. 188–206. [Google Scholar]
- Moe, R. Factors affecting flower abortion and malformation in roses. Physiol. Plant. 1971, 24, 291–300. [Google Scholar] [CrossRef]
- Darbyshire, R.; Webb, L.; Goodwin, I.; Barlow, S. Winter chilling trends for deciduous fruit trees in Australia. Agric. For. Meteorol. 2011, 151, 1074–1085. [Google Scholar] [CrossRef]
- Weinberger, J.H. Chilling requirements of peach cultivars. Proc. Am. Soc. Hortic. Sci. 1950, 56, 122–128. [Google Scholar]
- Richardson, E.A.; Seeley, S.D.; Walker, D.R.; Anderson, J.L.; Ashcroft, G.L. Pheno-climatography of spring peach bud development. HortScience 1975, 10, 236–237. [Google Scholar] [CrossRef]
- Fishman, S.; Erez, A.; Couvillon, G.A. The temperature dependence of dormancy breaking in plants: Computer simulation of pro-cesses studied under controlled temperatures. J. Theor. Biol. 1987, 126, 309–321. [Google Scholar] [CrossRef]
- Erez, A. Bud dormancy; phenomenon, problems and solutions in the tropics and subtropics. In Temperate Fruit Crops in Warm Climates; Springer: Dordrecht, The Netherlands, 2000; pp. 17–48. [Google Scholar]
- Erez, A.; Couvillon, G.A. Characterization of the influence of moderate temperatures on rest completion in peach. J. Am. Soc. Hortic. Sci. 1987, 112, 677–680. [Google Scholar] [CrossRef]
- Erez, A.; Couvillon, G.A.; Hendershott, C.H. The effect of cycle length on chilling negation by high temperatures in dormant peach leaf buds. J. Am. Soc. Hortic. Sci. 1979, 104, 573–576. [Google Scholar] [CrossRef]
- Ou, S. Chilling requirement of local mume trees in Taiwan. J. Beijing For. Univ. 1999, 21, 73–77. [Google Scholar]
- Zhuang, W.; Zhang, Z.; Shi, T.; Wang, P.; Shao, J.; Luo, X.; Gao, Z. Advance on chilling requirement and its chilling models in deciduous fruit crops. J. Fruit Sci. 2012, 29, 447–453. [Google Scholar]
- Yang, Y.; Li, Q. Chilling requirement of Prunus mume cultivars. J. Beijing For. Univ. 2013, 35, 47–51. [Google Scholar]
- Gao, Z.; Zhuang, W.; Wang, L.; Shao, J.; Luo, X.; Cai, B.; Zhang, Z. Evaluation of chilling and heat requirements in Japanese apricot with three models. HortScience 2012, 47, 1826–1831. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Sagisaka, S.; Araki, T. Amino acid pools in perennial plants at the wintering stage and at the beginning of growth. Plant Cell Physiol. 1983, 24, 479–494. [Google Scholar]
- Karssen, C.M.; Brinkhorst-Van der Swan, D.L.C.; Breekland, A.E.; Koornneef, M. Induction of dormancy during seed development by endoge-nous abscisic acid: Studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 1983, 157, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Lang, A. The effect of gibberellin upon flower formation. Proc. Natl. Acad. Sci. USA 1957, 43, 709–717. [Google Scholar] [CrossRef]
- Wang, L.; Hu, N. Chilling requirement of Peach cultivars. J. Fruit Sci. 1992, 9, 39–42. [Google Scholar]
- Li, H. Modern Plant Physiology, 3rd ed.; Higher Education Press: Beijing, China, 2012. [Google Scholar]
- Gao, J. Plant Physiology Guide (Paperback); Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Parkes, H.; Darbyshire, R.; White, N. Chilling requirements of apple cultivars grown in mild Australian winter conditions. Sci. Hortic. 2020, 260, 108858. [Google Scholar] [CrossRef]
- Li, Z.; Liu, N.; Zhang, W.; Wu, C.; Jiang, Y.; Ma, J.; Li, M.; Sui, S. Integrated transcriptome and proteome analysis provides insight into chilling-induced dormancy breaking in Chimonanthus praecox. Hortic. Res. 2020, 7, 198. [Google Scholar] [CrossRef]
- Yamane, H.; Kashiwa, Y.; Kakehi, E.; Yonemori, K.; Mori, H.; Hayashi, K.; Iwamoto, K.; Tao, R.; Kataoka, I. Differential expression of dehydrin in flower buds of two Japanese apricot cultivars re-quiring different chilling requirements for bud break. Tree Physiol. 2006, 26, 1559–1563. [Google Scholar] [CrossRef]
- Wang, X.Y.; He, H.L.; Liang, G.S.; Zhao, C.X. Study on the dormancy of mume cultivars in southern subtropical area of China. J. Fruit Sci. 2007, 24, 308–312. [Google Scholar]
- Chao, W.S.; Serpe, M.D. Changes in the expression of carbohydrate metabolism genes during three phases of bud dormancy in leafy spurge. Plant Mol. Biol. 2010, 73, 227–239. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, M.; Rageau, R.; Lacointe, A.; Gendraud, M. Influences of cold deprivation during dormancy on carbohydrate contents of vegetative and floral primordia and nearby structures of peach buds (Prunus persica L. Batch). Sci. Hortic. 2005, 105, 223–240. [Google Scholar] [CrossRef]
- Hernández, J.A.; Díaz-Vivancos, P.; Acosta-Motos, J.R.; Alburquerque, N.; Martínez, D.; Carrera, E.; García-Bruntón, J.; Barba-Espín, G. Interplay among antioxidant system, hormone profile and carbohydrate metabolism during bud dormancy breaking in a high-chill peach variety. Antioxidants 2021, 10, 560. [Google Scholar] [CrossRef]
- Walton, E.; Boldingh, H.; McLaren, G.; Williams, M.; Jackman, R. The dynamics of starch and sugar utilisation in cut peony (Paeonia lactiflora Pall.) stems during storage and vase life. Postharvest Biol. Technol. 2010, 58, 142–146. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Hernández, J.A.; Díaz-Vivancos, P.; Martínez-Sánchez, G.; Alburquerque, N.; Martínez, D.; Barba-Espín, G.; Acosta-Motos, J.R.; Carrera, E.; García-Bruntón, J. Physiological and biochemical characterization of bud dormancy: Evolution of carbohydrate and antioxidant metabolisms and hormonal profile in a low chill peach variety. Sci. Horticul-Turae 2021, 281, 109957. [Google Scholar] [CrossRef]
- Źróbek-Sokolnik, A. Temperature stress and responses of plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: Berlin/Heidelberg, Germany, 2012; pp. 113–134. [Google Scholar]
- Gubler, F.; Millar, A.A.; Jacobsen, J.V. Dormancy release, ABA and pre-harvest sprouting. Curr. Opin. Plant Biol. 2005, 8, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Moyle, R.; Bhalerao, R.; Hertzberg, M.; Lundeberg, J.; Nilsson, P.; Bhalerao, R.P. Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. Plant J. 2004, 40, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.H.; Zhong, W.J.; Huo, X.M.; Zhuang, W.B.; Ni, Z.J.; Gao, Z.H. Expression analysis of ABA-and GA-related genes during four stages of bud dormancy in Japanese apricot (Prunus mume Sieb. et Zucc). J. Hortic. Sci. Biotechnol. 2016, 91, 362–369. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhuo, X.; Zhao, K.; Zheng, T.; Han, Y.; Yuan, C.; Zhang, Q. Transcriptome profiles reveal the crucial roles of hormone and sugar in the bud dormancy of Prunus mume. Sci. Rep. 2018, 8, 5090. [Google Scholar] [CrossRef] [PubMed]




| Release Date | CR (CU) | FB Germination Rate (%) | |||
|---|---|---|---|---|---|
| ‘Gulihong’ | ‘Nanjing Gongfen’ | ‘Zaoyudie’ | ‘Zaohualve’ | ||
| 9-17 | 252 | 9.52 | 0 | 20.57 | 0 |
| 9-19 | 276 | 15.19 | 0 | 18.20 | 26.12 |
| 9-21 | 348 | 18.10 | 12.34 | 31.58 | 50.82 |
| 9-23 | 396 | 40.25 | 49.91 | 63.53 | 58.40 |
| 9-25 | 420 | 62.10 | 40.21 | 53.29 | 61.27 |
| 9-27 | 492 | 75.36 | 63.52 | 78.54 | 60.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ma, K.; Li, Q. Research on Chilling Requirements and Physiological Mechanisms of Prunus mume. Horticulturae 2023, 9, 603. https://doi.org/10.3390/horticulturae9050603
Zhang Y, Ma K, Li Q. Research on Chilling Requirements and Physiological Mechanisms of Prunus mume. Horticulturae. 2023; 9(5):603. https://doi.org/10.3390/horticulturae9050603
Chicago/Turabian StyleZhang, Yuhan, Kaifeng Ma, and Qingwei Li. 2023. "Research on Chilling Requirements and Physiological Mechanisms of Prunus mume" Horticulturae 9, no. 5: 603. https://doi.org/10.3390/horticulturae9050603





