Differential Response of Olive Cultivars to Leaf Spot Disease (Fusicladium oleagineum) under Climate Warming Conditions in Morocco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plant Materiel Sampling
2.3. Assessment of Disease Incidence
2.4. Assessment of Disease Severity
- yi+1 is the cumulative disease incidence in the i observation,
- ti is the time at the observation and,
- n is the total number of observations.
2.5. Assessment of Chlorophyll Content
2.6. Assessment of Total Phenolic and Flavonoid Contents
2.7. Data analysis
3. Results
3.1. Field Observation and Disease Symptoms Identification
3.2. Assessment of Disease Incidence and Severity
3.3. Determination of Total Chlorophyll, Phenolic and Flavonoid Contents
3.4. OLS Disease Progression over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouraich, I.; Tyner, W.E. Moroccan agriculture, climate change, and the moroccan green plan: A CGE analysis. Afr. J. Agric. Resour. Econ. 2014, 13, 307–330. [Google Scholar]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Portner, H.O.; Roberts, D.C.; Tignor, M.M.B.; Poloczanska, E.; Mintenbeck, K.; Alegria, A.; Craig, M.; Langsdorf, S.; Loschke, S.; Moller, V.; et al. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2022. Available online: https://www.ipcc.ch/report/ar6/wg2/ (accessed on 28 February 2022).
- Barbuzano, J. Climate change will reduce spanish olive oil production. Eos 2020, 101. [Google Scholar] [CrossRef]
- Kaniewski, D.; Van Campo, E.; Boiy, T.; Terral, J.F.; Khadari, B.; Besnard, G. Primary domestication and early uses of the emblematic olive tree: Palaeo botanical, historical and molecular evidences from the Middle East. Biol. Rev. 2012, 87, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Cannon, J. Cradle of transformation: The Mediterranean and Climate Change. Mongabay. 2022. Available online: https://news.mongabay.com/2022/04/cradle-of-transformation-the-mediterranean-and-climate-change/ (accessed on 28 April 2022).
- Abdou, Z.S. Impact of climate change on plant diseases and IPM strategies. In Plant Diseases-Current Threats and Management Trends; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Rodrigues, Â.M.; Valentim, C.; Margarida, A.; Eugénia, G.; Raimundo, S.; Carlos, M.; Correia, A.; Albino, B. The effect of nitrogen fertilization on the incidence of olive fruit fly, olive leaf spot and olive anthracnose in two olive cultivars grown in rainfed conditions. J. Sci. Hortic. 2019, 256, 108658. [Google Scholar] [CrossRef]
- Castagne, J.L.M. Catalogue des Plantes Qui Croissent Naturellement Aux Environs de Marseille; Nicot et Pardigon: Pont-Moreau, France, 1845; p. 220. [Google Scholar]
- González-Lamothe, R.; Segura, R.; Trapero, A.; Baldoni, L.; Botella, M.A.; Valpuesta, V. Phylogeny of the fungus Spilocaea oleagina, the causal agent of peacock leaf spot in olive. FEMS Microbiol. Lett. 2002, 210, 149–155. [Google Scholar] [CrossRef]
- Renaud, P. Ecologie de la maladie de l’œil de paon et résistance variétale dans leurs incidences sur la culture de l’olivier dans le pays. Afr. Med. Al-Awamia 1968, 26, 55–74. [Google Scholar]
- Issa, T.; Almadi, L.; Jarrar, S.; Tucci, M.; Buonaurio, R.; Famiani, F. Factors affecting Venturia oleaginea infections on olive and effects of the disease on floral biology. Phytopathol. Med. 2019, 58, 221–229. [Google Scholar]
- ONSSA: Office National de Sécurité Sanitaire et Alimentaire. Bulletin de Veille Phytosanitaire; 2016. Available online: https://www.onssa.gov.ma/evaluation-des-risques/surveillance-des-risques-phytosanitaires/ (accessed on 28 February 2022).
- Rhimini, Y.; Chliyeh, M.; Ouazzani, A.; Chahdi, A.; Touati, J.; Ouazzani, A.; Touhami, R.; Benkirane, R.; Douira, A. Influence of certain cultural practices and variable climatic factors on the manifestation of Spilocaea oleagina, olive peacock spot agent in the north western region of Morocco. Inter J. Pure Appl. Biosci. 2014, 2, 1–9. [Google Scholar]
- Ater, M.; Essalou, L.; Ilbert, H.; Moukhli, A.; Khadari, B. L’Oléiculture au maroc de la préhistoire à nos jours: Pratiques, diversité, adaptation, usages, commerce et politiques. Options Méditerranéennes Séminaires Méditerranéennes 2016, 2, 224. [Google Scholar]
- Couanon, W.; Le Verge, S.; Pinatel, C. L’OEIL DE PAON, les Fiches Techniques de l’AFIDOL; Centre Technique de l’Olivier: Aix-en-Provence, France, 2018; pp. 1–2. [Google Scholar]
- Roubal, C. Tavelures du Pommier et de L’olivier: Réalisation de Modèles Epidémiologiques Par des Méthodes Exploitant des Observations Biologiques Acquises au Verger. Sciences Agricoles; Université d’Avignon: Avignon, France, 2017; p. 186. [Google Scholar]
- Trapero, A.; Roca, R.; Luis, F.; Moral, J.; Trapero, C.; López-Escudero, F.J. El Olivar Ecologico, Chapter: Capítulo 7: Las Enfermedades y su Manejo en el Olivar Ecológico; Junta de Andalucía: Andalusia, Spain, 2011; pp. 206–256.
- Salman, M.; Hawamda, A.; Amarni, A.; Rahil, M.; Hajjeh, H.; Natsheh, B.; Abuamsha, R. Evaluation of the incidence and severity of olive leaf spot caused by Spilocaea oleagina on olive trees in Palestine. Am. J. Plant Sci. 2011, 2, 457–460. [Google Scholar] [CrossRef]
- Bartolini, G.; Prevost, G.; Messeri, C.; Carignani, C. Olive germplasm: Cultivars and world-wide collections. In FAO SaPGRSo; FAO: Rome, Italy, 2005. [Google Scholar]
- Khadari, B.; Charafi, J.; Moukhli, A.; Ater, M. Substantial genetic diversity in cultivated Moroccan olive despite a single major cultivar: A paradoxical situation evidenced by the use of SSR loci. Tree Genet. Genomes 2008, 4, 213–221. [Google Scholar] [CrossRef]
- Barguigua, A.; Zahir, I.; Youss, S.; Fikri, N.; Youss, B. Prospection des maladies microbiennes de l’olivier dans la région Tadla-Azilal. Rev. Mar. Sci. Agron. Vét. 2020, 8, 331–338. [Google Scholar]
- El Bakkali, A.; Mekkaoui, A.; El Iraqui, E.S.; Essarioui, A.; Khadari, B. Addressing the challenge of cultivars identification and authentication in mediterranean olive collections: A case study in morocco. Eur. Sci. J. 2020, 16, 339–355. [Google Scholar] [CrossRef]
- D-map. Available online: https://d-maps.com/index.php?lang=en (accessed on 31 August 2009).
- Google-Earth. Available online: https://www.google.com/intl/fr/earth/ (accessed on 20 March 2021).
- Teviotdale, B.L.; Sibbett, G.S.; Harper, D. Several copper fungicides control olive leaf spot. Cali. Agric. 1989, 43, 30–31. [Google Scholar]
- Shabi, E.; Birger, R.; Lavee, S. Leaf spot (Spilocaea oleagina) of olive in israel and it control. Acta Hortic. 1994, 356, 390–394. [Google Scholar] [CrossRef]
- Macdonald, A.J.; Walter, M.; Trought, M.; Frampton, C.M.; Burnip, G. Survey of olive leaf spot in New Zealand. Plant Prot. 2000, 53, 126–132. [Google Scholar] [CrossRef]
- Jeger, M.J.; Viljanen-Rollinson, S.L.H. The use of the area under disease-progress curve (AUDPC) to assess quantitative disease resistance in crop cultivars. Theor. Appl. Genet. 2001, 102, 32–40. [Google Scholar] [CrossRef]
- Singh, P.V.; Billore, R.P. Relationship between chlorophyll and energy contents of the Andropogon grassland community. Photosynthetica 1975, 9, 93–95. [Google Scholar]
- Sanders, T.H.; McMichael, R.W.; Hendrix, K.W. Occurrence of resveratrol in edible peanuts. J. Agric. Food Chem. 2020, 48, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Bolling, B.W. Characterisation of stilbenes in California almonds (Prunus dulcis) by UHPLC–MS. Food Chem. 2014, 148, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of total phenolics. Food Annal. Chem. 2002, 6, 111–118. [Google Scholar]
- Barreira, J.C.; Ferreira, I.C.; Oliveira, M.; Pereira, J.A. Antioxidant activities of the extracts from chestnut fower leaf skins and fruit. Food Chem. 2018, 107, 1106–1113. [Google Scholar] [CrossRef]
- Bock, C.H.; Chiang, K.S.; Del Ponte, E.M. Plant disease severity estimated visually: A century of research, best practices, and opportunities for improving methods and practices to maximize accuracy. Tropi. Plant Pathol. 2021, 47, 25–42. [Google Scholar] [CrossRef]
- Seem, R. Disease incidence and severity relationships. Ann. Rev. Phytopathol. 1984, 22, 133–150. [Google Scholar] [CrossRef]
- Sanei, S.J.; Razavi, S.E. Differentiation of olive Colletotrichum gloeosporioides populations on the basis of vegetative compatibility and pathogenicity. Afri. J. Agricul. Res. 2011, 6, 2099–2107. [Google Scholar]
- Kleef, F.; Salman, M. Antifungal effect of Ambrosia artemisiifolia L. Extract and chemical fungicide against Spilocaea oleagina causing olive leaf spot. Arab. J. Sci. Eng. 2022, 43, 113–117. [Google Scholar] [CrossRef]
- Obanor, F.O.; Jaspers, M.; Jones, E.; Walter, M. Green house and field evaluation of fungicides for control of olive leaf spot in New Zealand. Crop. Prot. 2008, 120, 211–222. [Google Scholar]
- Al-Khatib, M.; Alhussaen, K.; El-Banna, N.; Zyadeh, M. Biological control of olive leaf spot (peacock spot disease) caused by Cycloconium oleaginum (Spilocea oleagina). J. Microbiol. 2010, 2, 64–67. [Google Scholar]
- Guechi, A.; Girre, L. Sources of Cycloconium oleaginum (Cast.) conidia for infection of olive leaves and conditions determining leaf spot disease development in the region of Setif (Algeria). J. Mycopathol. 1994, 125, 163–171. [Google Scholar] [CrossRef]
- Obanor, F.O.; Walter, M.; Jones, E.; Jaspers, M.V. Sources of variation in a field evaluation of the incidence and severity of olive leaf spot. N. Z. Plant. Prot. 2005, 58, 273–277. [Google Scholar] [CrossRef]
- Graniti, A. Olive scab: A review. Bull. OEPP 1993, 23, 377–384. [Google Scholar] [CrossRef]
- Roca, L.F.; Juan, M.M.; Jose, R.V.; Arantxa, A.; Rodrigues, O.; Trapero, A. Copper Fungicides in the Control of Olive Diseases; FAO Olive Network: Rome, Italy, 2007. [Google Scholar]
- Benítez, Y.; Botella, M.A.; Trapero, A.; Alsalimiya, M.; Caballero, J.L.; Dorado, G.; Muñoz-Blanco, J. Molecular analysis of the interaction between Olea europaea and the biotrophic fungus Spilocaea oleagina. Mol. Plant Pathol. 2005, 6, 425–438. [Google Scholar] [CrossRef]
- Bartolini, G. OLEA Databases. Available online: http://www.oleadb.it/ (accessed on 31 August 2009).
- Rahioui, B.; Aissam, S.; Messaouri, H.; Moukhli, A.; Khadari, B.; El Modafar, C. Role of phenolic metabolism in the defense of the olive-tree against leaf-spot disease caused by Spilocaea oleagina. Int. J. Agric. Biol. 2013, 15, 273–278. [Google Scholar]
- Ouerghi, F.; Rhouma, A.; Rassaa, N.; Hennachi, I.; Bouzid, N. Factors affecting resistance of two olive cultivars to leaf spot disease in the North-West of Tunisia. Eur. J. Adv. Res. Biol. Life Sci. 2017, 4, 39–51. [Google Scholar]
- Barranco, N.D.; Cimato, A.; Fiorino, P.; Rallo, R.L.; Touzani, A.; Castañeda, C.; Serafini, E.; Trujillo, N.I. World Catalogue of Olive Varieties, COI; Mundi-Prensa: Madrid, Spain, 2000; p. 360. [Google Scholar]
- Carla, M.R.; Varanda, A.; Patrick, M.; Miguel, L.; Maria, D.; Campos, A.; Maria, R. Fungal communities associated with peacock and cercospora leaf spots in olive. Plant 2019, 8, 169. [Google Scholar]
- Rallo, L.; Barranco, D.; Caballero, J.M.; Del Rio, C.; Martin, A.; Tous, J.; Trujillo, I. Variedades de Olivoen Espana; Mundi-Prensa: Madrid, Spain, 2005; p. 480. (In Spanish) [Google Scholar]
- Hadiddou, A.; Oukabli, A.; Moudaffar, C.; Mamouni, A.; Gaboun, F.; Mekaoui, A.; El Fechtali, M. Evaluation des performances de production de 14 variétés de l’olivier (Olea europaea L.) Nationales et méditerranéennes dans deux systèmes contrastés de culture (pluvial et irrigué) au Maroc. Al Awamia 2013, 127, 22–43. [Google Scholar]
- Moral, J.; Ávila, L.M.; López-Doncel, M.; Alsalimiya, R.; Oliveira, F.; Gutiérrez, N.; Benali, A.; Roca, L.F.; Navarro, N.; Bouhmidi, K.; et al. Resistencia a los repilos de distintas variedades de olivo. Vida Rural. 2005, 208, 34–41. [Google Scholar]
- Adawi, A.; Jarrar, S.; Almadi, L.; Alkowni, R.; Gallo, M.; D’Onghia, A.M.; Buonaurio, R.; Famiani, F. Effectiveness of low copper-containing chemicals against olive leaf spot disease caused by Venturia oleaginea. Agriculture 2022, 12, 326. [Google Scholar] [CrossRef]
- Trigui, M.; Ben Hsouna, A.; Hammami, I.; Culioli, G.; Ksantini, M.; Tounsi, S.; Jaoua, S. Efficacy of Lawsonia inermis leaves extract and its phenolic compounds against olive knot and crown gall diseases. Crop. Prot. 2013, 45, 83–88. [Google Scholar] [CrossRef]
- López-Doncel, L.M.; Viruega, J.R.; Trapero, A. Respuesta del olivo a la inoculación con Spilocaea oleagina, agente del repilo. Bol. San. Veg. Plagas. 2000, 26, 349–363. [Google Scholar]
- Sergeeva, V.; Braun, U.; Spooner-Hart, R.; Nair, N. Observations on spot caused by Fusicladium oleagineum on olives (Olea europaea) in new south Wales, Australia. Australas. Plant Dis. 2009, 4, 26–28. [Google Scholar]
- Jayaramraja, P.R.; Pius, P.K.; Manian, S.; Meenakshi, T.S. Certain factors associated with blister blight resistance in Camellia sinensis (L.). Physiol. Mol. Plant Pathol. 2005, 67, 291–295. [Google Scholar] [CrossRef]
- Rhouma, A.; Chttaoui, M.; Krid, S.; Elbsir, H.; Msallem, M.; Triki, M.A. Evaluation of susceptibility of an olive progeny (Picholine x Meski) to olive leaf spot disease caused by Fusicladium oleagineum. Eur. J. Plant Pathol. 2013, 135, 23–33. [Google Scholar] [CrossRef]
- Lanza, B.; Di Serio, M.G.; Di Giovacchino, L. Long-term spreading of olive mill wastewater on olive orchard: Effects on olive production, oil quality, and soil properties. Commun. Soil. Sci. Plant Anal. 2017, 48, 2420–2433. [Google Scholar] [CrossRef]
- Viruega, J.R.; Roca, L.F.; Moral, J.; Trapero, A. Factors affecting infection and disease development on olive leaves inoculated with Fusicladium oleagineum. Plant Dis. 2011, 95, 1139–1146. [Google Scholar] [CrossRef]
- Tajnari, H. Étude bio-écologique d’Euphyllura olivina Costa (Hom. Psyllidae) Dans les Régions du Haouz et d’Essaouira: Mise en Evidence d ‘un état de Diapause Ovarienne. Master’s Thesis, École Nationale D’agriculture, Meknes, Maroc, 1992; 153p. [Google Scholar]
- Veresoglou, S.D.; Barto, E.K.; Menexes, G.; Rillig, M.G. Fertilization affects severity of disease caused by fungal plant pathoges. Plant Pathol. 2012, 62, 961–969. [Google Scholar] [CrossRef]
- Fernandez, E.R.; Beltran, G.; Sanchez-Zamora, M.A.; García-Novelo, J.; Aguilera, M.; Uceda, M. Olive oil quality decreases with nitrogen over fertilization. Hort. Sci. 2006, 41, 215–219. [Google Scholar]

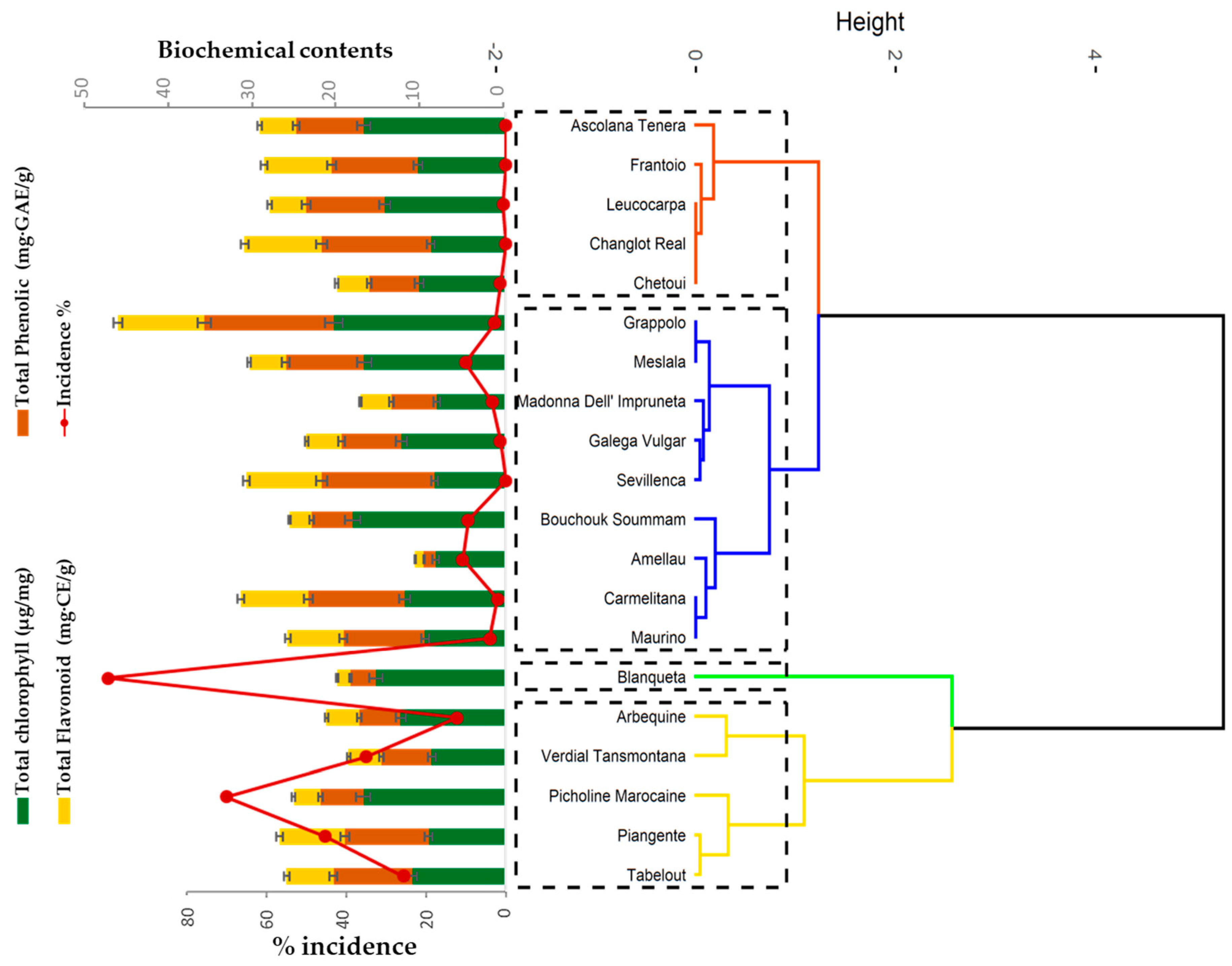
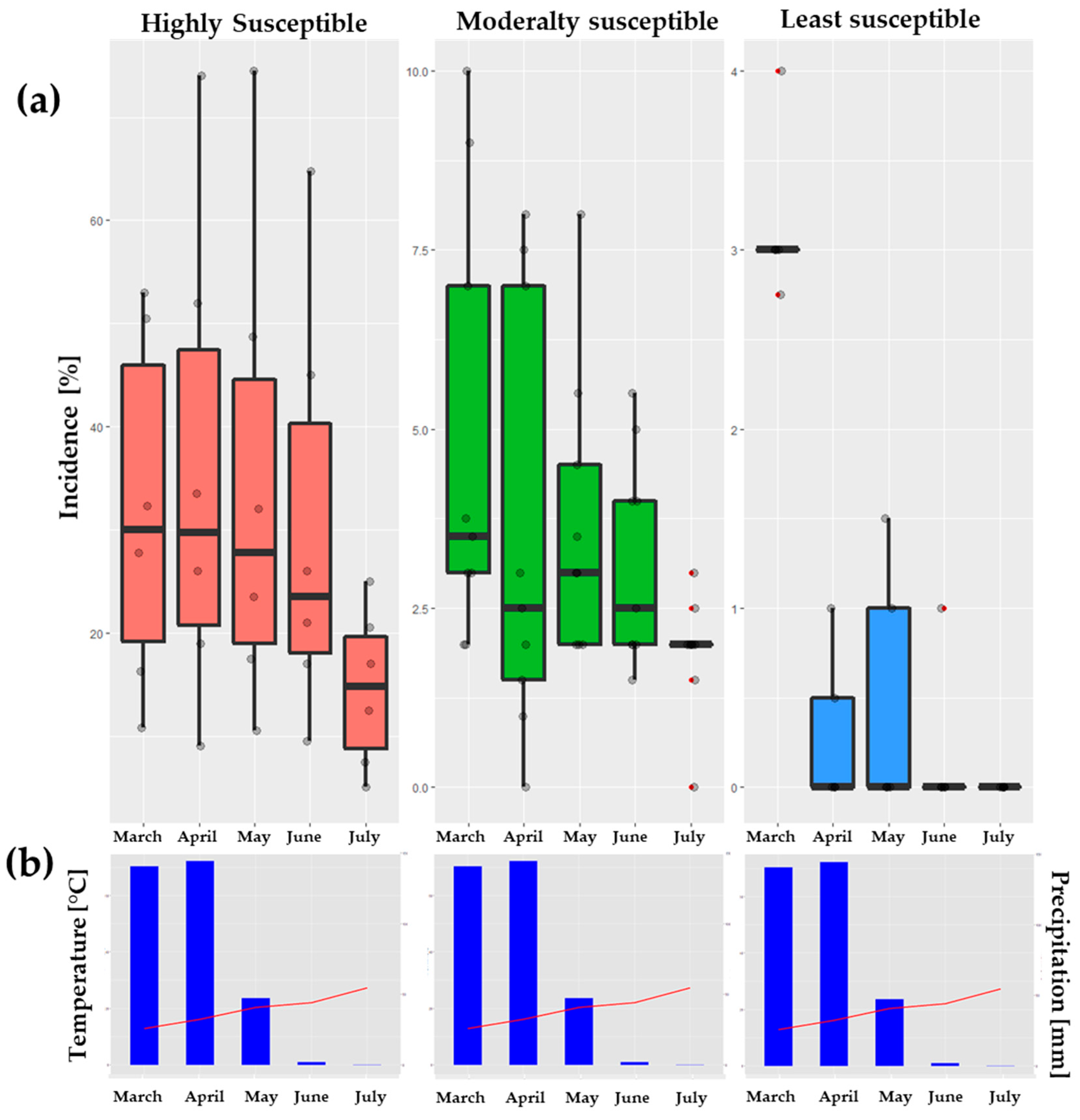
| Incidence (%) | Severity (%) | |
|---|---|---|
| Cultivars | 352.34 *** | 94.36 *** |
| Month | 1180.59 *** | 242.77 *** |
| Cultivars—Month | 113.19 *** | 31.30 ** |
| Error | 59.834 | 16.843 |
| R2 | 0.772 | 0.755 |
| Incidence (%) | Severity (%) | |
|---|---|---|
| Cultivars | 693,963.05 * | 223,763.39 * |
| Error | 304,815.19 | 96,429.702 |
| R2 | 0.684 | 0.688 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habbadi, K.; Maafa, I.; Benbouazza, A.; Aoujil, F.; Choukri, H.; El Houssaini, S.E.I.; El Bakkali, A. Differential Response of Olive Cultivars to Leaf Spot Disease (Fusicladium oleagineum) under Climate Warming Conditions in Morocco. Horticulturae 2023, 9, 589. https://doi.org/10.3390/horticulturae9050589
Habbadi K, Maafa I, Benbouazza A, Aoujil F, Choukri H, El Houssaini SEI, El Bakkali A. Differential Response of Olive Cultivars to Leaf Spot Disease (Fusicladium oleagineum) under Climate Warming Conditions in Morocco. Horticulturae. 2023; 9(5):589. https://doi.org/10.3390/horticulturae9050589
Chicago/Turabian StyleHabbadi, Khaoula, Ilyass Maafa, Abdellatif Benbouazza, Faiçal Aoujil, Hasnae Choukri, Salma El Iraqui El Houssaini, and Ahmed El Bakkali. 2023. "Differential Response of Olive Cultivars to Leaf Spot Disease (Fusicladium oleagineum) under Climate Warming Conditions in Morocco" Horticulturae 9, no. 5: 589. https://doi.org/10.3390/horticulturae9050589







