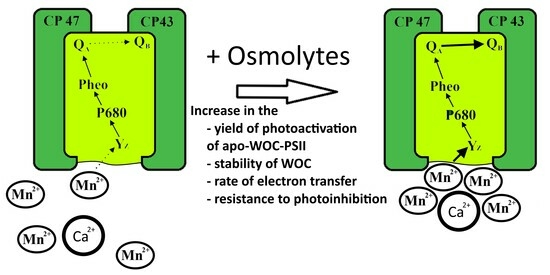
Effect of Osmolytes on Photoassembly of Functionally Active Mn4CaO5 Cluster in Mn-Depleted Photosystem II Preparations Isolated from Spinach Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Treatment of PSII Preparations
2.2. Procedure for Photoassembly of Functionally Active Mn4CaO5 Cluster in apo-WOC-PSII Preparations and Measurement of Oxygen-Evolving Activity
2.3. Mn2+ Photooxidation in apo-WOC-PSII and Mn-Dependent Protection of apo-WOC-PSII Preparations against Photoinhibition
2.4. Determination of Stability of CaCl2-PSII Preparations
2.5. Estimation of Heat-Induced Inhibition of Electron Transfer in PSII
2.6. Ultrasonic Interferometry and Refractometry
2.7. Statistical Analysis
3. Results
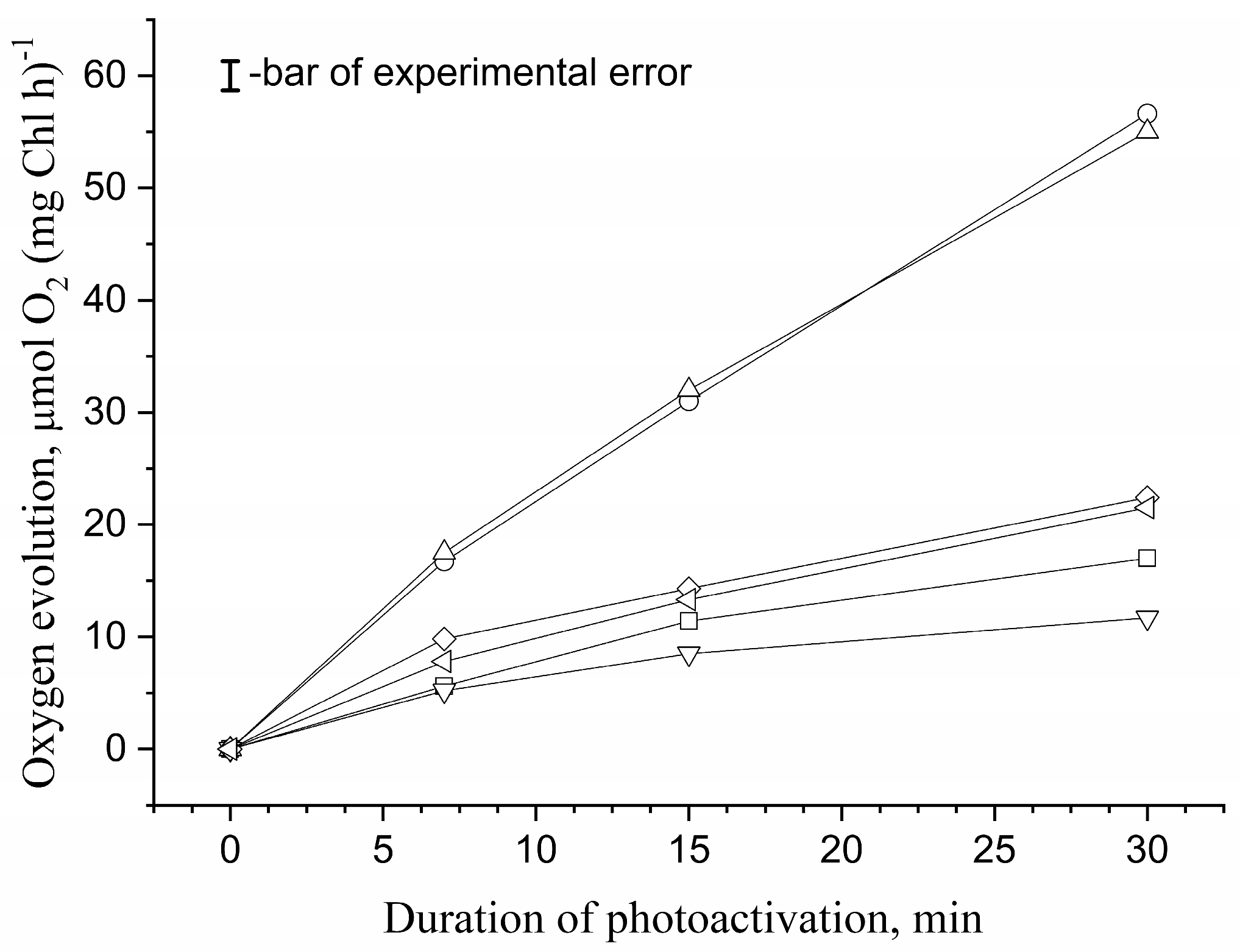
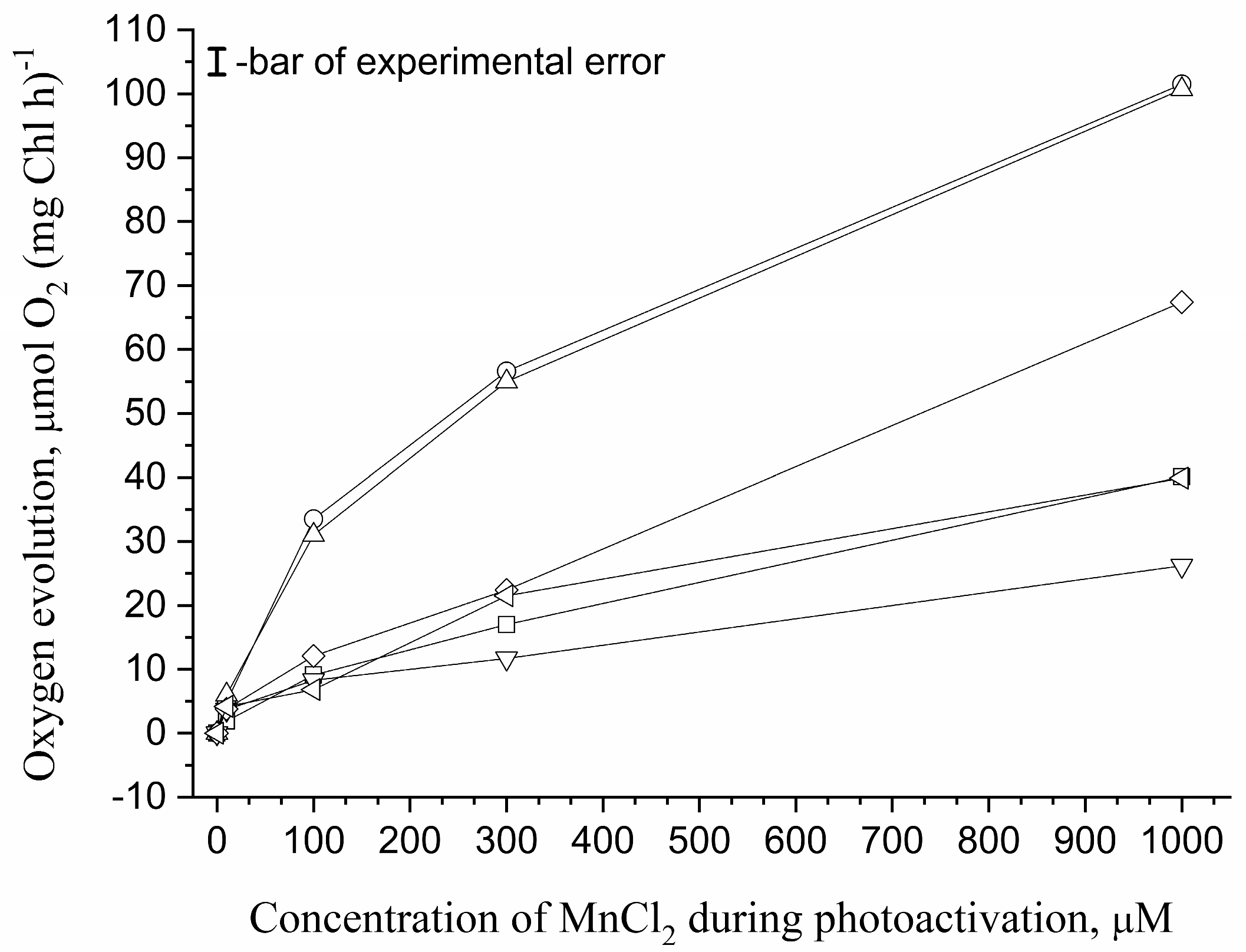
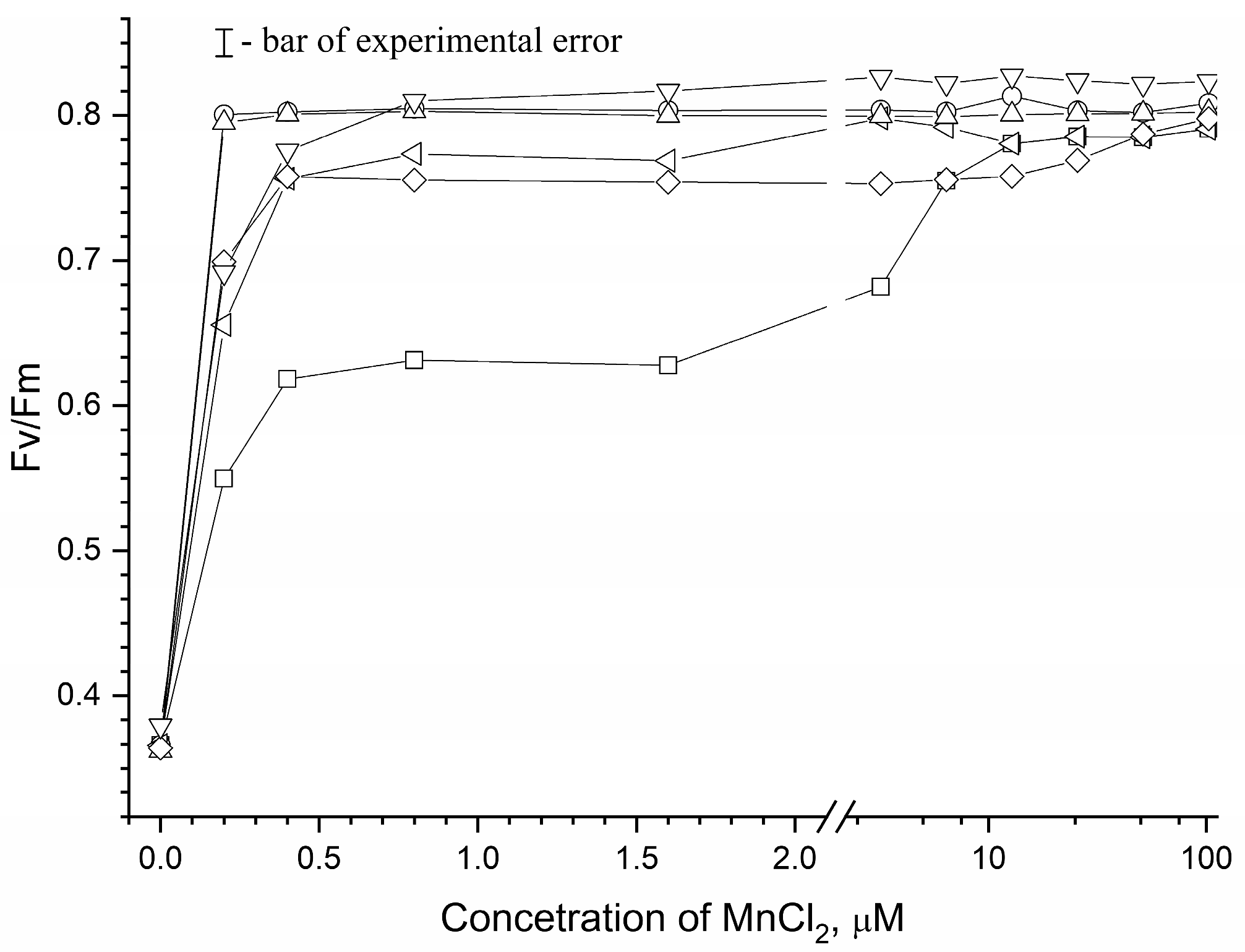
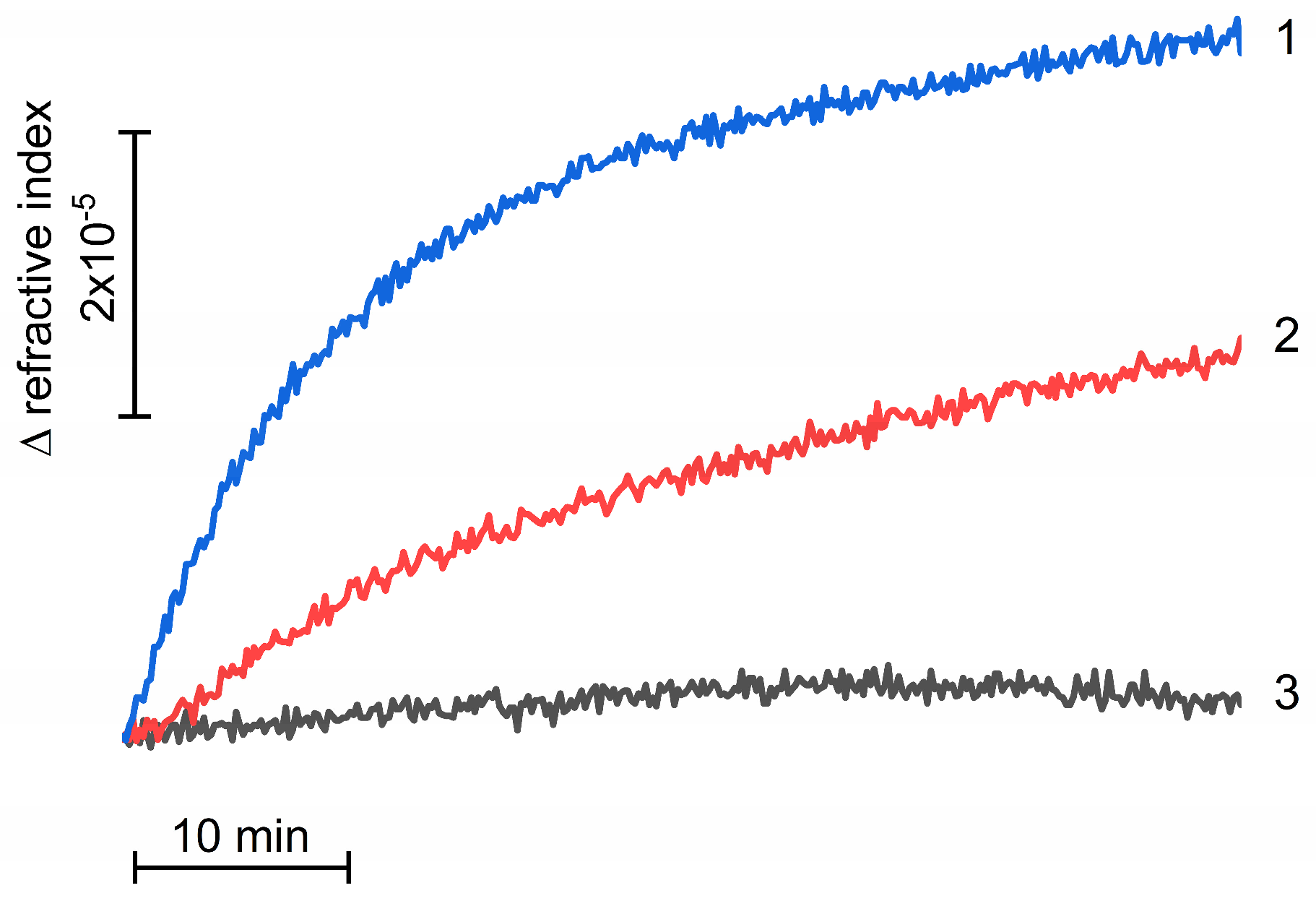
3.1. Effect of Osmolytes on Photoassembly of Mn4CaO5 Cluster in apo-WOC-PSII Preparations
3.2. Effect of Osmolytes on Mn2+ Photooxidation in apo-WOC-PSII Preparations
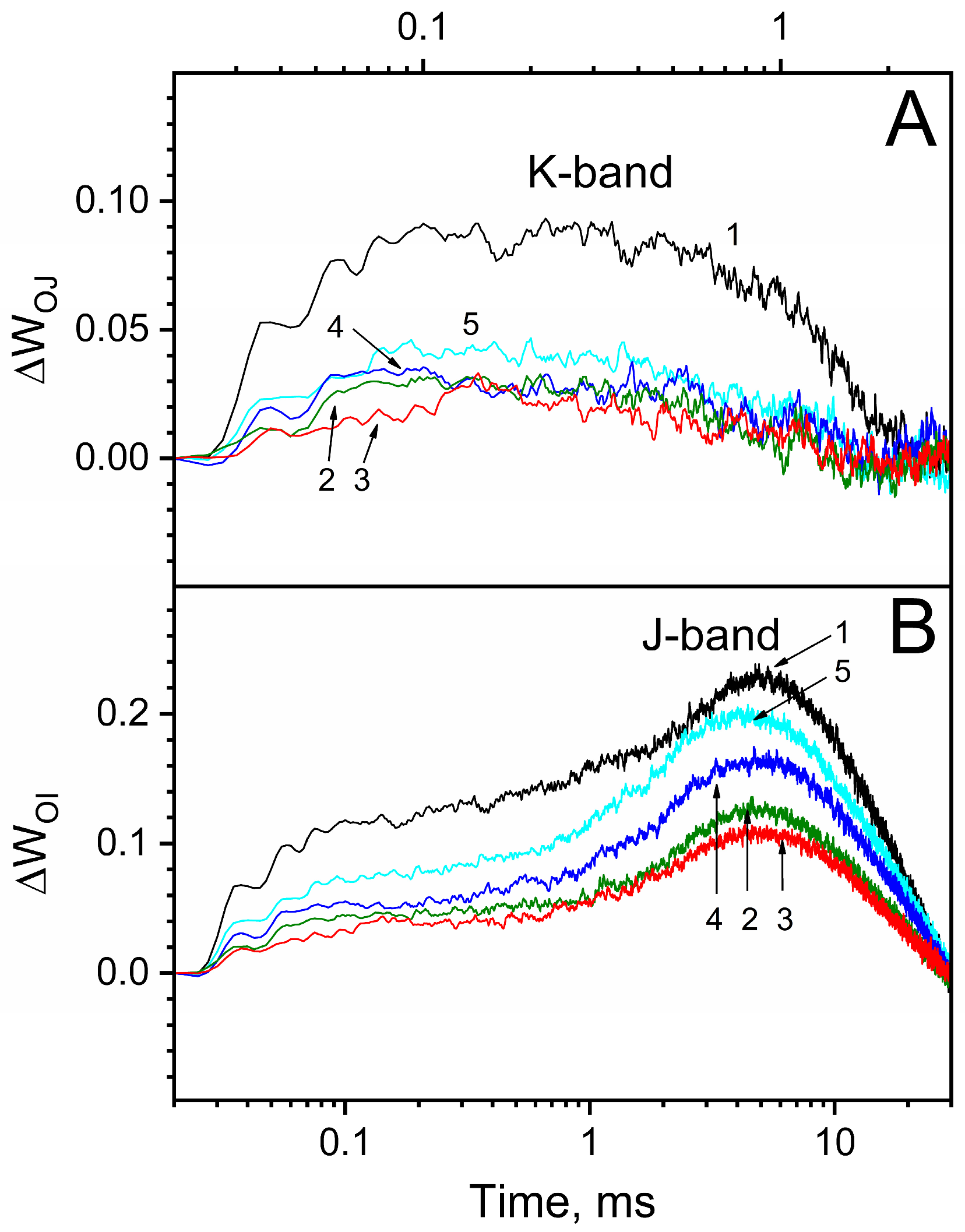
3.3. Effect of Osmolytes on Mn-Induced Protection of apo-WOC-PSII against Photoinhibition
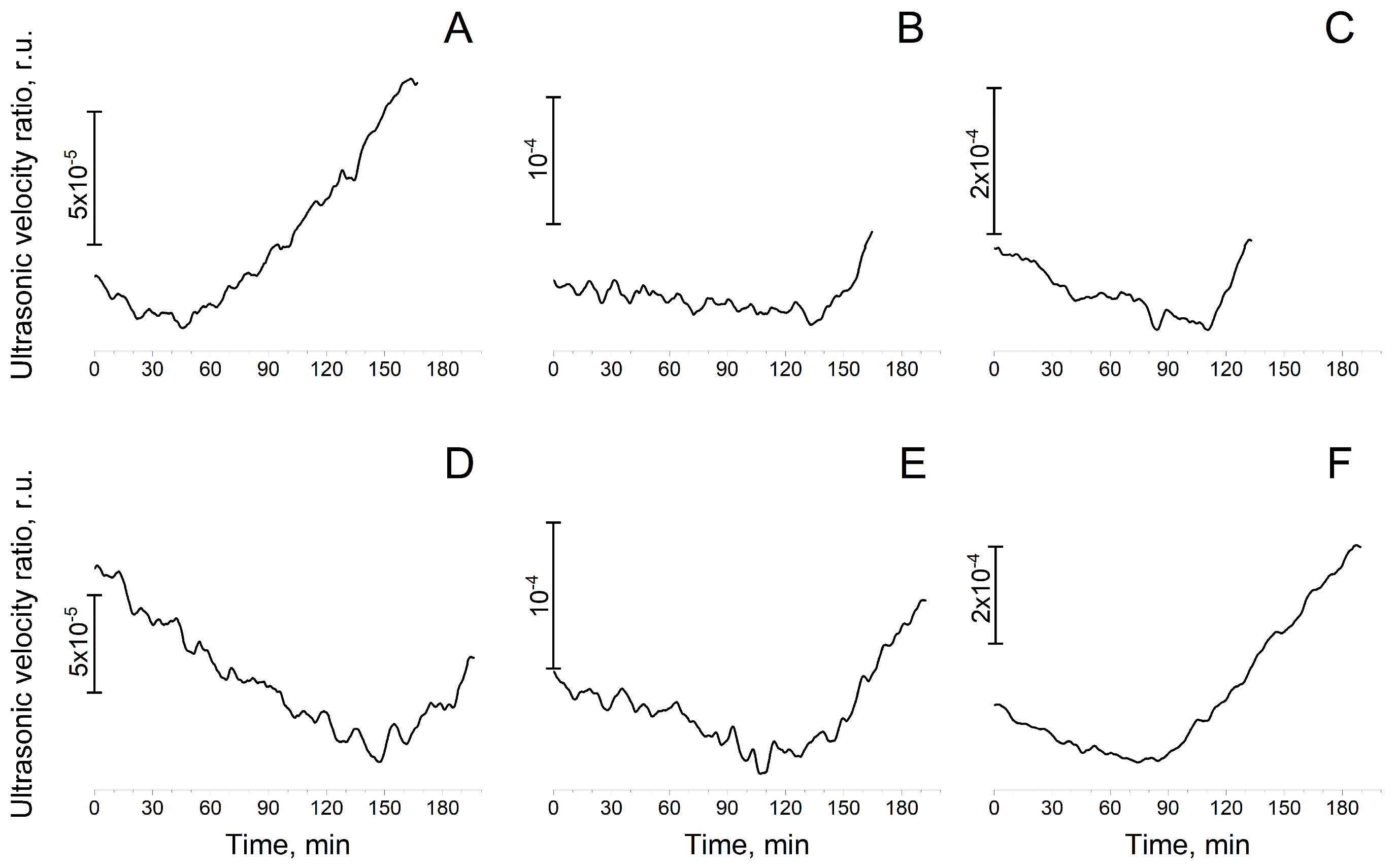
3.4. Osmolyte Effect on Stability of Mn4CaO5 Cluster in CaCl2-PSII Preparations
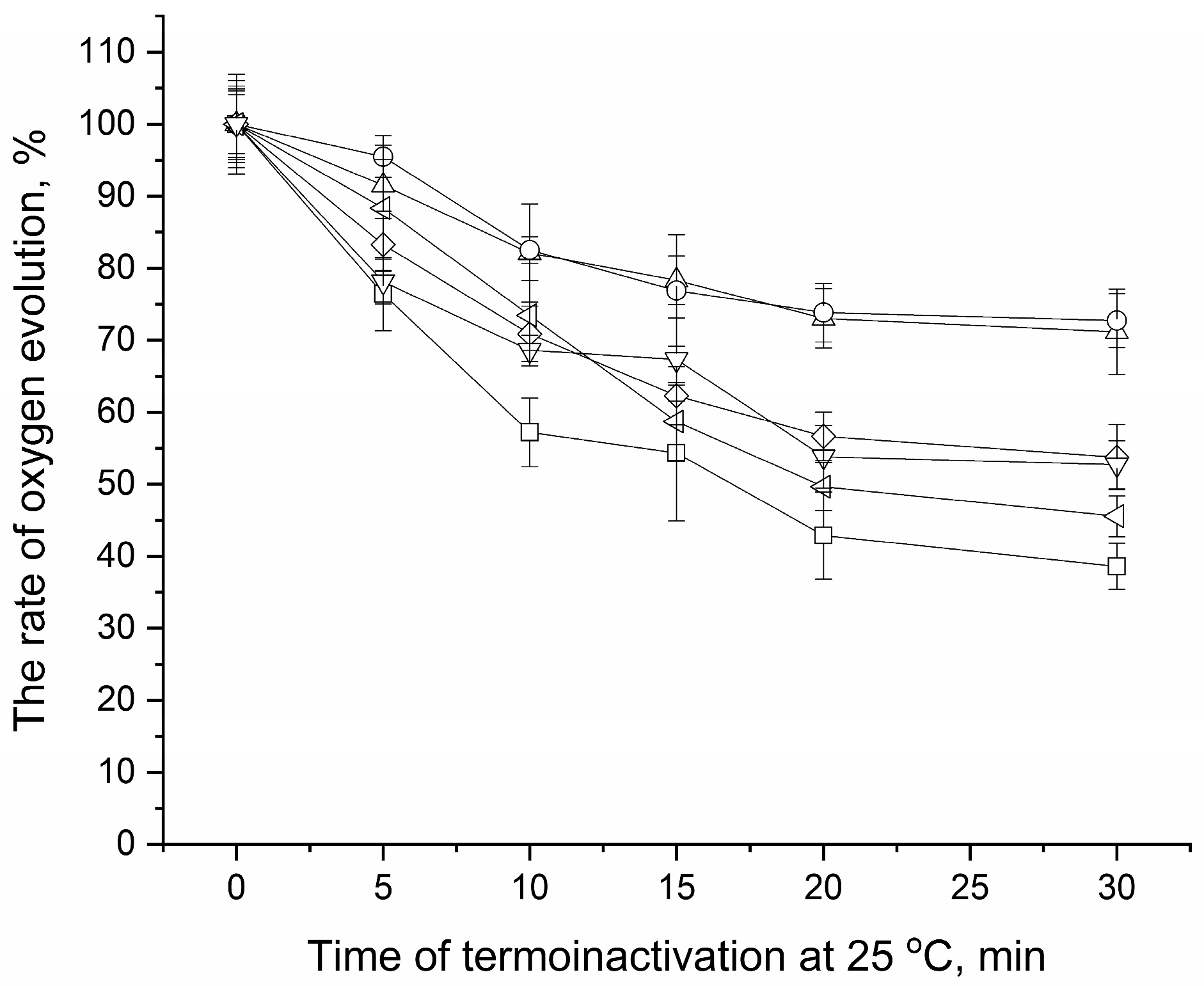
3.5. Effect of Osmolytes on Heat-Induced Inhibition of Electron Transfer in PSII
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimov, V.; Allakhverdiev, S.; Demeter, S.; Krasnovsky, A. Photoreduction of pheophytin in chloroplast photosystem II as a function of the redox potential of the medium. Dokl. Akad. Nauk SSSR 1979, 249, 227–230. [Google Scholar]
- Ishikita, H.; Loll, B.; Biesiadka, J.; Saenger, W.; Knapp, E.-W. Redox potentials of chlorophylls in the photosystem II reaction center. Biochemistry 2005, 44, 4118–4124. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Tomo, T.; Shimada, Y.; Kindo, H.; Nagao, R.; Klimov, V.V.; Mimuro, M. Redox potential of pheophytin a in photosystem II of two cyanobacteria having the different special pair chlorophylls. Proc. Natl. Acad. Sci. USA 2010, 107, 3924–3929. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M.M. Light Stress at High Temperature; Elsevier: New York, NY, USA, 1987. [Google Scholar]
- Masojídek, J.; Trivedi, S.; Halshaw, L.; Alexiou, A.; Hall, D.O. The synergistic effect of drought and light stresses in sorghum and pearl millet. Plant Physiol. 1991, 96, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Ögren, E. The significance of photoinhibition for photosynthetic productivity. In Photoinhibition of Photosynthesis from Molecular Mechanisms to the Field; Bios Scientific Publishers: Oxford, UK, 1994; pp. 433–447. [Google Scholar]
- Goh, C.-H.; Ko, S.-M.; Koh, S.; Kim, Y.-J.; Bae, H.-J. Photosynthesis and environments: Photoinhibition and repair mechanisms in plants. J. Plant Biol. 2012, 55, 93–101. [Google Scholar] [CrossRef]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Tikkanen, M.; Grieco, M.; Nurmi, M.; Rantala, M.; Suorsa, M.; Aro, E.-M. Regulation of the photosynthetic apparatus under fluctuating growth light. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3486–3493. [Google Scholar] [CrossRef]
- Powles, S.B. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 1984, 35, 15–44. [Google Scholar] [CrossRef]
- Aro, E.-M.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta (BBA)—Bioenerg. 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar]
- Radmer, R.; Cheniae, G.M. Photoactivation of the manganese catalyst of O2 evolution. II. A two-quantum mechanism. Biochim. Biophys. Acta (BBA)—Bioenerg. 1971, 253, 182–186. [Google Scholar] [CrossRef]
- Tamura, N.; Cheniae, G. Requirements for the photoligation of Mn2+ in PS II membranes and the expression of water-oxidizing activity of the polynuclear Mn-catalyst. FEBS Lett. 1986, 200, 231–236. [Google Scholar] [CrossRef]
- Tamura, N.; Cheniae, G. Photoactivation of the water-oxidizing complex in photosystem II membranes depleted of Mn and extrinsic proteins. I. Biochemical and kinetic characterization. Biochim. Biophys. Acta (BBA)—Bioenerg. 1987, 890, 179–194. [Google Scholar] [CrossRef]
- Miller, A.F.; Brudvig, G.W. Manganese and calcium requirements for reconstitution of oxygen-evolution activity in manganese-depleted photosystem II membranes. Biochemistry 1989, 28, 8181–8190. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Inoue, Y.; Cheniae, G.M. Photoactivation of the water-oxidizing complex in Photosystem II membranes depleted of Mn, Ca and extrinsic proteins: II. Studies on the functions of Ca2+. Biochim. Biophys. Acta (BBA)—Bioenerg. 1989, 976, 173–181. [Google Scholar] [CrossRef]
- Miyao, M.; Inoue, Y. An improved procedure for photoactivation of photosynthetic oxygen evolution: Effect of artificial electron acceptors on the photoactivation yield of NH2OH-treated wheat photosystem II membranes. Biochim. Biophys. Acta (BBA)—Bioenerg. 1991, 1056, 47–56. [Google Scholar] [CrossRef]
- Ananyev, G.M.; Dismukes, G.C. Assembly of the tetra-Mn site of photosynthetic water oxidation by photoactivation: Mn stoichiometry and detection of a new intermediate. Biochemistry 1996, 35, 4102–4109. [Google Scholar] [CrossRef]
- Ananyev, G.M.; Dismukes, G.C. High-resolution kinetic studies of the reassembly of the tetra-manganese cluster of photosynthetic water oxidation: Proton equilibrium, cations, and electrostatics. Biochemistry 1996, 35, 14608–14617. [Google Scholar] [CrossRef]
- Ananyev, G.M.; Dismukes, G.C. Calcium induces binding and formation of a spin-coupled dimanganese (II,II) center in the apo-water oxidation complex of photosystem II as precursor to the functional tetra-Mn/Ca cluster. Biochemistry 1997, 36, 11342–11350. [Google Scholar] [CrossRef]
- Zaltsman, L.; Ananyev, G.M.; Bruntrager, E.; Dismukes, G.C. Quantitative kinetic model for photoassembly of the photosynthetic water oxidase from its inorganic constituents: Requirements for manganese and calcium in the kinetically resolved steps. Biochemistry 1997, 36, 8914–8922. [Google Scholar] [CrossRef]
- Baranov, S.V.; Ananyev, G.M.; Klimov, V.V.; Dismukes, G.C. Bicarbonate accelerates assembly of the inorganic core of the water-oxidizing complex in manganese-depleted photosystem II: A proposed biogeochemical role for atmospheric carbon dioxide in oxygenic photosynthesis. Biochemistry 2000, 39, 6060–6065. [Google Scholar] [CrossRef] [PubMed]
- Baranov, S.; Tyryshkin, A.; Katz, D.; Dismukes, G.; Ananyev, G.; Klimov, V. Bicarbonate is a native cofactor for assembly of the manganese cluster of the photosynthetic water oxidizing complex. Kinetics of reconstitution of O2 evolution by photoactivation. Biochemistry 2004, 43, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Miyao-Tokutomi, M.; Inoue, Y. Improvement by benzoquinones of the quantum yield of photoactivation of photosynthetic oxygen evolution: Direct evidence for the two-quantum mechanism. Biochemistry 1992, 31, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Khorobrykh, A. A possible relationship between the effect of factors on photoactivation of photosystem II depleted of functional Mn and cytochrome b559. Biochim. Biophys. Acta (BBA)—Bioenerg. 2023, 1864, 148997. [Google Scholar] [CrossRef] [PubMed]
- Khorobrykh, A.A.; Yanykin, D.V.; Klimov, V.V. Enhancement of photoassembly of the functionally active water-oxidizing complex in Mn-depleted photosystem II membranes upon transition to anaerobic conditions. J. Photochem. Photobiol. B Biol. 2016, 163, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, J.; Ananyev, G.M.; Dismukes, G.C. Photoassembly of the water-oxidizing complex in photosystem II. Coord. Chem. Rev. 2008, 252, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Burnap, R.L. Photoactivation: The light-driven assembly of the water oxidation complex of photosystem II. Front. Plant Sci. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bommer, M.; Chatterjee, R.; Hussein, R.; Yano, J.; Dau, H.; Kern, J.; Dobbek, H.; Zouni, A. Structural insights into the light-driven auto-assembly process of the water-oxidizing Mn4CaO5-cluster in photosystem II. eLife 2017, 6, e26933. [Google Scholar] [CrossRef]
- Klimov, V.; Shafiev, M.; Allakhverdiev, S. Photoinactivation of the reactivation capacity of photosystem II in pea subchloroplast particles after a complete removal of manganese. Photosynth. Res. 1990, 23, 59–65. [Google Scholar] [CrossRef]
- Jegerschoeld, C.; Virgin, I.; Styring, S. Light-dependent degradation of the D1 protein in photosystem II is accelerated after inhibition of the water splitting reaction. Biochemistry 1990, 29, 6179–6186. [Google Scholar] [CrossRef]
- Telfer, A.; De Las Rivas, J.; Barber, J. β-Carotene within the isolated Photosystem II reaction centre: Photooxidation and irreversible bleaching of this chromophore by oxidised P680. Biochim. Biophys. Acta (BBA)—Bioenerg. 1991, 1060, 106–114. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Chapman, D.J.; Barber, J. Inhibition of water splitting increases the susceptibility of photosystem II to photoinhibition. Plant Physiol. 1992, 99, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Allakhverdiev, S.I.; Hurry, V.; Hüner, N.P. Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth. Res. 2015, 126, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Yu, X.; Kikuchi, A.; Asahina, M.; Watanabe, K.N. Genetic engineering of glycine betaine biosynthesis to enhance abiotic stress tolerance in plants. Plant Biotechnol. 2009, 26, 125–134. [Google Scholar] [CrossRef]
- Jewell, M.C.; Campbell, B.C.; Godwin, I.D. Transgenic plants for abiotic stress resistance. In Transgenic Crop Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 67–132. [Google Scholar]
- Kumar, V.; Khare, T. Individual and additive effects of Na+ and Cl− ions on rice under salinity stress. Arch. Agron. Soil Sci. 2015, 61, 381–395. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Sicher, R.C.; Timlin, D.; Bailey, B. Responses of growth and primary metabolism of water-stressed barley roots to rehydration. J. Plant Physiol. 2012, 169, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Whittaker, A.; Bochicchio, A.; Vazzana, C.; Lindsey, G.; Farrant, J. Changes in leaf hexokinase activity and metabolite levels in response to drying in the desiccation-tolerant species Sporobolus stapfianus and Xerophyta viscosa. J. Exp. Bot. 2001, 52, 961–969. [Google Scholar] [CrossRef]
- Moore, J.P.; Hearshaw, M.; Ravenscroft, N.; Lindsey, G.G.; Farrant, J.M.; Brandt, W.F. Desiccation-induced ultrastructural and biochemical changes in the leaves of the resurrection plant Myrothamnus flabellifolia. Aust. J. Bot. 2007, 55, 482–491. [Google Scholar] [CrossRef]
- Peters, S.; Mundree, S.G.; Thomson, J.A.; Farrant, J.M.; Keller, F. Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): Both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. J. Exp. Bot. 2007, 58, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, T. In situ localization of glucose and sucrose in dehydrating leaves of Sporobolus stapfianus. J. Plant Physiol. 2008, 165, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.; Walton, L.J.; Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 2005, 388, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Crowe, L.M. Lessons from nature: The role of sugars in anhydrobiosis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Francia, F.; Malferrari, M.; Sacquin-Mora, S.; Venturoli, G. Charge recombination kinetics and protein dynamics in wild type and carotenoid-less bacterial reaction centers: Studies in trehalose glasses. J. Phys. Chem. B 2009, 113, 10389–10398. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef]
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Anhydrobiosis. Annu. Rev. Physiol. 1992, 54, 579–599. [Google Scholar] [CrossRef]
- Jun, S.-S.; Choi, H.J.; Lee, H.Y.; Hong, Y.-N. Differential protection of photosynthetic capacity in trehalose-and LEA protein-producing transgenic plants under abiotic stresses. J. Plant Biol. 2008, 51, 327–336. [Google Scholar] [CrossRef]
- Williams, B.; Njaci, I.; Moghaddam, L.; Long, H.; Dickman, M.B.; Zhang, X.; Mundree, S. Trehalose accumulation triggers autophagy during plant desiccation. PLoS Genet. 2015, 11, e1005705. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Hossain, M.A.; Fujita, M.; Tran, L.-S.P. Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci. Rep. 2015, 5, 11433. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Schluepmann, H.; Delatte, T.L.; Wingler, A.; Silva, A.B.; Fevereiro, P.S.; Jansen, M.; Fiorani, F.; Wiese-Klinkenberg, A.; Paul, M.J. Regulation of growth by the trehalose pathway: Relationship to temperature and sucrose. Plant Signal. Behav. 2013, 8, e26626. [Google Scholar] [CrossRef] [PubMed]
- Zentella, R.; Mascorro-Gallardo, J.O.; Van Dijck, P.; Folch-Mallol, J.; Bonini, B.; Van Vaeck, C.; Gaxiola, R.; Covarrubias, A.A.; Nieto-Sotelo, J.; Thevelein, J.M. A Selaginella lepidophylla trehalose-6-phosphate synthase complements growth and stress-tolerance defects in a yeast tps1 mutant. Plant Physiol. 1999, 119, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Fujimura, Y.; Murata, N. Protection of the oxygen-evolving photosystem II complex by glycinebetaine. Biochim. Biophys. Acta (BBA)—Bioenerg. 1991, 1057, 361–366. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Murata, N. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynth. Res. 1995, 44, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.; Hayashi, H.; Papageorgiou, G.; Murata, N. Stabilization of the Mn-cluster of the oxygen-evolving complex by glycinebetaine. Biochim. Biophys. Acta (BBA)—Bioenerg. 1993, 1144, 92–96. [Google Scholar] [CrossRef]
- Allakhverdiev, S.; Feyziev, Y.M.; Ahmed, A.; Hayashi, H.; Aliev, J.A.; Klimov, V.; Murata, N.; Carpentier, R. Stabilization of oxygen evolution and primary electron transport reactions in photosystem II against heat stress with glycinebetaine and sucrose. J. Photochem. Photobiol. B Biol. 1996, 34, 149–157. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Hayashi, H.; Nishiyama, Y.; Ivanov, A.G.; Aliev, J.A.; Klimov, V.V.; Murata, N.; Carpentier, R. Glycinebetaine protects the D1/D2/Cytb559 complex of photosystem II against photo-induced and heat-induced inactivation. J. Plant Physiol. 2003, 160, 41–49. [Google Scholar] [CrossRef]
- Klimov, V.V.; Allakhverdiev, S.I.; Nishiyama, Y.; Khorobrykh, A.A.; Murata, N. Stabilization of the oxygen-evolving complex of photosystem II by bicarbonate and glycinebetaine in thylakoid and subthylakoid preparations. Funct. Plant Biol. 2003, 30, 797–803. [Google Scholar] [CrossRef]
- Yanykin, D.; Khorobrykh, A.; Mamedov, M.; Klimov, V. Trehalose protects Mn-depleted photosystem 2 preparations against the donor-side photoinhibition. J. Photochem. Photobiol. B Biol. 2016, 164, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Halverson, K.M.; Barry, B.A. Sucrose and glycerol effects on photosystem II. Biophys. J. 2003, 85, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, M.; Hayashi, H.; Wada, H.; Mohanty, P.; Papageorgiou, G.; Murata, N. Glycinebetaine enhances and stabilizes the evolution of oxygen and the synthesis of ATP by cyanobacterial thylakoid membranes. FEBS Lett. 1991, 294, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Mohanty, P.; Hayashi, H.; Papageorgiou, G. Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Lett. 1992, 296, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Yanykin, D.; Malferrari, M.; Rapino, S.; Venturoli, G.; Semenov, A.Y.; Mamedov, M. Hydroxyectoine protects Mn-depleted photosystem II against photoinhibition acting as a source of electrons. Photosynth. Res. 2019, 141, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Hincha, D.K. Low concentrations of trehalose protect isolated thylakoids against mechanical freeze-thaw damage. Biochim. Biophys. Acta (BBA)—Biomembr. 1989, 987, 231–234. [Google Scholar] [CrossRef]
- Apostolova, E.; Bushova, M.; Tenchov, B.; Murata, N. Freezing damage and protective of photosystem 2 by sucrose and trehalose. In Research Photosynthesis; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2005; pp. 165–168. [Google Scholar]
- Mamedov, M.; Petrova, I.; Yanykin, D.; Zaspa, A.; Semenov, A.Y. Effect of trehalose on oxygen evolution and electron transfer in photosystem 2 complexes. Biochemistry 2015, 80, 61–66. [Google Scholar] [CrossRef]
- Williams, W.; Gounaris, K. Stabilisation of PS-II-mediated electron transport in oxygen-evolving PS II core preparations by the addition of compatible co-solutes. Biochim. Biophys. Acta (BBA)—Bioenerg. 1992, 1100, 92–97. [Google Scholar] [CrossRef]
- Mamedov, M.; Nosikova, E.; Vitukhnovskaya, L.; Zaspa, A.; Semenov, A.Y. Influence of the disaccharide trehalose on the oxidizing side of photosystem II. Photosynthetica 2018, 56, 236–243. [Google Scholar] [CrossRef]
- Francia, F.; Palazzo, G.; Mallardi, A.; Cordone, L.; Venturoli, G. Residual water modulates QA−-to-QB electron transfer in bacterial reaction centers embedded in trehalose amorphous matrices. Biophys. J. 2003, 85, 2760–2775. [Google Scholar] [CrossRef]
- Yanykin, D.; Khorobrykh, A.; Mamedov, M.; Klimov, V. Trehalose stimulation of photoinduced electron transfer and oxygen photoconsumption in Mn-depleted photosystem 2 membrane fragments. J. Photochem. Photobiol. B Biol. 2015, 152, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Polander, B.C.; Barry, B.A. A hydrogen-bonding network plays a catalytic role in photosynthetic oxygen evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 6112–6117. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, M.D.; Milanovsky, G.E.; Malferrari, M.; Vitukhnovskaya, L.A.; Francia, F.; Semenov, A.Y.; Venturoli, G. Trehalose matrix effects on electron transfer in Mn-depleted protein-pigment complexes of Photosystem II. Biochim. Biophys. Acta (BBA)—Bioenerg. 2021, 1862, 148413. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.; Evans, M. Isolation of a photosystem 2 preparation from higher plants with highly enriched oxygen evolution activity. FEBS Lett. 1983, 160, 159–164. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Ono, T.-A.; Inoue, Y. Mn-preserving extraction of 33-, 24-and 16-kDa proteins from O2-evolving PS II particles by divalent salt-washing. FEBS Lett. 1983, 164, 255–260. [Google Scholar] [CrossRef]
- Miyao, M.; Murata, N. Partial disintegration and reconstitution of the photosynthetic oxygen evolution system. Binding of 24 kilodalton and 18 kilodalton polypeptides. Biochim. Biophys. Acta (BBA)—Bioenerg. 1983, 725, 87–93. [Google Scholar] [CrossRef]
- Klimov, V.V.; Allakhverdiev, S.I.; Shuvalov, V.A.; Krasnovsky, A.A. Effect of extraction and re-addition of manganese on light reactions of photosystem-II preparations. FEBS Lett. 1982, 148, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Klimov, V.; Allakhverdiev, S.; Feyziev, Y.M.; Baranov, S. Bicarbonate requirement for the donor side of photosystem II. FEBS Lett. 1995, 363, 251–255. [Google Scholar] [CrossRef]
- Yanykin, D.V.; Astashev, M.E.; Khorobrykh, A.A.; Paskhin, M.O.; Serov, D.A.; Gudkov, S.V. Application of Fixed-Length Ultrasonic Interferometry to Determine the Kinetics of Light-/Heat-Induced Damage to Biological Membranes and Protein Complexes. Inventions 2022, 7, 87. [Google Scholar] [CrossRef]
- Klimov, V.; Hulsebosch, R.; Allakhverdiev, S.; Wincencjusz, H.; Van Gorkom, H.; Hoff, A. Bicarbonate may be required for ligation of manganese in the oxygen-evolving complex of photosystem II. Biochemistry 1997, 36, 16277–16281. [Google Scholar] [CrossRef]
- Haldimann, P.; Strasser, R.J. Effects of anaerobiosis as probed by the polyphasic chlorophyll a fluorescence rise kinetic in pea (Pisum sativum L.). Photosynth. Res. 1999, 62, 67–83. [Google Scholar] [CrossRef]
- Redillas, M.C.; Strasser, R.J.; Jeong, J.S.; Kim, Y.S.; Kim, J.-K. The use of JIP test to evaluate drought-tolerance of transgenic rice overexpressing OsNAC10. Plant Biotechnol. Rep. 2011, 5, 169–175. [Google Scholar] [CrossRef]
- Schansker, G.; Strasser, R.J. Quantification of non-Q B-reducing centers in leaves using a far-red pre-illumination. Photosynth. Res. 2005, 84, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-Y.; Huang, Y.-X. Dependence of Refractive Index on Concentration and Temperature in Electrolyte Solution, Polar Solution, Nonpolar Solution, and Protein Solution. J. Chem. Eng. Data 2015, 60, 2827–2833. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P. Cellular and molecular physiology of cell volume regulation. In Competable and Counteracting Solutes; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 1994; pp. 81–110. [Google Scholar]
- Ajithkumar, I.P.; Panneerselvam, R. ROS scavenging system, osmotic maintenance, pigment and growth status of Panicum sumatrense roth. under drought stress. Cell Biochem. Biophys. 2014, 68, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Aref, I.M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Iqbal, M. Glutathione and proline can coordinately make plants withstand the joint attack of metal (loid) and salinity stresses. Front. Plant Sci. 2014, 5, 662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Meng, Y.-L.; Nii, N. Changes in glycine betaine and related enzyme contents in Amaranthus tricolor under salt stress. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao—J. Plant Physiol. Mol. Biol. 2004, 30, 496–502. [Google Scholar]
- Conde, A.; Silva, P.; Agasse, A.; Conde, C.; Gerós, H. Mannitol transport and mannitol dehydrogenase activities are coordinated in Olea europaea under salt and osmotic stresses. Plant Cell Physiol. 2011, 52, 1766–1775. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Ningthoujam, M.; Habib, K.; Bano, F.; Zutshi, S.; Fatma, T. Exogenous osmolytes suppresses the toxic effects of malathion on Anabaena variabilis. Ecotoxicol. Environ. Saf. 2013, 94, 21–27. [Google Scholar] [CrossRef]
- Bakore, G.; Bararia, M. Kinetics of the Oxidation of D-Glucose by Manganese (III). Z. Phys. Chem. 1965, 229, 245–249. [Google Scholar] [CrossRef]
- Yanykin, D.V.; Khorobrykh, A.A.; Semenov, A.Y.; Mamedov, M.D. Effect of Trehalose on the Functional Properties of Photosystem II. In Photosynthesis: Molecular Approaches to Solar Energy Conversion; Shen, J.-R., Satoh, K., Allakhverdiev, S.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 447–464. [Google Scholar]
- Judy, E.; Kishore, N. Biological wonders of osmolytes: The need to know more. Biochem. Anal. Biochem 2016, 5, 1–5. [Google Scholar] [CrossRef]
- Voitsekhovskaja, O.V.; Koroleva, O.A.; Batashev, D.R.; Knop, C.; Tomos, A.D.; Gamalei, Y.V.; Heldt, H.-W.; Lohaus, G. Phloem loading in two Scrophulariaceae species. What can drive symplastic flow via plasmodesmata? Plant Physiol. 2006, 140, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Santarius, K.; Milde, H. Sugar compartmentation in frost-hardy and partially dehardened cabbage leaf cells. Planta 1977, 136, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Leidreiter, K.; Kruse, A.; Heineke, D.; Robinson, D.; Heldt, H.W. Subcellular volumes and metabolite concentrations in potato (Solanum tuberosum cv. Désirée) leaves 1. Bot. Acta 1995, 108, 439–444. [Google Scholar] [CrossRef]
- Schneider, T.; Keller, F. Raffinose in chloroplasts is synthesized in the cytosol and transported across the chloroplast envelope. Plant Cell Physiol. 2009, 50, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Nobel, P.S. Permeability of pea chloroplasts to alcohols and aldoses as measured by reflection coefficients. Biochim. Biophys. Acta (BBA)—Biomembr. 1971, 241, 200–212. [Google Scholar] [CrossRef]
- Schäfer, G.; Heber, U. Glucose transport into spinach chloroplasts. Plant Physiol. 1977, 60, 286–289. [Google Scholar] [CrossRef]
- Nägele, T.; Heyer, A.G. Approximating subcellular organisation of carbohydrate metabolism during cold acclimation in different natural accessions of Arabidopsis thaliana. New Phytol. 2013, 198, 777–787. [Google Scholar] [CrossRef]
- Patzke, K.; Prananingrum, P.; Klemens, P.A.W.; Trentmann, O.; Rodrigues, C.M.; Keller, I.; Fernie, A.R.; Geigenberger, P.; Bölter, B.; Lehmann, M.; et al. The Plastidic Sugar Transporter pSuT Influences Flowering and Affects Cold Responses. Plant Physiol. 2019, 179, 569–587. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanykin, D.V.; Kazantseva, D.V.; Khorobrykh, A.A. Effect of Osmolytes on Photoassembly of Functionally Active Mn4CaO5 Cluster in Mn-Depleted Photosystem II Preparations Isolated from Spinach Leaves. Horticulturae 2023, 9, 1339. https://doi.org/10.3390/horticulturae9121339
Yanykin DV, Kazantseva DV, Khorobrykh AA. Effect of Osmolytes on Photoassembly of Functionally Active Mn4CaO5 Cluster in Mn-Depleted Photosystem II Preparations Isolated from Spinach Leaves. Horticulturae. 2023; 9(12):1339. https://doi.org/10.3390/horticulturae9121339
Chicago/Turabian StyleYanykin, Denis V., Dina V. Kazantseva, and Andrey A. Khorobrykh. 2023. "Effect of Osmolytes on Photoassembly of Functionally Active Mn4CaO5 Cluster in Mn-Depleted Photosystem II Preparations Isolated from Spinach Leaves" Horticulturae 9, no. 12: 1339. https://doi.org/10.3390/horticulturae9121339