Optimized Fertilization Shifted Soil Microbial Properties and Improved Vegetable Growth in Facility Soils with Obstacles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Setup
2.3. Sample Analyses
2.3.1. Determination of Soil Chemical Properties
2.3.2. Determination of Soil Culturable Microorganisms
2.3.3. Analysis of Soil Microbial Community Structure
2.4. Statistical Analysis
3. Results
3.1. Effects of Different Treatments on Soil Basic Chemical Properties
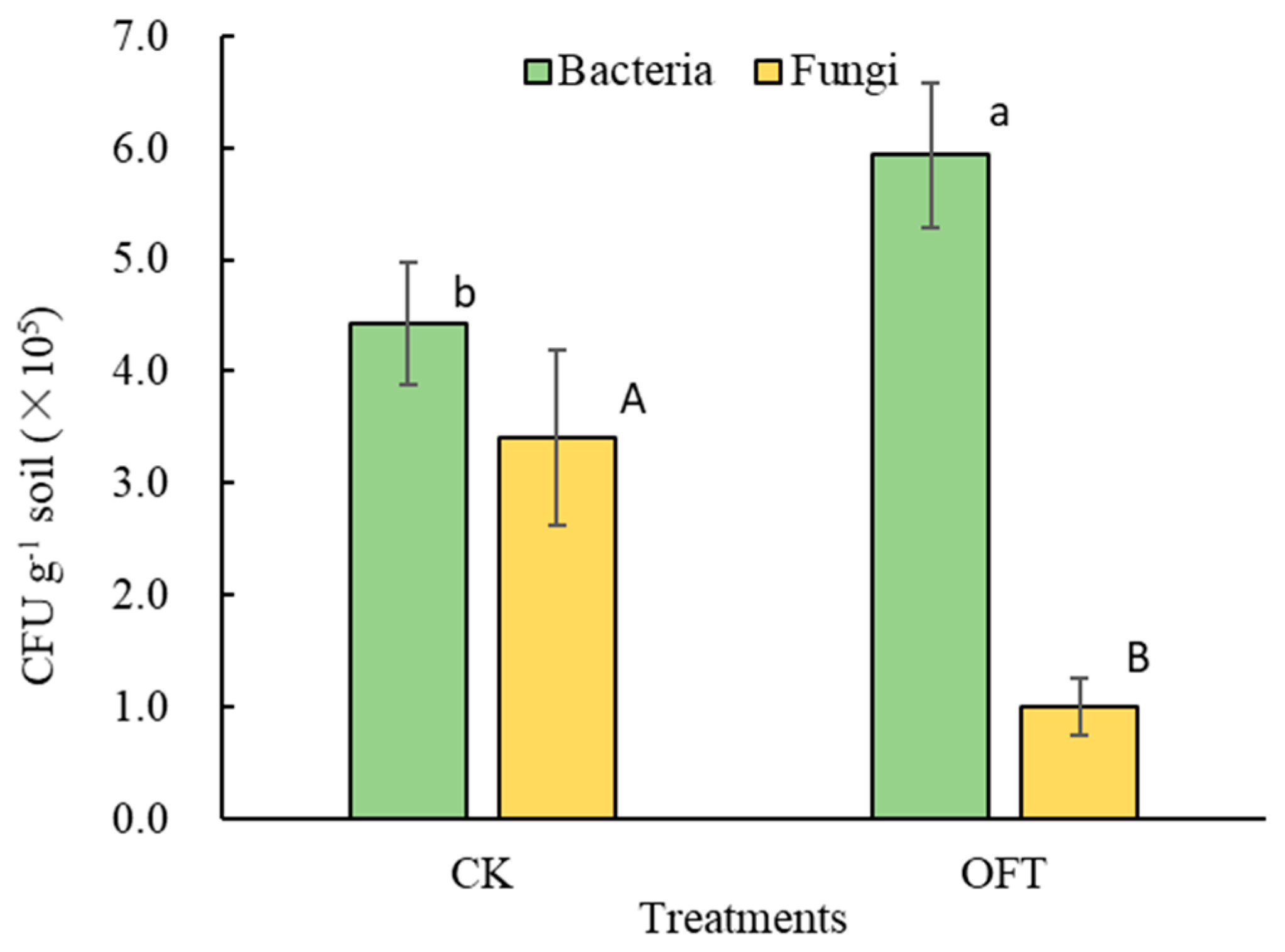
3.2. Effects of Formula Fertilization Treatment on Culturable Microorganisms in Soil
3.3. Effects of Optimized Fertilization on Soil Microbial Community Shift
3.3.1. Soil Microbial Diversity
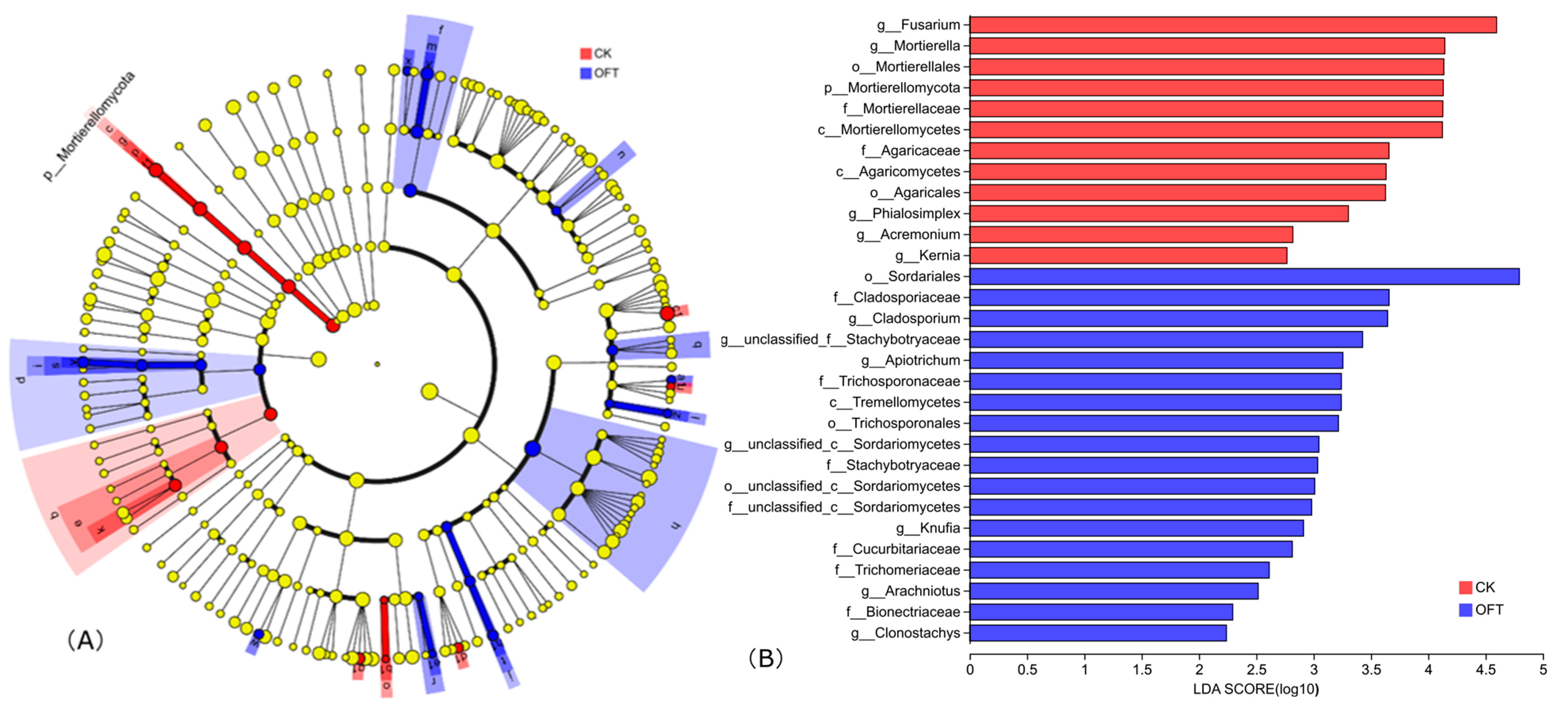
3.3.2. Soil Microbial Community Composition
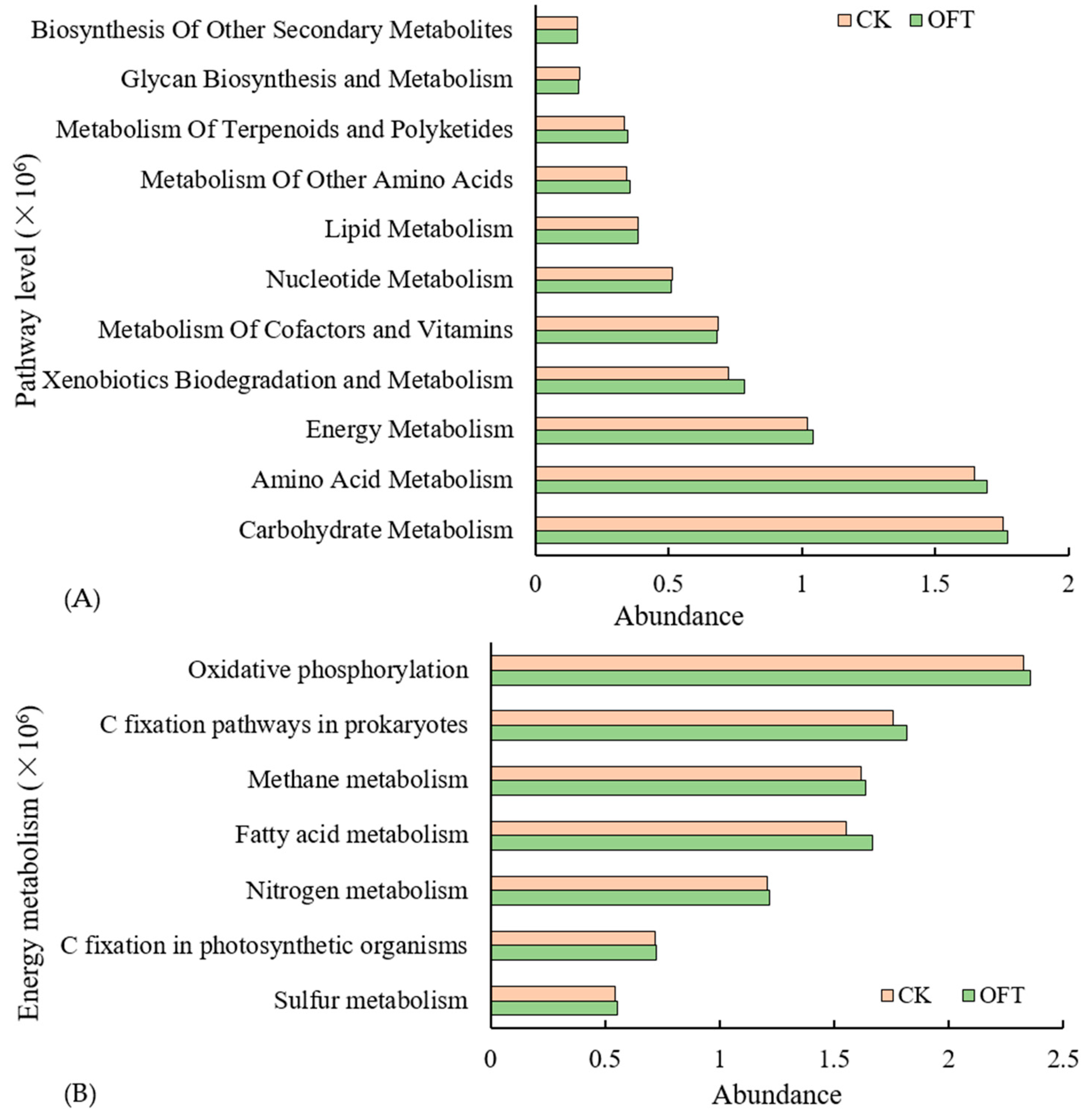
3.3.3. Functional Prediction of Soil Bacterial Groups
3.4. Relationship between Soil Environmental Factors and Microbial Groups
3.5. Effects of Different Treatments on the Growth Amount of Chinese Cabbage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.; Bai, X.; Wang, H.; Yang, M.; Bao, L.; Deng, X.; Chen, Z.; Zhou, J. Optimizing nitrogen and water management for sustainable greenhouse vegetable production with less greenhouse gas emissions. Agr. Ecosyst. Environ. 2023, 352, 108529. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Zhang, W.; Chen, B.; Song, D. Sustainability evaluation of protected vegetables production in China based on emergy analysis. J. Clean. Prod. 2023, 388, 135928. [Google Scholar] [CrossRef]
- Zikeli, S.; Deil, L.; Möller, K. The challenge of imbalanced nutrient flows in organic farming systems: A study of organic greenhouses in Southern Germany. Agr. Ecosyst. Environ. 2017, 244, 1–13. [Google Scholar] [CrossRef]
- Shen, W.; Hu, M.; Qian, D.; Xue, H.; Gao, N.; Lin, X. Microbial deterioration and restoration in greenhouse-based intensive vegetable production systems. Plant Soil. 2021, 463, 1–18. [Google Scholar] [CrossRef]
- Bai, X.; Gao, J.; Wang, S.; Cai, H.; Chen, Z.; Zhou, J. Excessive nutrient balance surpluses in newly built solar greenhouses over five years leads to high nutrient accumulations in soil. Agr. Ecosyst. Environ. 2020, 288, 106717. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Y.; Cayuela, M.L.; Sánchez-Monedero, M.A.; Wang, Q. Compost biochemical quality mediates nitrogen leaching loss in a greenhouse soil under vegetable cultivation. Geoderma 2020, 358, 113984. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production–A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Z.; Liu, X.; Zhai, B.; Powlson, D. Nitrate accumulation in soil profiles under seasonally open ‘sunlight greenhouses’ in northwest China and potential for leaching loss during summer fallow. Soil. Use Manage 2010, 26, 332–339. [Google Scholar] [CrossRef]
- Min, J.; Zhao, X.; Shi, W.; Xing, G.; Zhu, Z. Nitrogen Balance and Loss in a Greenhouse Vegetable System in Southeastern China. Pedosphere 2011, 21, 464–472. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Tang, Y.; Zhu, R.; Li, X.; Gruda, N.; Dong, J.; Duan, Z. Plastic shed soil salinity in China: Current status and next steps. Agric. Ecosyst. Environ. 2021, 317, 107474. [Google Scholar] [CrossRef]
- Shi, W.; Yao, J.; Yan, F. Vegetable cultivation under greenhouse conditions leads to rapid accumulation of nutrients, acidification and salinity of soils and groundwater contamination in South-Eastern China. Nutr. Cycl. Agroecosys. 2008, 83, 73–84. [Google Scholar] [CrossRef]
- Wang, S.; Bai, X.; Chen, Z.; Zhou, J. Nutrient balance and accumulation in soils of solar greenhouses. Res. Environ. Sci. 2015, 28, 1091–1098. [Google Scholar]
- Rodríguez, A.; Peña-Fleitas, M.T.; Gallardo, M. Sweet pepper and nitrogen supply in greenhouse production: Critical nitrogen curve, agronomic responses and risk of nitrogen loss. Eur. J. Agron. 2020, 117, 126046. [Google Scholar] [CrossRef]
- Qasim, W.; Xia, L.; Lin, S.; Wan, L.; Zhao, Y.; Butterbach-Bahl, K. Global greenhouse vegetable production systems are hotspots of soil N2O emissions and nitrogen leaching: A meta-analysis. Environ. Pollut. 2021, 272, 116372. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Zhang, S.; Sun, Z.; Ding, X. Assessment of magnesium deficiency in greenhouse tomato crops grown on calcareous soil. Soil Use Manag. 2023. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, J.; Jia, Z.; Zhang, T.; Zhang, W.; Wei, M. Physiological and nutrient responses to nitrogen, phosphorus, or potassium deficiency of hydroponically grown strawberry. Hortiscience 2023, 58, 628–634. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; Goss, E.M.; Havelaar, A.; van Diepeningen, A.D.; Finckh, M.R.; Morris, J.G., Jr. One Health-Cycling of diverse microbial communities as a connecting force for soil, plant, animal, human and ecosystem health. Sci. Total Environ. 2019, 664, 927–937. [Google Scholar] [CrossRef]
- Ferris, H.; Tuomisto, H. Unearthing the role of biological diversity in soil health. Soil. Boil. Biochem. 2015, 85, 101–109. [Google Scholar] [CrossRef]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease Suppressive Soils: New Insights from the Soil Microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef]
- Akbaba, M.; Ozden, E. Salt tolerance of endophytic root bacteria and their effects on seed germination and viability on tomato plants. Braz. J. Microbiol. 2023, 1–16. [Google Scholar] [CrossRef]
- Hao, J.; Xu, W.; Song, J.; Gao, G.; Bai, J.; Yu, Q.; Ren, G.; Feng, Y.; Wang, X. Adaptability of agricultural soil microbial nutrient utilization regulates community assembly under mulching measures on the Loess Plateau. Agri. Ecosyst. Environ. 2023, 357, 108702. [Google Scholar] [CrossRef]
- Dang, K.; Ji, L.; Slaughter, L.C.; Hou, J.; Shen, M.; Li, J.; Dong, Y. Synergistic changes of rhizosphere bacterial community and soil properties in greenhouse soils under long-term tomato monoculture. Appl. Soil Ecol. 2023, 183, 104738. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Boil. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Song, Y.; Xu, M.; Li, X.; Bian, Y.; Wang, F.; Yang, X.; Gu, C.; Jiang, X. Long-Term Plastic Greenhouse Cultivation Changes Soil Microbial Community Structures: A Case Study. J. Agric. Food Chem. 2018, 66, 8941–8948. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Ni, Y.; Gao, N.; Bian, B.; Zheng, S.; Lin, X.; Chu, H. Bacterial community composition is shaped by soil secondary salinization and acidification brought on by high nitrogen fertilization rates. Appl. Soil Ecol. 2016, 108, 76–83. [Google Scholar] [CrossRef]
- Sahab, S.; Suhani, I.; Srivastava, V.; Chauhan, P.; Singh, R.P.; Prasad, V. Potential risk assessment of soil salinity to agroecosystem sustainability: Current status and management strategies. Sci. Total Environ. 2021, 764, 144164. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Ren, X.; Chen, B.; Zhang, Y.; Shen, C.; Wang, F.; Wu, D. Long-term greenhouse cucumber production alters soil bacterial community structure. J. Soil Sci. Plant Nutr. 2019, 20, 306–321. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, D.; Zhang, Y. Investigating the Effects of Greenhouse Vegetable Cultivation on Soil Fertility in Lhasa, Tibetan Plateau. Chin. Geogr. Sci. 2020, 30, 456–465. [Google Scholar] [CrossRef]
- Zhao, Y.; Mao, X.; Zhang, M.; Yang, W.; Di, H.; Ma, L.; Liu, W.; Li, B. Response of soil microbial communities to continuously mono-cropped cucumber under greenhouse conditions in a calcareous soil of north China. J. Soil Sediment. 2020, 20, 2446–2459. [Google Scholar] [CrossRef]
- Mikkelsen, R.L. The “4R” nutrient stewardship framework for horticulture. HortTechnology 2011, 21, 658–662. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, X.; Wang, J.; Liu, F.; Ma, X.; Cao, H.; Chen, X.; Wang, X. Sustainable Nitrogen Management for Vegetable Production in China. Front. Agric. Sci. Eng. 2022, 9, 373–385. [Google Scholar]
- Watts, D.B.; Torbert, H.A.; Feng, Y. Soil microbial community dynamics as influenced by composted dairy manure, soil properties, and landscape position. Soil Sci. 2010, 175, 474–486. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Wang, C.; Wu, R.; Li, Y.; Qin, Y.; Li, Y.; Meng, F.; Wang, L.; Xu, F. Effects of pesticide residues on bacterial community diversity and structure in typical greenhouse soils with increasing cultivation years in Northern China. Sci. Total Environ. 2020, 710, 136321. [Google Scholar] [CrossRef]
- Han, J.; Luo, Y.; Yang, L.; Liu, X.; Wu, L.; Xu, J. Acidification and salinization of soils with different initial pH under greenhouse vegetable cultivation. J. Soil Sediment. 2014, 14, 1683–1692. [Google Scholar] [CrossRef]
- Bao, S. Soil Agro-Chemistrical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2007. [Google Scholar]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Y.; Hess, F.; Huang, B.; Chen, Z. Nutrient balance and soil changes in plastic greenhouse vegetable production. Nutr. Cycl. Agroecosys. 2020, 117, 77–92. [Google Scholar] [CrossRef]
- Chu, H.; Lin, X.; Fujii, T.; Morimoto, S.; Yagi, K.; Hu, J.; Zhang, J. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Boil. Biochem. 2007, 39, 2971–2976. [Google Scholar] [CrossRef]
- Chaurasia, A.; Meena, B.R.; Tripathi, A.N.; Pandey, K.K.; Rai, A.B.; Singh, B. Actinomycetes: An unexplored microorganisms for plant growth promotion and biocontrol in vegetable crops. World J. Microbiol. Biotechnol. 2018, 34, 132. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Ouhdouch, Y.; Hafidi, M.; Kouisni, L. Multifunctional role of Actinobacteria in agricultural production sustainability: A review. Microbiol. Res. 2022, 261, 127059. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech. 2015, 5, 353–377. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, G.; Ren, S.; Li, L.; Li, C.; Li, Y.; Yu, X.; Yin, Y.; Liu, T.; Liu, X. Responses of soil microbial community structure, potential ecological functions, and soil physicochemical properties to different cultivation patterns in cucumber. Geoberma 2023, 429, 116237. [Google Scholar] [CrossRef]
- Gutierrez-Sanchez, A.; Plasencia, J.; Monribot-Villanueva, J.L.; Rodriguez-Haas, B.; Ruiz-May, E.; Guerrero-Analco, J.A.; Sanchez-Rangel, D. Virulence factors of the genus Fusarium with targets in plants. Microbiol. Res. 2023, 227, 127506. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting Fungi present in the agricultural soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Mares-Ponce de León, Y.; Muñoz-Castellanos, L.N.; Ruiz-Cisneros, M.F.; Pérez-Corral, D.A.; Ornelas-Paz, J.J.; Acosta-Muñiz, C.H.; Berlanga-Reyes, D.I.; Rios-Velasco, C. Morphological and molecular identification of Mortierella species associated to rhizosphere of apple trees with symptoms of root diseases. Mex. J. Phytopathol. 2017, 36, 184–195. [Google Scholar]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Jousset, A.; Bienhold, C.; Chatzinotas, A. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef]
- Fetzer, I.; Johst, K.; Schaewe, R.; Banitz, T.; Harms, H.; Chatzinotas, A. The extent of functional redundancy changes as species’ roles shift in different environments. Proc. Natl. Acad. Sci. USA 2015, 112, 14888–14893. [Google Scholar] [CrossRef]





| Treatment | pH | EC (μS cm−1) | OM (g kg−1) | NN (mg kg−1) | Ca (mg kg−1) | Mg (mg kg−1) | B (mg kg−1) | Mo (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| CK | 5.76 ± 0.14 | 894.8 ± 25.8 | 37.9 ± 1.18 | 41.9 ± 6.89 | 10.9 ± 3.0 | 6.4 ± 1.89 | 1. 0 ± 0.19 | 0.23 ± 0.01 |
| OFT | 5.98 ± 0.30 | 764.7 ± 18.46 | 38. 3 ± 1.29 | 40.71 ± 5.01 | 15.3 ± 1.94 | 7.2 ± 0.97 | 1.50 ± 0.15 | 0.25 ± 0.02 |
| Treatment | Sobs Index | ACE Index | Chao Index | Shannon Index | Simpson Index | Coverage% | |
|---|---|---|---|---|---|---|---|
| Bacteria | CK | 1038 ± 113.2 | 1356.6 ± 150.8 | 1356.2 ± 145.8 | 5.42 ± 0.16 | 0.012 ± 0.003 | 98.42 |
| OFT | 1107.7 ± 89.76 | 1492.8 ± 103.2 | 1494.5 ± 141.4 | 5.34 ± 0.12 | 0.015 ± 0.001 | 98.17 | |
| Fungi | CK | 167.7 ± 22.2 | 191.3 ± 29.7 | 191.3 ± 25.5 | 2.9 ± 0.3 | 0.105 ± 0.03 | 99.90 |
| OFT | 181.7 ± 12.9 | 210.9 ± 12.5 | 208.4 ± 9.41 | 2.9 ± 0.21 | 0.118 ± 0.038 | 99.88 |
| Phylum | pH | EC | NN | Ca | Mg | B | Mo | |
|---|---|---|---|---|---|---|---|---|
| Bacteria | Patescibacteria | −0.49 | −0.77 | −0.66 | −0.89 * | −0.77 | −0.77 | −0.54 |
| Gemmatimonadota | 0.09 | 0.37 | 0.43 | 0.54 | 0.37 | 0.60 | 0.94 ** | |
| Verrucomicrobiota | 0.31 | −0.77 | −0.89 * | −0.60 | −0.77 | −0.71 | 0.03 | |
| unclassified_k__norank_d__Bacteria | −0.03 | −0.75 | −0.84 * | −0.81 * | −0.75 | −0.90 ** | −0.75 | |
| Abditibacteriota | −0.47 | −0.88 * | −0.79 | −0.97 ** | −0.88 * | −0.88 * | −0.50 | |
| Armatimonadota | 0.89 * | 0.31 | 0.09 | 0.37 | 0.31 | 0.14 | 0.14 | |
| Deinococcota | −0.31 | −1.00 *** | −0.94 ** | −0.94 ** | −1.00 *** | −0.89 * | −0.20 | |
| Fungi | Chytridiomycota | −0.09 | 0.60 | 0.77 | 0.66 | 0.60 | 0.83 * | 0.43 |
| unclassified_k__Fungi | −0.14 | 0.83 * | 0.94 * | 0.71 | 0.83 | 0.83 | 0.14 | |
| Rozellomycota | 0.49 | 0.77 | 0.66 | 0.89 * | 0.77 | 0.77 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, Y.; Ma, Y.; Wang, G.; Qian, X.; Wang, J. Optimized Fertilization Shifted Soil Microbial Properties and Improved Vegetable Growth in Facility Soils with Obstacles. Horticulturae 2023, 9, 1303. https://doi.org/10.3390/horticulturae9121303
Lang Y, Ma Y, Wang G, Qian X, Wang J. Optimized Fertilization Shifted Soil Microbial Properties and Improved Vegetable Growth in Facility Soils with Obstacles. Horticulturae. 2023; 9(12):1303. https://doi.org/10.3390/horticulturae9121303
Chicago/Turabian StyleLang, Yiqian, Yun Ma, Guiliang Wang, Xiaoqing Qian, and Juanjuan Wang. 2023. "Optimized Fertilization Shifted Soil Microbial Properties and Improved Vegetable Growth in Facility Soils with Obstacles" Horticulturae 9, no. 12: 1303. https://doi.org/10.3390/horticulturae9121303





