Establishment and Optimization of Flavonoid Extraction and Detection System for Hemerocallis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Equipment and Reagents
2.2.1. Instrument and Equipment
2.2.2. Reagents and Standards
2.3. Extraction Method
2.3.1. Organic Solvent Extraction
2.3.2. Ultrasonic-Assisted Extraction
2.4. HPLC Analysis of Flavonoids in Hemerocallis Flower Buds
2.4.1. HPLC Conditions
2.4.2. Preparation of the Mixed Standard Solution
2.4.3. Standard Curve Generation
2.4.4. Extraction and Detection of the Flavonoid Content in Hemerocallis
2.5. Optimization of the Ultrasonic-Assisted Extraction System for Extracting Flavonoids from Hemerocallis Flower Buds
2.5.1. Optimization of the Extraction System for Total Flavonoid Determination by Ultraviolet Spectrophotometry
2.5.2. Single-Factor Experiment
2.5.3. Response Surface Test
2.5.4. Antioxidant Activity
2.5.5. Ultrasonic-Assisted Extraction and HPLC Analysis of Flavonoid Content in Hemerocallis
2.6. Comparing of the Flavonoid Content Obtained via Ultrasonic-Assisted and Organic Solvent Extraction Methods
3. Results
3.1. Determination and HPLC Analysis of Hemerocallis Flavonoids Obtained via Organic Solvent Extraction
3.1.1. Precision Test of HPLC
3.1.2. Stability Test of HPLC
3.1.3. The Chromatographic Results of Mixed Standard and the Standard Curve Plot
3.1.4. The Content of Six Flavonoids Obtained by Organic Solvent Extraction
3.2. Optimization of the Single-Factor Experiment by Ultrasonic-Assisted Extraction Method
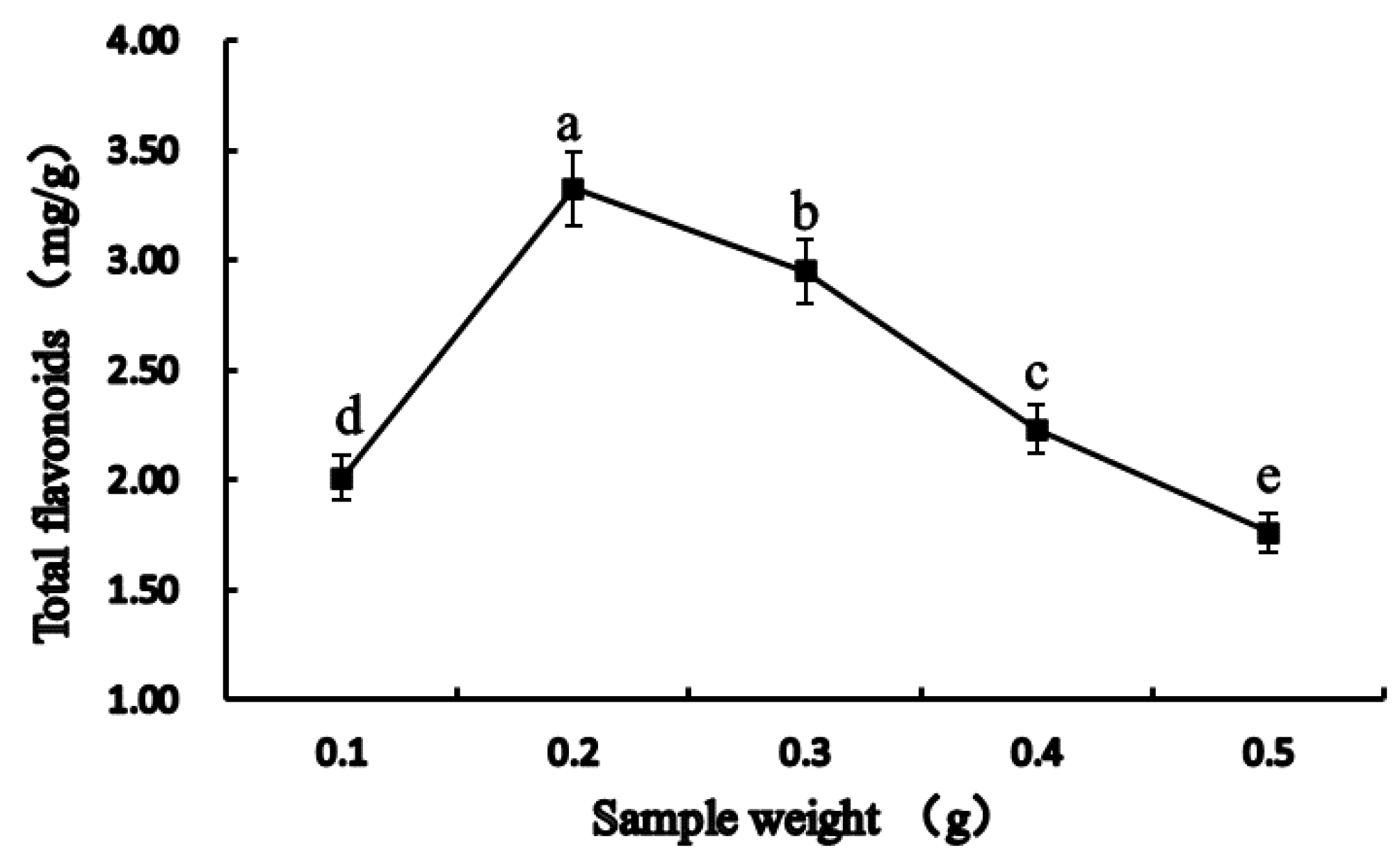
3.2.1. Effect of Sample Weight on Total Flavonoids Extraction
3.2.2. Effect of Ethanol Volume Fraction on the Total Flavonoid Extraction
3.2.3. Effect of Ethanol Volume on Total Flavonoids Extraction
3.2.4. Effect of Ultrasonic Extraction Time on Total Flavonoids Extraction
3.3. Response Surface Test
3.3.1. Response Surface Experimental Design
3.3.2. The Response Surface Test and Variance Analysis
3.3.3. The Content of Six Flavonoids in Hemerocallis Obtained by Ultrasonic-Assisted Extraction
3.3.4. Evaluation of Antioxidant Activity of Flavonoids
3.3.5. The Content of Six Flavonoids in Hemerocallis Obtained by Ultrasonic-Assisted Extraction
3.4. Comparison of the Contents of the Six Flavonoids Obtained Using the Two Extraction Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, P. Angiosperm Phylogeny Website; Missouri Botanical Garden: St. Louis, MO, USA, 2020. [Google Scholar]
- Zhao, X.C.; Du, J.L.; Xie, Y.G.; Zhang, Y.; Jin, H.Z. Chemical constituents of the flowers of Hemerocallis minor. Chem. Nat. Compd. 2018, 54, 556–558. [Google Scholar] [CrossRef]
- Yu, L.; Yao, G.; Ma, W. Rhein: A novel potential antitumor drug. Chin. Pharm. J. 2016, 25, 321–328. [Google Scholar]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Biochemistry, N. The capacity of antioxidant protection during modulated ageing of bean (Phaseolus vulgaris L.) cotyledons. 1. The antioxidant enzyme activities. Cell Biochem. Funct. 2007, 25, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, J.; Huang, X.; Tu, Y.; Ni, K. Identification of polymethoxylated flavones from green tangerine peel (Pericarpium Citri Reticulatae Viride) by chromatographic and spectroscopic techniques. J. Pharm. Biomed. Anal. 2007, 44, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Salin, O.; Törmäkangas, L.; Leinonen, M.; Saario, E.; Hagström, M.; Ketola, R.A.; Saikku, P.; Vuorela, H.; Vuorela, P.M. Corn mint (mentha arvensis) extract diminishes acute chlamydia pneumoniae infection in vitro and in vivo. J. Agric. Food Chem. 2011, 59, 12836–12842. [Google Scholar] [CrossRef]
- Fliegmann, J.; Furtwngler, K.; Malterer, G.; Cantarello, C.; Mithfer, A. Flavone synthase II (CYP93B16) from soybean (Glycine max L.). Phytochemistry 2010, 71, 508–514. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Xu, F.; Wang, Y. Advances in the study of flavonoids in Ginkgo biloba leaves. J. Med. Plants Res. 2009, 3, 1248–1252. [Google Scholar]
- Abd-Alla, H.I.; Albalawy, M.A.; Aly, H.F.; Shalaby, N.M.M.; Shaker, K.H. Flavone composition and antihypercholesterolemic and antihyperglycemic activities of Chrysanthemum coronarium L. Z. Für Naturforschung C J. Biosci. 2014, 69, 199–208. [Google Scholar] [CrossRef]
- Matsui, K.; Walker, A.R. Biosynthesis and regulation of flavonoids in buckwheat. Breed. Sci. 2020, 70, 74–84. [Google Scholar] [CrossRef]
- Juan, M.Y.; Chou, C.C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Hibatallah, J.; Carduner, C.; Poelman, M.C. In-vivo and in-vitro assessment of the free-radical-scavenger activity of Ginkgo flavone glycosides at high concentration. J. Pharm. Pharmacol. 2010, 51, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Lu, C.K.; Huang, Y.J.; Chen, H.J. Antioxidative caffeoylquinic acids and flavonoids from hemerocallis fulva flowers. J. Agric. Food Chem. 2011, 59, 8789–8795. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Sun, Y.; Wang, L.; Wang, J.; Wu, B.; Yan, T.; Jia, Y. An Investigation of the Anti-Depressive Properties of Phenylpropanoids and Flavonoids in Hemerocallis citrina Baroni. Molecules 2022, 27, 5809. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Verri, W.A., Jr. Therapeutic potential of flavonoids in pain and inflammation: Mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Devi, S.; Salkini, M.A.; Alam, P. Rutin Improves Anxiety and Reserpine-Induced Depression in Rats. Molecules 2022, 27, 7313. [Google Scholar] [CrossRef]
- Anjomshoa, M.; Boroujeni, S.N.; Ghasemi, S.; Lorigooini, Z.; Amiri, A.; Balali-Dehkordi, S.; Amini-Khoei, H. Rutin via increase in the CA3 diameter of the hippocampus exerted antidepressant-like effect in mouse model of maternal separation stress: Possible involvement of NMDA receptors. Behav. Neurol. 2020, 2020, 4813616. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Xi, J.; Wang, C.Z. High hydrostatic pressure extraction of flavonoids from propolis. J. Chem. Technol. Biotechnol. 2005, 80, 50–54. [Google Scholar]
- Duan, L.; Zhang, W.H.; Zhang, Z.H.; Liu, E.H.; Guo, L. Evaluation of natural deep eutectic solvents for the extraction of bioactive flavone c-glycosides from flos trollii. Microchem. J. 2018, 145, 180–186. [Google Scholar] [CrossRef]
- Zhou, T.; Xiao, X.; Li, G.; Cai, Z.W. Study of polyethylene glycol as a green solvent in the microwave-assisted extraction of flavone and coumarin compounds from medicinal plants. J. Chromatogr. A 2011, 1218, 3608–3615. [Google Scholar] [CrossRef] [PubMed]
- Hendrawan, Y.; Sabrinauly, S.; Hawa, L.C.; Argo, B.D.; Rachmawati, M. Analysis of the phenol and flavonoid content from basil leaves (Ocimum Americanum L.) extract using pulsed electric field (PEF) pre-treatment. Agric. Eng. Int. CIGR J. 2019, 21, 149–158. [Google Scholar]
- Numa, V.; Crampon, C.; Bellon, A.; Mouahid, A.; Badens, E. Valorization of food side streams by supercritical fluid extraction of compounds of interest from apple pomace. J. Supercrit. Fluids 2023, 202, 106056. [Google Scholar] [CrossRef]
- Zhang, G.; He, L.; Hu, M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov. Food Sci. Emerg. Technol. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Swift, L.J. Thin-layer chromatographic-spectrophotometric analysis for neutral fraction flavones in orange peel juice. J. Agric. Food Chem. 1967, 15, 99–101. [Google Scholar] [CrossRef]
- Dong, S.F.; Han, L.Q.; Zhao, W.X.; Dong, H.B.; Liu, J.H. Analysis and study of total flavone and trace element in Carthmus tinctorius L. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2008, 28, 225–227. [Google Scholar]
- Sithisarn, P.; Rojsanga, P.; Sithisarn, P. Inhibitory effects on clinical isolated bacteria and simultaneous HPLC quantitative analysis of flavone contents in extracts from Oroxylum indicum. Molecules 2019, 24, 1937. [Google Scholar] [CrossRef]
- Wenkert, E.; Gottlieb, H.E. Carbon-13 nuclear magnetic resonance spectroscopy of flavonoid and isoflavonoid compounds. Phytochemistry 1977, 16, 1811–1816. [Google Scholar] [CrossRef]
- Cui, H.L.; Zhang, Y.Y.; Shi, X.L.; Gong, F.F.; Xiong, X.; Kang, X.P.; Li, S. The numerical classification and grading standards of daylily (Hemerocallis) flower color. PLoS ONE 2019, 14, e0216460. [Google Scholar] [CrossRef]
- Kirana, S.Y.; Febrianti, R.; Amananti, W. Determination of Total Flavonoid Content of Bajakah Tampala and Kalalawit Roots Using the Reflux. Indones. J. Chem. Sci. Technol. (IJCST) 2023, 6, 56–64. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Sarg, T.M.; Salem, S.A.; Farrag, N.M.; Abdel-Aal, M.M.; Ateya, A.M. Phytochemical and Antimicrobial Investigation of Hemerocallis fulva L. grown in Egypt+. Int. J. Crude Drug Res. 1990, 28, 153–156. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, X.; Wu, J.; Su, H.; Chen, C.; Chen, Y. Qualitative and quantitative analysis of chemical constituents in traditional Chinese medicinal formula Tong-Xie-Yao-Fang by high-performance liquid chromatography/diode array detection/electrospray ionization tandem mass spectrometry. Anal. Chim. Acta 2011, 691, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Garofulić, I.E.; Zorić, Z.; Pedisić, S.; Dragović Uzelac, V. Retention of polyphenols in encapsulated sour cherry juice in dependence of drying temperature and wall material. LWT-Food Sci. Technol. 2017, 83, 110–117. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Li, Q.; Jin, W.; Chen, W.; Han, J.; Zhang, Y. Simultaneous optimization for ultrasound-assisted extraction and antioxidant activity of flavonoids from Sophora flavescens using response surface methodology. Molecules 2018, 24, 112. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Liu, Q.; Lin, Y.; Zhang, Z.; Li, S. Study on Extraction and Antioxidant Activity of Flavonoids from Hemerocallis fulva (Daylily) Leaves. Molecules 2022, 27, 2916. [Google Scholar] [CrossRef]
- Prabhu, D.; Prabhu, P.R.; Rao, P. Thermodynamics, adsorption, and response surface methodology investigation of the corrosion inhibition of aluminum by Terminalia chebula Ritz. extract in H3PO4. Chem. Pap. 2021, 75, 653–667. [Google Scholar] [CrossRef]
- Wang, G.H.; Zhang, B.X.; Nie, Q.X.; Li, H.; Zang, C. Optimal extraction of nuciferine and flavone from lotus leaf based on central composite design and response surface methodology. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2008, 33, 2332–2335. [Google Scholar]
- Ferro, M.; Seigneurin-Berny, D.; Rolland, N.; Chapel, A.; Salvi, D.; Garin, J.; Joyard, J. Organic solvent extraction as a versatile procedure to identify hydrophobic chloroplast membrane proteins. Electrophoresis 2000, 21, 3517–3526. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, M.; Weng, H.; Xu, Y.; Zeng, L. Optimization of ultrasound assisted extraction (UAE) of Kinsenoside compound from Anoectochilus roxburghii (Wall.) Lindl by response surface methodology (RSM). Molecules 2020, 25, 193. [Google Scholar] [CrossRef]
- Zhang, L.F.; Liu, Z.L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar]
- Yang, R.F.; Geng, L.L.; Lu, H.Q.; Fan, X.D. Ultrasound-synergized electrostatic field extraction of total flavonoids from Hemerocallis citrina baroni. Ultrason. Sonochem. 2017, 34, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gu, Y. Diffusion coefficients and oil swelling factors of carbon dioxide, methane, ethane, propane, and their mixtures in heavy oil. Fluid Phase Equilibria 2016, 243, 64–73. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Effect of extraction conditions on phenolic content, anthocyanin content and antioxidant activity of bran extracts from Thai rice cultivars. J. Cereal Sci. 2019, 86, 86–91. [Google Scholar] [CrossRef]
- Liu, Z.T.; Zhang, Y.; Zhang, X.J.; Zhang, T.T.; Zhang, J.S.; Chen, X.Q. Optimization of ultrasound-assisted extraction of flavonoids from Portulaca oleracea L., the extraction kinetics and bioactivity of the extract. J. Appl. Res. Med. Aromat. Plants 2023, 37, 100512. [Google Scholar] [CrossRef]
- Hao, Z.; Liang, L.; Liu, H.; Yan, Y.; Zhang, Y. Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity. Molecules 2022, 27, 2964. [Google Scholar] [CrossRef]
- Hou, M.; Hu, W.; Wang, A.; Xiu, Z.; Shi, Y.; Hao, K.; Sun, J. Ultrasound-assisted extraction of total flavonoids from Pteris cretica L.: Process optimization, HPLC analysis, and evaluation of antioxidant activity. Antioxidants 2019, 8, 425. [Google Scholar] [CrossRef]
- Liao, J.; Guo, Z.; Yu, G. Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrason. Sonochem. 2021, 76, 105661. [Google Scholar] [CrossRef]






| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 15.33 | 14 | 1.09 | 106.57 | <0.0001 | significant |
| A | 2.64 | 1 | 2.64 | 257.15 | <0.0001 | |
| B | 0.40 | 1 | 0.40 | 39.27 | <0.0001 | |
| C | 0.44 | 1 | 0.44 | 42.92 | <0.0001 | |
| D | 1.05 | 1 | 1.05 | 102.24 | <0.0001 | |
| AB | 0.16 | 1 | 0.16 | 15.58 | 0.0015 | |
| AC | 0.87 | 1 | 0.87 | 85.11 | <0.0001 | |
| AD | 0.44 | 1 | 0.44 | 42.41 | <0.0001 | |
| BC | 0.16 | 1 | 0.16 | 15.58 | 0.0015 | |
| BD | 0.15 | 1 | 0.15 | 14.81 | 0.0018 | |
| CD | 0.26 | 1 | 0.26 | 24.83 | 0.0002 | |
| A2 | 2.32 | 1 | 2.32 | 226.26 | <0.0001 | |
| B2 | 5.19 | 1 | 5.19 | 505.65 | <0.0001 | |
| C2 | 3.25 | 1 | 3.25 | 315.95 | <0.0001 | |
| D2 | 2.65 | 1 | 2.65 | 257.51 | <0.0001 | |
| Residual | 0.14 | 14 | 0.01 | |||
| Lack of Fit | 0.13 | 10 | 0.01 | 5.20 | 0.0632 | not significant |
| Pure Error | 0.01 | 4 | 0.00 | |||
| Cor Total | 15.47 | 28 | ||||
| R2 = 0.9907 | Adj R2 = 0.9814 | |||||
| C.V.% = 3.16 | ||||||
| Number | Rutin (µg/g) | Isoquercetin (µg/g) | Myricetin (µg/g) | Quercetin (µg/g) | Apigenin (µg/g) | Diosmetin (µg/g) |
|---|---|---|---|---|---|---|
| H0001 | 82.40 **1 | 42.42 ** | −11.59 ** | −7.22 ** | −1.08 * | −3.97 ** |
| H0004 | 879.50 ** | 28.60 ** | −8.40 ** | −4.61 ** | −1.92 ** | 14.05 ** |
| H0006 | 752.72 ** | 6.34 ** | −8.32 ** | −9.34 ** | 4.69 ** | −2.63 ** |
| H0015 | 615.38 ** | −189.86 ** | −11.79 ** | 10.84 ** | 12.58 ** | 30.55 ** |
| H0019 | 2115.45 ** | −61.65 ** | −10.10 ** | −0.08 | 3.24 ** | 14.16 ** |
| H0021 | 591.38 ** | −5.63 ** | −9.98 ** | −16.34 ** | 55.62 ** | −5.33 ** |
| H0024 | 43.96 * | −0.35 | −13.64 ** | −29.58 ** | −3.81 ** | 9.90 ** |
| H0025 | 327.62 ** | 20.27 * | −13.24 ** | −27.10 ** | −0.40 | 14.98 ** |
| H0026 | 318.46 ** | 21.13 ** | −8.27 ** | −7.47 ** | −0.84 | 0.62 |
| H0034 | 110.75 ** | 1.75 | −8.83 ** | −15.26 ** | −1.00 ** | 3.24 ** |
| H0039 | 62.72 ** | −15.33 ** | −9.83 ** | −16.36 ** | 0.44 ** | −20.36 ** |
| H0056 | 703.66 ** | 39.15 ** | −8.82 ** | −9.93 ** | 1.43 ** | −0.56 |
| H0057 | 1702.13 ** | 88.28 ** | −9.83 * | −7.88 ** | −1.87 ** | 23.52 ** |
| H0058 | 1001.59 ** | 112.17 ** | −2.89 ** | −0.43 | −0.90 ** | 24.18 ** |
| H0059 | 550.41 ** | −14.85 | 2.37 | 167.25 ** | 3.81 ** | 8.60 ** |
| H0060 | 186.67 ** | 4.08 * | 12.13 ** | −7.60 ** | −2.21 ** | −0.86 |
| H0061 | 892.89 ** | 53.59 ** | −8.75 ** | −4.99 ** | 4.21 ** | 1.19 * |
| H0062 | 669.14 ** | 28.00 ** | −9.61 ** | −25.02 ** | −2.88 ** | −13.38 ** |
| H0065 | 93.19 ** | 32.20 ** | −6.96 ** | 5.72 | 3.90 ** | 7.63 ** |
| H0066 | 1187.78 ** | 39.81 ** | −7.60 ** | −5.82 ** | −2.52 ** | 7.71 ** |
| H0070 | 1243.81 ** | 36.47 ** | −8.42 ** | −8.46 ** | 2.54 ** | 28.71 ** |
| H0071 | 937.79 ** | 30.72 ** | −10.21 ** | −17.42 ** | 14.83 ** | 71.25 ** |
| H0072 | 337.38 ** | 37.47 ** | −6.92 ** | −19.31 ** | −5.10 ** | 2.63 |
| H0073 | 488.41 ** | 11.40 ** | −12.66 ** | −9.09 ** | 3.87 ** | 16.01 ** |
| H0075 | 634.58 ** | 81.23 ** | −12.62 ** | −3.76 ** | 6.42 ** | 11.04** |
| H0076 | 51.51 ** | −7.12 ** | −8.77 ** | −19.39 ** | −0.65 ** | 16.02 ** |
| H0079 | 1069.82 ** | 124.43 | −6.57 ** | −14.81 ** | −1.88 ** | 1.67 ** |
| H0081 | 1095.43 ** | 85.28 ** | −4.86 * | 1.88 ** | 0.21 ** | 22.08 ** |
| H0083 | 293.34 ** | 2.93 ** | −5.74 ** | 9.08 ** | −0.65 ** | 48.49 ** |
| H0084 | 808.58 ** | 55.21 ** | −7.52 ** | 17.62 ** | 1.39 ** | 7.73 ** |
| H0085 | 810.76 ** | 133.51 ** | −7.31 ** | 26.60 ** | 3.53 ** | 17.71 ** |
| H0086 | 1102.36 ** | −295.26 ** | −7.04 ** | −16.40 ** | 5.39 ** | 28.56 ** |
| H0087 | 1100.68 ** | 343.76 ** | −7.97 ** | 48.37 ** | 2.07 ** | 14.57 ** |
| H0088 | 1085.94 ** | 63.98 ** | −9.97 ** | −4.99 ** | 2.25 ** | 17.44 ** |
| H0089 | 547.08 ** | −51.63 ** | −8.97 ** | −11.08 ** | −3.59 ** | 1.35 ** |
| H0091 | 1239.87 ** | 85.19 ** | −5.17 ** | −18.17 ** | −0.47 ** | 16.86 ** |
| H0092 | 902.46 ** | 93.16 ** | 0.31 ** | −19.73 ** | 1.87 ** | 93.05 ** |
| H0094 | 995.74 ** | 35.25 ** | 2.38 ** | −6.83 * | 2.10 ** | −21.49 ** |
| H0095 | 960.26 ** | 279.09 ** | −7.66 ** | 111.23 ** | 0.73 ** | −3.15 ** |
| H0120 | 849.07 ** | 112.83 ** | −9.91 ** | −26.57 ** | 1.59 ** | 0.98 ** |
| H0122 | 602.21 ** | 93.57 ** | −1.30 | 43.85 ** | 1.67 ** | −2.08 ** |
| H0123 | 624.16 ** | 4.45 ** | −6.51 ** | −6.02 ** | 0.78 ** | 2.38 ** |
| H0142 | 948.45 ** | 143.06 ** | −6.04 ** | 22.34 ** | 0.08 | 187.18 ** |
| H0143 | 984.06 ** | 19.85 ** | −1.98 ** | −8.20 ** | −4.22 ** | 6.87 ** |
| H0144 | 243.47 ** | 67.16 ** | −10.94 ** | −23.87 ** | −2.42 ** | −3.78 ** |
| H0145 | 476.54 ** | −69.00 ** | −8.98 ** | −3.05 ** | −4.18 ** | −3.08 ** |
| H0160 | 228.04 ** | 4.76 ** | −9.27 ** | −3.68 ** | 0.13 * | −1.00 ** |
| H0161 | 1051.03 ** | −1.77 | −7.77 ** | −3.62 ** | 35.87 ** | 2.48 ** |
| H0162 | 899.06 ** | 102.31 ** | −7.93 ** | −2.76 ** | −2.52 ** | 149.46 ** |
| H0164 | 378.81 ** | 15.24 ** | −9.74 ** | 1.64 ** | −0.79 ** | 82.36 ** |
| H0166 | 343.13 ** | 16.22 ** | −10.24 ** | −0.65 | −2.39 ** | 71.06 ** |
| H0167 | 540.51 ** | 37.97 ** | −5.50 ** | 0.86 | −0.01 | 134.11 ** |
| H0170 | 381.29 ** | 38.96 ** | −7.89 ** | 6.35 ** | 0.91 * | 72.68 ** |
| H0172 | 506.77 ** | 31.32 ** | −8.72 ** | 6.25 ** | 1.48 ** | 9.95 ** |
| H0173 | 535.18 ** | 23.73 ** | −5.49 ** | 5.47 ** | 0.88 ** | 119.85 ** |
| H0174 | 417.86 ** | 38.03 ** | −8.86 ** | 8.85 ** | 0.72 ** | 9.66 ** |
| H0178 | 363.75 ** | 718.11 ** | −10.72 ** | −13.20 ** | 2.95 ** | 7.26 ** |
| H0179 | 494.72 ** | 47.75 ** | −9.39 ** | 1.84 ** | 1.43 ** | 10.98 ** |
| H0181 | 1368.60 ** | 34.64 ** | −10.20 ** | −0.98 ** | 3.60 ** | 17.60 ** |
| H0182 | 402.46 ** | 38.77 ** | −9.31 ** | 4.33 ** | 3.04 ** | 4.74 ** |
| H0186 | 915.21 ** | 152.63 ** | −8.42 ** | 21.98 ** | 1.04 ** | 12.39 ** |
| H0187 | 32.99 ** | −42.98 ** | −7.77 ** | 1.65 ** | 37.48 ** | 2.22 |
| H0190 | 1445.00 ** | −167.30 ** | −9.42 ** | −10.76 ** | 0.91 ** | 14.94 ** |
| H0191 | 2038.34 ** | 130.10 ** | −10.32 ** | 46.24 ** | 3.44 ** | 76.87 ** |
| H0192 | 526.62 ** | 20.04 ** | −8.74 ** | −1.18 ** | −3.19 ** | 4.97 ** |
| H0205 | 972.48 ** | 42.60 ** | −8.39 ** | −8.72 ** | 5.79 ** | 15.41 ** |
| H0206 | 827.41 ** | 354.73 ** | −9.21 ** | 38.29 ** | 0.23 ** | 5.05 ** |
| H0207 | 390.52 ** | 85.30 ** | −11.18 ** | 47.99 ** | −37.71 ** | 9.19 ** |
| H0209 | −63.30 ** | −38.13 ** | −10.42 ** | −0.57 | −0.62 ** | 1.62 * |
| H0210 | −420.90 ** | 41.37 ** | 32.10 ** | 24.56 ** | −4.84 ** | −9.56 ** |
| GPD 2 | 68 | 56 | 5 | 25 | 42 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Jing, M.; Zhang, L.; Liu, C.; Xue, N.; Yang, W.; Zhang, W.; Hou, F.; Zhang, X.; Wang, Y.; et al. Establishment and Optimization of Flavonoid Extraction and Detection System for Hemerocallis. Horticulturae 2023, 9, 1233. https://doi.org/10.3390/horticulturae9111233
Su J, Jing M, Zhang L, Liu C, Xue N, Yang W, Zhang W, Hou F, Zhang X, Wang Y, et al. Establishment and Optimization of Flavonoid Extraction and Detection System for Hemerocallis. Horticulturae. 2023; 9(11):1233. https://doi.org/10.3390/horticulturae9111233
Chicago/Turabian StyleSu, Jie, Mengyue Jing, Lijuan Zhang, Chenzhi Liu, Niping Xue, Wenjing Yang, Wei Zhang, Feifan Hou, Xiaomin Zhang, Yanfang Wang, and et al. 2023. "Establishment and Optimization of Flavonoid Extraction and Detection System for Hemerocallis" Horticulturae 9, no. 11: 1233. https://doi.org/10.3390/horticulturae9111233




