Impact of Cover Cropping on Temporal Nutrient Distribution and Availability in the Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Management
2.3. Determination of Soil Nutrient Concentration
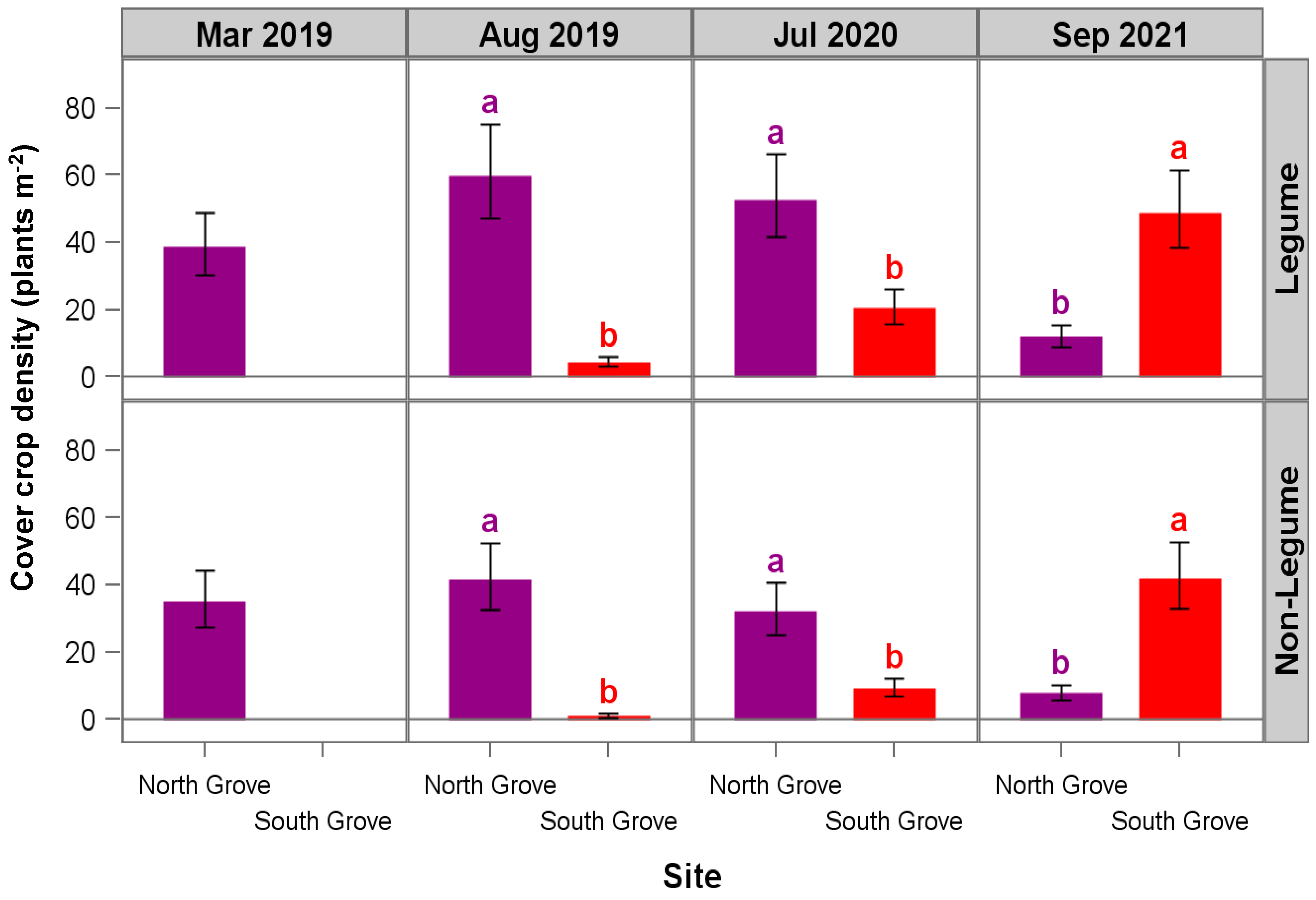
2.4. Cover Crop Density Assessment
2.5. Statistical Analyses
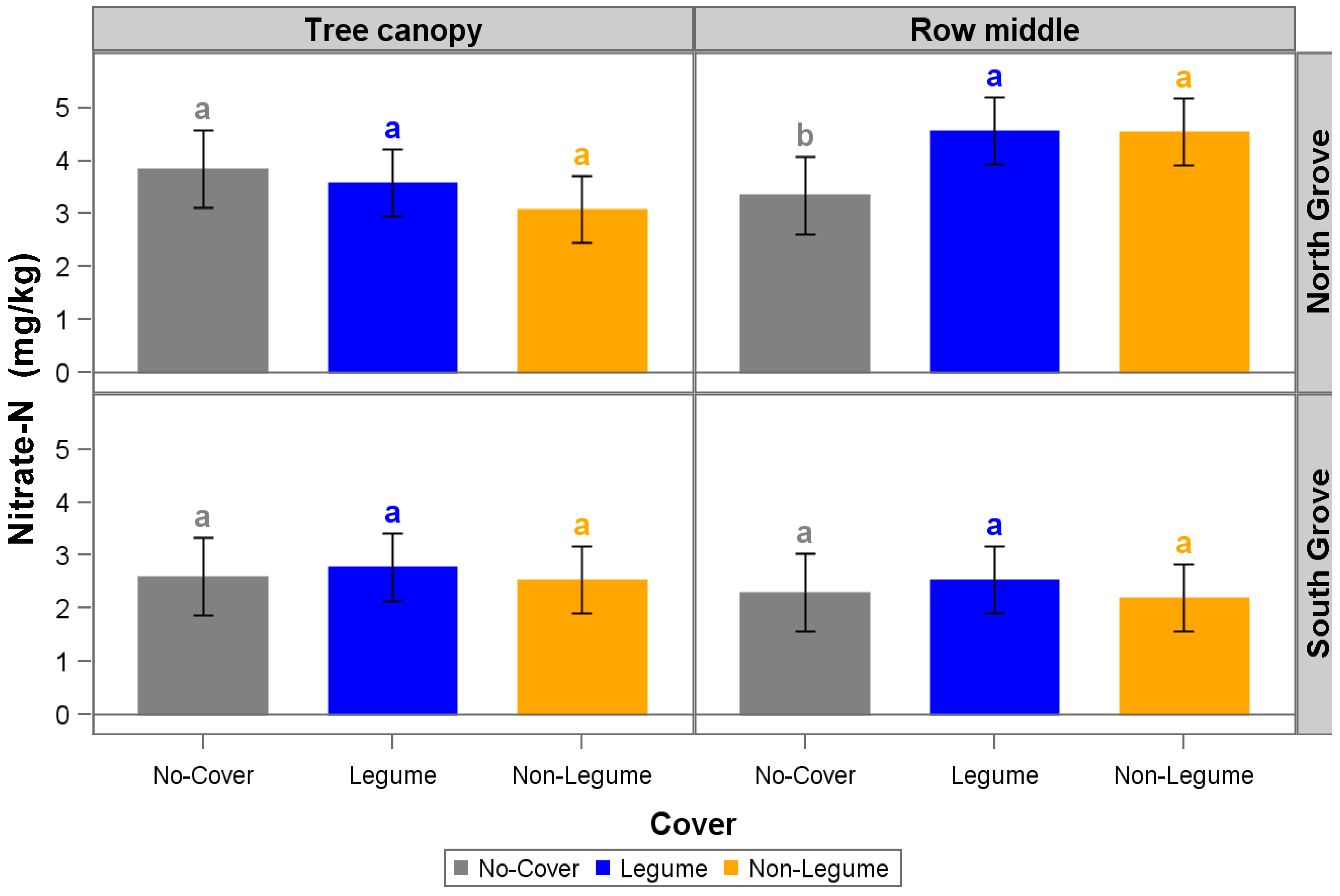
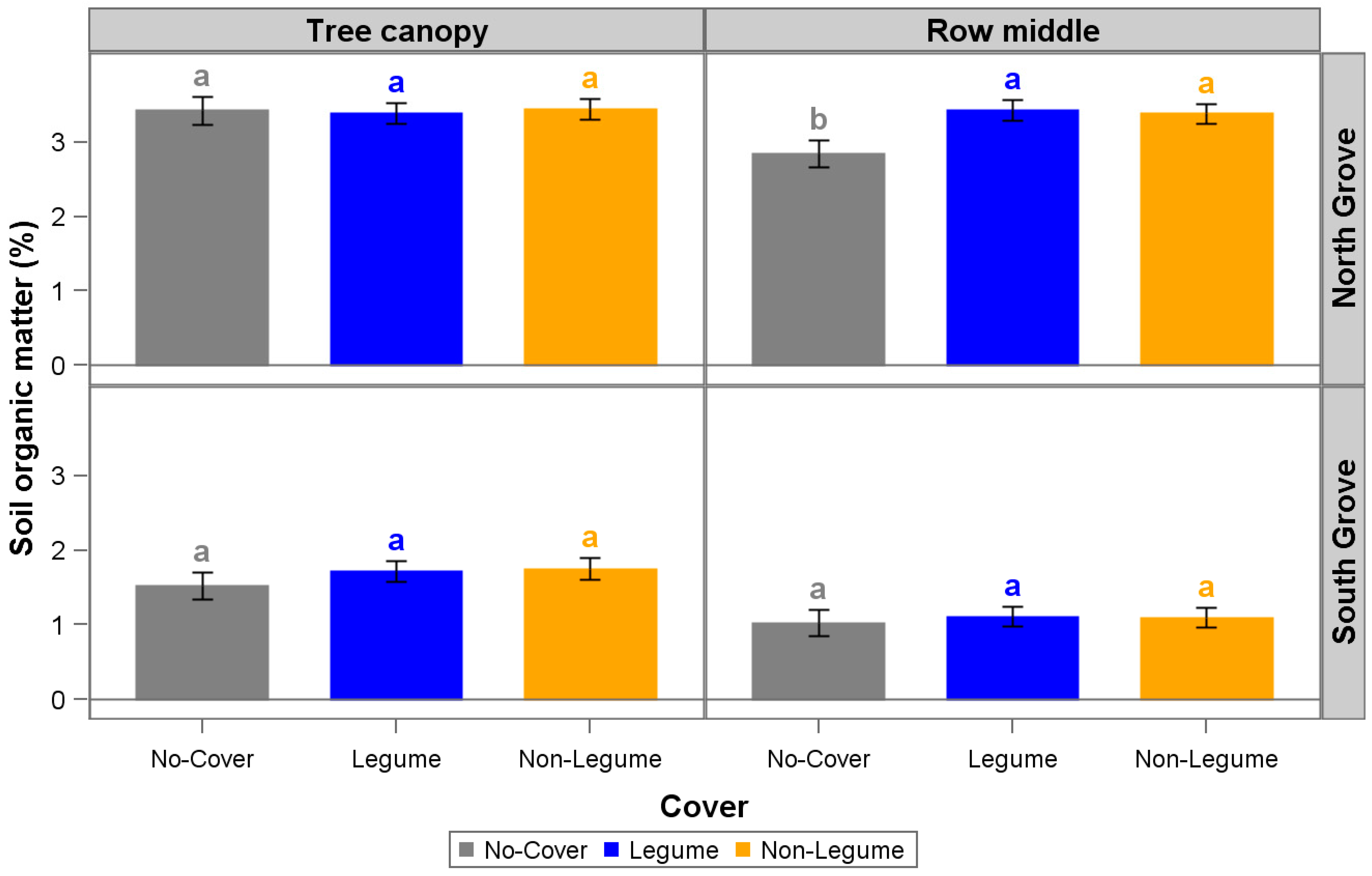
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Weil, R.; Hill, R. Effects of compaction and cover crops on soil least limiting water range and air permeability. Soil Till. Res. 2014, 136, 61–69. [Google Scholar] [CrossRef]
- Dabney, S.M.; Delgado, J.A.; Reeves, D.W. Using winter cover crops to improve soil and water quality. Commun. Soil Sci. Plant Anal. 2001, 32, 1221–1250. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Meersmans, J.; Serlet, L. Cover crops and their erosion-reducing effects during concentrated flow erosion. Catena 2011, 85, 237–244. [Google Scholar] [CrossRef]
- Wick, A.; Berti, M.; Lawley, L.; Liebig, M. Integration of annual and perennial cover crops for improving soil health. In Soil Health and Intensification of Agroecosystems; Al-Kaisi, M.M., Lowery, B., Eds.; Academic Press: Oxford, UK, 2017; pp. 127–150. [Google Scholar]
- Buscot, F.; Varma, A. Microorganisms in Soils: Roles in Genesis and Functions, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Baitsaid, A.; Schaffer, B.; Vargas, A.I.; Li, Y.; Liu, G. Effect of plant age on in soil decomposition and nitrogen content of sunn hemp tissue. Commun. Soil Sci. Plant Anal. 2018, 49, 2680–2688. [Google Scholar] [CrossRef]
- Stallings, A.M.; Balkcom, K.S.; Wood, C.W.; Guertal, E.A.; Weaver, D.B. Nitrogen mineralization from ‘AU Golden’ sunn hemp residue. J. Plant Nutr. 2017, 40, 50–62. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Hons, F.M.; Zuberer, D.A. Long-term changes in soil carbon and nitrogen pools in wheat management systems. Soil Sci. Soc. Am. J. 1994, 58, 1639–1645. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Tian, Y.; Wu, X.; Yang, J.; Luo, Y.; Li, H.; Kumar Awasthi, M.; Zhao, Z. Temporal and spatial variation of soil microorganisms and nutrient under white clover cover. Soil Tillage Res. 2020, 202, 104666. [Google Scholar] [CrossRef]
- Obreza, T.A.; Collins, M.E. Common Soils Used for Citrus Production in Florida; SL 193; University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Fares, A.; Dogan, A.; Abbas, F.; Parsons, L.; Obreza, T.; Morgan, K. Water balance components in a mature citrus orchard. Soil Sci. Soc. Am. J. 2008, 72, 578–585. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, Y.; Hu, C.; Zhan, T.; Zhang, S.; Cai, M.; Zhao, X. Soil applied Ca, Mg and B altered phyllosphere and rhizosphere bacterial microbiome and reduced Huanglongbing incidence in Gannan Navel Orange. Sci. Total Environ. 2021, 791, 148046. [Google Scholar] [CrossRef]
- Delgado, J.A.; Dillon, M.A.; Sparks, R.T.; Essah, S.Y.C. Decade of advances in cover crops: Cover crops with limited irrigation can increase yields, crop quality, and nutrient and water use efficiencies while protecting the environment. J. Soil Water Conserv. 2007, 62, 110A–117A. [Google Scholar]
- Henis, Y. Soil microorganisms, soil organic matter and soil fertility. In The Role of Organic Matter in Modern Agriculture; Chen, Y., Avnimelech, Y., Eds.; Springer: Dordrecht, The Netherlands, 1986; Volume 25. [Google Scholar]
- U.S. Department of Agriculture; Natural Resources Conservation Service (USDA-NRCS). Soil Health. 2021. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/health/ (accessed on 4 January 2022).
- Tully, K.L.; McAskill, C. Promoting soil health in organically managed systems: A review. Org. Agric. 2019, 10, 339–358. [Google Scholar] [CrossRef]
- Soil Health Institute. Soil Health Indicators and Methods to Be Assessed. 2021. Available online: https://soilhealthinstitute.org/north-american-project-to-evaluate-soil-health-measurements/ (accessed on 8 August 2021).
- Hux, B.; DeLaune, P.; Schirmarcher, M.; Gentry, T.; Mubvumba, P. Winter cover crop impact on soil health and nutrients in Texas rolling plains dryland cotton. Agrosystems Geosci. Environ. 2023, 6, e20352. [Google Scholar] [CrossRef]
- Koppen, W. Das Geographisca System der Klimate. In Handbuch der Klimatologie; Koppen, W., Geiger, G., Eds.; Gebrüder Borntraeger Verlagsbuchhandlung: Stuttgart, Germany, 1936; pp. 1–44. [Google Scholar]
- USDA–NRCS. Web Soil Survey. 2015. Available online: https://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx (accessed on 9 October 2019).
- Obreza, T.A.; Zekri, M.; Hanlon, E.W. Soil and leaf tissue testing. In Nutrition of Florida Citrus Trees, 2nd ed.; Obreza, T.A., Morgan, K.T., Eds.; University of Florida: Gainesville, FL, USA, 2020; pp. 24–32. [Google Scholar]
- Hanlon, E.A.; Gonzalez, J.S.; Bartos, J.M. Mehlich-3 extractable P, K, Ca, Mg, Cu, Mn, Zn, and Fe. In IFAS Extension Soil Testing Laboratory (ESTL) and Analytical Research Laboratory (ARL) Chemical Procedures and Training Manual; University of Florida: Gainesville, FL, USA, 1997. [Google Scholar]
- Castellano-Hinojosa, A.; Kanissery, R.; Strauss, S.L. Cover crops in citrus orchards impact soil nutrient cycling and the soil microbiome after three years but effects are site-specific. Biol. Fertil. Soils 2023, 59, 659–678. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Martens-Habbena, W.; Smyth, A.R.; Kadyampakeni, D.M.; Strauss, S.L. Short-term effects of cover crops on soil properties and the abundance of N-cycling genes in citrus agroecosystems. Appl. Soil Ecol. 2022, 172, 104341. [Google Scholar] [CrossRef]
- Hanlon, E.A.; Gonzalez, J.S.; Bartos, J.M. Potassium chloride (1 M) extractable NH4-N, NO3−-N, and Al. In IFAS Extension Soil Testing Laboratory (ESTL) and Analytical Research Laboratory (ARL) Chemical Procedures and Training Manual; University of Florida: Gainesville, FL, USA, 1997. [Google Scholar]
- Cambardella, C.; Gajda, A.; Doran, J.; Wienhold, B.; Kettler, T. Estimation of particulate and total organic matter by weight loss-on-ignition. In Assessment Methods for Soil Carbon; Lal, R., Kimble, J.M., Follet, R.J., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 349–359. [Google Scholar]
- Abbasi, M.K.; Tahir, M.M.; Shah, A.H.; Batool, F. Mineral nutrient composition of different ecotypes of white clover and their nutrient credit to soil at Rawalkot Azad Jammu and Kashmir. Pak. J. Bot. 2009, 41, 41–51. [Google Scholar]
- O’Connell, S.; Shi, W.; Grossman, J.M.; Hoyt, G.D.; Fager, K.L.; Creamer, N.G. Short-term nitrogen mineralization from warm-season cover crops in organic farming systems. Plant Soil 2015, 396, 353–367. [Google Scholar] [CrossRef]
- Alva, A.K.; Tucker, D.P. Soils and citrus nutrition. In Citrus Health Management; Timmer, L.W., Duncan, L.W., Eds.; APS Press: St. Paul, MN, USA, 1999; pp. 59–71. [Google Scholar]
- Marcondes, J.; Lemos, E. Nitrogen metabolism in citrus based on expressed tag analysis. In Advances in Citrus Nutrition; Springer: Berlin/Heidelberg, Germany, 2012; pp. 245–255. [Google Scholar]
- Mattos, D., Jr.; Quaggio, J.; Cantarella, H.; Alva, A. Nutrient content of biomass components of Hamlin sweet orange trees. Sci. Agric. 2003, 60, 155–160. [Google Scholar] [CrossRef]
- Morgan, K.T.; Scholberg, J.M.S.; Obreza, T.A.; Wheaton, T.A. Size, biomass, and nitrogen relationships with sweet orange tree growth. J. Am. Soc. Hortic. Sci. 2006, 131, 149–156. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W. Biomass, nutrient accumulation and tree size relationships for drip-and microsprinkler-irrigated orange trees. J. Plant Nutr. 2016, 39, 589–599. [Google Scholar] [CrossRef]
- Qin, W.; Assinck, F.; Heinen, M.; Oenema, O. Water and nitrogen use efficiencies in citrus production: A meta-analysis. Agric. Ecosyst. Environ. 2016, 222, 103–111. [Google Scholar] [CrossRef]
- Quaggio, J.; Souza, T.; Bachiega, Z.; Marcelli, B.; Mattos, D. Nitrogen-fertilizer forms affect the nitrogen-use efficiency in fertigated citrus groves. J. Plant Nutr. Soil Sci. 2014, 177, 404–411. [Google Scholar] [CrossRef]
- Brewer, M. Citrus Row-Middle Management Using Cover Crops for Suppressing Weeds and Improving Soil. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2023. [Google Scholar]
- Steenwerth, K.; Belina, K. Cover crops enhance soil organic matter, carbon dynamics and microbiological function in a vineyard agroecosystem. Appl. Soil Ecol. 2008, 40, 359–369. [Google Scholar] [CrossRef]
- Oliveira, F.E.R.; Oliveira, J.M.; Xavier, F.A.S. Changes in soil organic carbon fractions in response to cover crops in an orange orchard. Rev. Bras. Cienc. Solo 2016, 40, e0150105. [Google Scholar] [CrossRef]
- Repullo-Ruibérriz de Torres, M.A.; Carbonell-Bojollo, R.M.; Moreno-García, M.; Ordóñez-Fernández, R.; Rodríguez-Lizana, A. Soil organic matter and nutrient improvement through cover crops in a Mediterranean olive orchard. Soil Tillage Res. 2021, 210, 104977. [Google Scholar] [CrossRef]
- Novara, A.; Minacapilli, M.; Santoro, A.; Rodrigo-Comino, J.; Carrubba, A.; Sarno, M.; Venezia, G.; Gristina, L. Real cover crops contribution to soil organic carbon sequestration in sloping vineyard. Sci. Total Environ. 2019, 652, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Sainju, U.M.; Singh, B.P.; Whitehead, W.F. Long-term effects of tillage, cover crops, and nitrogen fertilization on organic carbon and nitrogen concentrations in sandy loam soils in Georgia, USA. Soil Tillage Res. 2002, 63, 167–179. [Google Scholar] [CrossRef]
- Abera, G.; Wolde-Meskel, E.; Bakken, L. Unexpected high decomposition of legume residues in dry season soils from tropical coffee plantations and crop lands. Agron. Sustain. Dev. 2014, 34, 667–676. [Google Scholar] [CrossRef]
- Gijsman, A.J.; Alarcón, H.F.; Thomas, R.J. Root decomposition in tropical grasses and legumes, as affected by soil texture and season. Soil Biol. Biochem. 1997, 29, 1443–1450. [Google Scholar] [CrossRef]
- Vieira, F.C.B.; Bayer, C.; Zanatta, J.A.; Mielniczuk, J.; Six, J. Building up organic matter in a subtropical pleudult under legume cover-crop-based rotations. Soil Sci. Soc. Am. J. 2009, 73, 1699–1706. [Google Scholar] [CrossRef]
- Boyd, N.; Van Acker, R. The effects of depth and fluctuating soil moisture on the emergence of eight annual and six perennial plant species. Weed Sci. 2003, 51, 725–730. [Google Scholar] [CrossRef]
- Roth, G.; Curran, W.; Dillon, C.; Houser, C.; Harkcom, W. Cover Crop Interseeder and Applicator; Pennsylvania State University: University Park, PA, USA, 2015. [Google Scholar]
- Wilson, M.; Baker, J.; Allan, D. Factors affecting successful establishment of aerially seeded winter rye. Agron. J. 2013, 105, 6–1868. [Google Scholar] [CrossRef]


| Treatment | Category of Cover Crop § | October 2018 | January 2019 | June 2019 | November 2019 | May 2020 | November 2020 | June 2021 | November 2021 | Seeding Rate kg ha−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| LG + NL | Legumes | Crotalaria juncea L., Sesbania grandiflora L. Poir., Alysicarpus vaginalis L. DC., Trifolium incarnatum L., Melilotus officinalis L. Lam | Helianthus annuus L., T. repens L., T. incarnatum L. | C. juncea L. | C. juncea L., S. grandiflora L., A. vaginalis L., T. incarnatum L., M. officinalis L. Lam | C. juncea L., S. grandiflora L., A. vaginalis L., T. incarnatum L., M. officinalis L. Lam | C. juncea L., Pisum sativum L. | C. juncea L., Vigna unguiculata L. Walp. | C. juncea L. | 50–100 |
| Non-legumes | Raphanus sativus L., Avena sativa L., Secale cereale L., Panicum miliaceum L. | R. sativus L., S. cereale L., Fagopyrum esculentum Moench | F. esculentum M., P. miliaceum L., Urochloa ramosa (L.) T. Q. Nguyen | R. sativus L., A. sativa L., S. cereale L., P. miliaceum L. | R. sativus L., A. sativa L., S. cereale L., P. miliaceum L. | R. sativus L., A. sativa L., S. cereale L. | F. esculentum M., Urochloa ramosa (L.) T. Q. Nguyen | R. sativus L., A. sativa L., S. cereale L. | 150–200 | |
| NL | Non-legumes | R. sativus L., A. sativa L., S. cereale L., P. miliaceum L. | R. sativus L., S. cereale L., F. esculentum M. | F. esculentum M., P. miliaceum L., Urochloa ramosa (L.) T. Q. Nguyen | R. sativus L., A. sativa L., S. cereale L., P. miliaceum L. | R. sativus L., A. sativa L., S. cereale L., P. miliaceum L. | R. sativus L., A. sativa L., S. cereale L. | F. esculentum M., Urochloa ramosa (L.) T. Q. Nguyen | R. sativus L., A. sativa L., S. cereale L. | 150–200 |
| Site | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North Grove | South Grove | |||||||||||
| Treatment | ||||||||||||
| Soil Properties | LG + NL | NL | GSC | LG + NL | NL | GSC | ||||||
| Baseline 2018 | 3-year 2021 | Baseline 2018 | 3-year 2021 | Baseline 2018 | 3-year 2021 | Baseline 2018 | 3-year 2021 | Baseline 2018 | 3-year 2021 | Baseline 2018 | 3-year 2021 | |
| NH4+-N (mg kg−1) | 6.2 | 6.3 | 5.6 | 4.7 | 4.6 | 4.61 | NA | 2.8 | NA | 3.3 | NA | 2.9 |
| NO3−-N (mg kg−1) | 2.2 | 7.6 | 2.6 | 7 | 2.3 | 4.9 | 2.7 | 1.8 | 2.8 | 1.1 | 3 | 1.2 |
| P (mg kg−1) | 950 | 360 | 909 | 477 | 869 | 441 | 79 | 59 | 88 | 55 | 91 | 53 |
| Ca (mg kg−1) | 4428 | 2457 | 3995 | 2672 | 3752 | 2758 | 576 | 921 | 524 | 549 | 703 | 700 |
| Mg (mg kg−1) | 337 | 254 | 316 | 245 | 313 | 246 | 50 | 44 | 48 | 43 | 57 | 45 |
| Organic Matter (%) | 2.88 | 3.41 | 2.79 | 3.39 | 2.29 | 2.5 | 0.7 | 1 | 0.8 | 0.9 | 0.7 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brewer, M.; Kanissery, R.G.; Strauss, S.L.; Kadyampakeni, D.M. Impact of Cover Cropping on Temporal Nutrient Distribution and Availability in the Soil. Horticulturae 2023, 9, 1160. https://doi.org/10.3390/horticulturae9101160
Brewer M, Kanissery RG, Strauss SL, Kadyampakeni DM. Impact of Cover Cropping on Temporal Nutrient Distribution and Availability in the Soil. Horticulturae. 2023; 9(10):1160. https://doi.org/10.3390/horticulturae9101160
Chicago/Turabian StyleBrewer, Miurel, Ramdas G. Kanissery, Sarah L. Strauss, and Davie M. Kadyampakeni. 2023. "Impact of Cover Cropping on Temporal Nutrient Distribution and Availability in the Soil" Horticulturae 9, no. 10: 1160. https://doi.org/10.3390/horticulturae9101160






