The Influence of the Spectral Composition and Light Intensity on the Morphological and Biochemical Parameters of Spinach (Spinacia oleracea L.) in Vertical Farming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Spinach Variety
2.2. Cultivation Conditions
2.3. Determination of Biometric and Biochemical Indicators
2.4. Fluorescence Measurement
2.5. Data Analysis
3. Results and Discussion
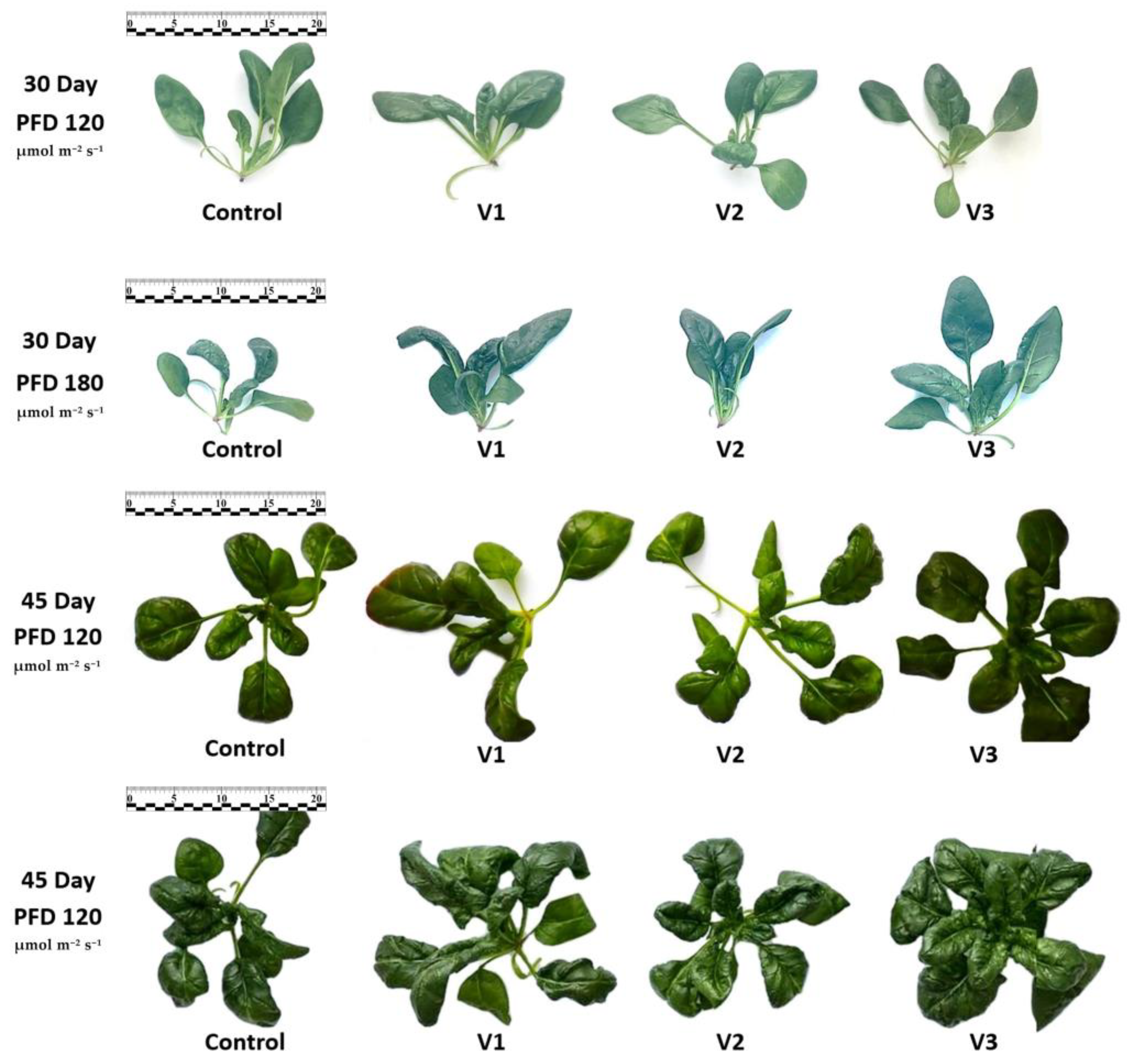
3.1. Morphological Parameters of Spinach Plants
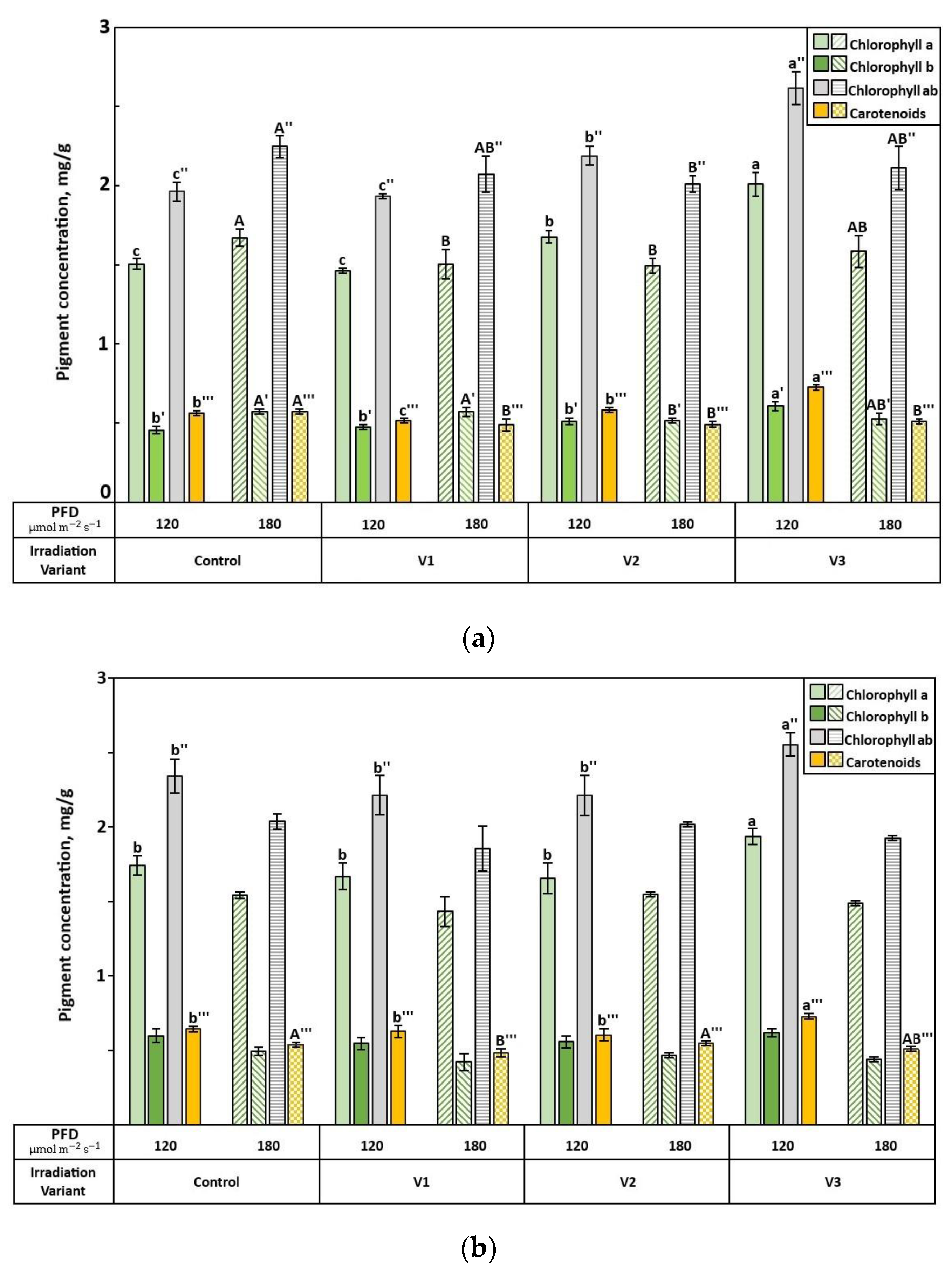
3.2. Biochemical Parameters of Spinach Plants
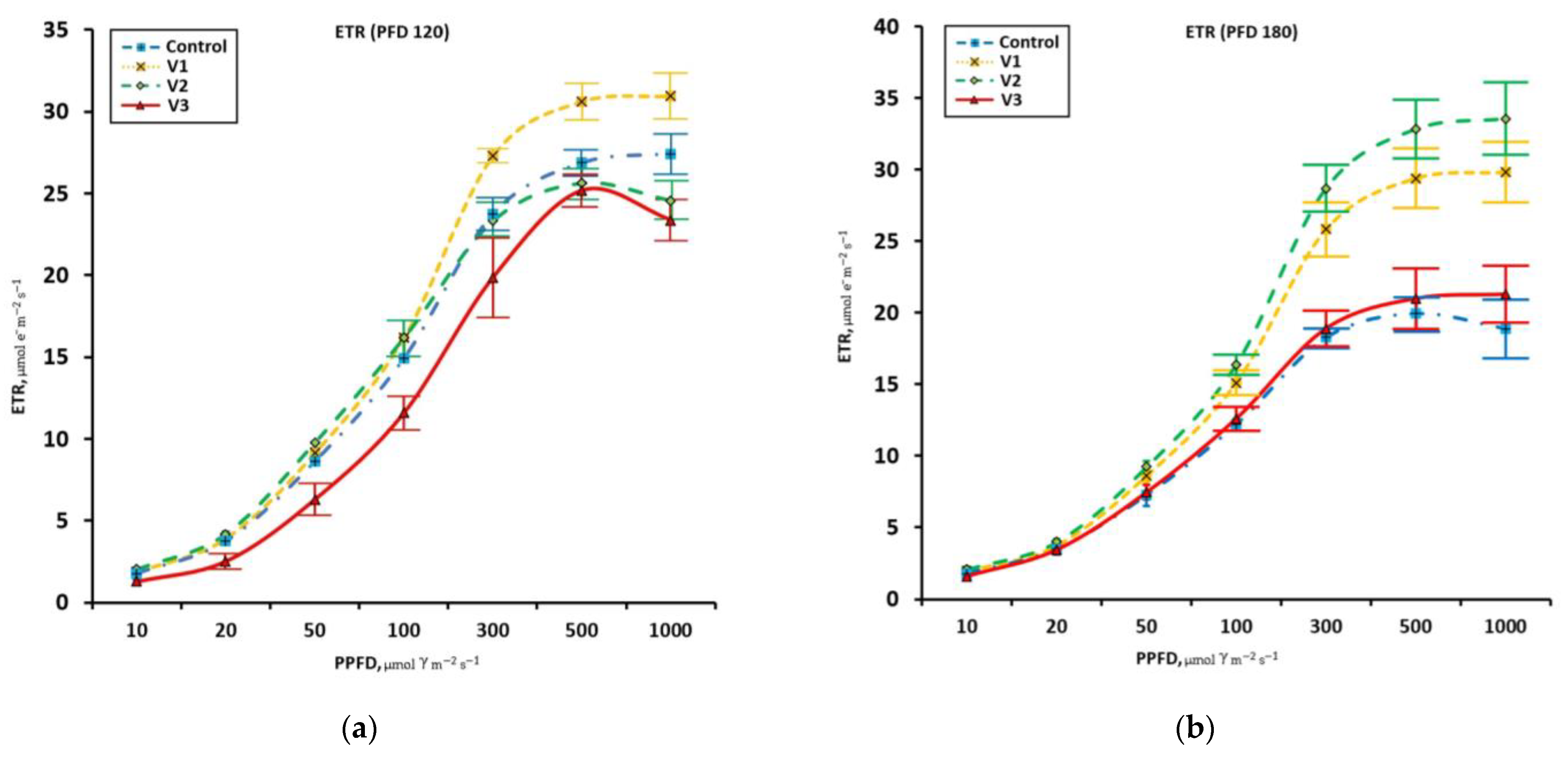
3.3. Chlorophyll Fluorescence Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roberts, J.L.; Moreau, R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Li, J.; Neja, S.; Kapoor, S.; Perez, J.E.T.; Tripathi, C.; Menon, R.; Jayaraman, A.; Lee, K.; Dashwood, W.M.; et al. Metabolomics of Acute vs. Chronic Spinach Intake in an Apc-Mutant Genetic Background: Linoleate and Butanoate Metabolites Targeting HDAC Activity and IFN-γ Signaling. Cells 2022, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Park, J.H.; Kim, S.Y.; Lee, S.W.; Chun, S.S.; Park, E. Antioxidant Effects of Spinach (Spinacia oleracea L.) Supplementation in Hyperlipidemic Rats. Prev. Nutr. Food. Sci. 2014, 19, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.M.P.; Velazquez, E.G.; Carrera, S.P.P. Spinacia oleracea Linn Considered as One of the Most Perfect Foods: A Pharmacological and Phytochemical Review. Mini Rev. Med. Chem. 2019, 19, 1666–1680. [Google Scholar] [CrossRef]
- EWG’s 2022 Shopper’s Guide to Pesticides in Produce. 2022. Available online: https://www.ewg.org/foodnews/summary.php (accessed on 7 September 2023).
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef]
- Najera, C.; Urrestarazu, M. Effect of the intensity and spectral quality of LED light on yield and nitrate accumulation in vegetables. HortScience 2019, 54, 1745–1750. [Google Scholar] [CrossRef]
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Nguyen, T.-P.-D.; Vu, N.-T.; Tran, T.-T.-H.; Nguyen, Q.-T.; Cao, P.-B.; Kim, I.-S.; Jang, D.-C. Growth and Quality of Hydroponic Cultivated Spinach (Spinacia oleracea L.) Affected by the Light Intensity of Red and Blue LEDs. Sains Malays. 2022, 51, 473–483. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of Light Quality on Growth and Vegetable Quality in Leaf Lettuce, Spinach and Komatsuna. Environ. Control. Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Zou, T.; Huang, C.; Wu, P.; Ge, L.; Xu, Y. Optimization of Artificial Light for Spinach Growth in Plant Factory Based on Orthogonal Test. Plants 2020, 9, 490. [Google Scholar] [CrossRef]
- Lebedeva, O.S.; Osipova, G.S. The Influence of Irradiation Power on the Growth, Development and Productivity of Spinach Varieties When Grown in Closed Systems. Intellectual Potential of Young Scientists as a Driver for the Development of the Agro-Industrial Complex: Proceedings of the International Scientific and Practical Conference of Young Scientists and Students; St. Petersburg State Agrarian University: St Petersburg, Russia, 2021; pp. 97–100. [Google Scholar]
- Agarwal, A.; Gupta, S.D.; Barman, M.; Mitra, A. Photosynthetic apparatus plays a central role in photosensitive physiological acclimations affecting spinach (Spinacia oleracea L.) growth in response to blue and red photon flux ratios. Environ. Exp. Bot. 2018, 156, 170–182. [Google Scholar] [CrossRef]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Kurata, K. Analysis of the relationship between blue-light photon flux density and the photosynthetic properties of spinach (Spinacia oleracea L.) leaves with regard to the acclimation of photosynthesis to growth irradiance. Soil Sci. Plant Nutr. 2007, 53, 459–465. [Google Scholar] [CrossRef]
- Spinach. 7 Components of Cultural Success. Available online: https://gavrishprof.ru/info/publications/shpinat-7-sostavlyayushchih-uspeha-kultury (accessed on 7 September 2023).
- Semenova, N.A.; Smirnov, A.A.; Dorokhov, A.S.; Proshkin, Y.A.; Ivanitskikh, A.S.; Chilingaryan, N.O.; Dorokhov, A.A.; Yanykin, D.V.; Gudkov, S.V.; Izmailov, A.Y. Evaluation of the Effectiveness of Different LED Irradiators When Growing Red Mustard (Brassica juncea L.) in Indoor Farming. Energies 2022, 15, 8076. [Google Scholar] [CrossRef]
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–434. [Google Scholar] [CrossRef] [PubMed]
- GOST 29270-95–2012; Interstate Standard for Processed Fruits and Vegetables. Methods for Determining Nitrates. Interstate Council for Standardization, Metrology and Certification: Moscow, Russia, 2012; p. 11.
- Padhi, B.; Chauhan, G.; Kandoi, D.; Stirbet, A.; Tripathy, B.C.; Govindjee, G. A comparison of chlorophyll fluorescence transient measurements, using Handy PEA and FluorPen fluorometers. Photosynthetica 2021, 59, 399–408. [Google Scholar] [CrossRef]
- Puglielli, G.; Redondo-Gómez, S.; Gratani, L.; Mateos-Naranjo, E. Highlighting the differential role of leaf paraheliotropism in two Mediterranean Cistus species under drought stress and well-watered conditions. J. Plant Physiol. 2017, 213, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lin, N.; Liu, X.; Yang, S.; Wang, W.; Wan, X. From Chloroplast Biogenesis to Chlorophyll Accumulation: The Interplay of Light and Hormones on Gene Expression in Camellia sinensis cv. Shuchazao Leaves. Front. Plant Sci. 2020, 11, 256. [Google Scholar] [CrossRef]
- Kappel, F.; Flore, J.A. Effect of Shade on Photosynthesis, Specific Leaf Weight, Leaf Chlorophyll Content, and Morphology of Young Peach Trees. J. Am. Soc. Hortic. Sci. 1983, 108, 541–544. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef]
- Naznin, M.; Lefsrud, M.; Gravel, V.; Azad, M. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Liu, H.; Zhang, Y.; Hao, Y.; Song, S.; Lei, B. Effect of supplemental blue light intensity on the growth and quality of Chinese kale. Hortic. Environ. Biotechnol. 2018, 60, 49–57. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Vitale, L.; Vitale, E.; Guercia, G.; Turano, M.; Arena, C. Effects of different light quality and biofertilizers on structural and physiological traits of Spinach plants. Photosynthetica 2020, 58, 932–943. [Google Scholar] [CrossRef]
- Resolution of the Chief State Sanitary Doctor of the Russian Federation Dated November 14, 2001 N 36 “On the Implementation of Sanitary Rules” (as Amended on 31 May, 20 August 2002, 15 April 2003). Available online: https://soeks.ru/informaciya/normy_pdk (accessed on 7 September 2023).
- Technical Regulations of the Customs Union TR CU 021/2011 “On the Safety of Food Products” (as Amended on 14 July 2021). Available online: https://docs.cntd.ru/document/902320560 (accessed on 7 September 2023).
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A Review of Environment Effects on Nitrate Accumulation in Leafy Vegetables Grown in Controlled Environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef]
- Bian, Z.H.; Cheng, R.F.; Wang, Y.; Yang, Q.C.; Lu, C.G. Effect of green light on nitrate reduction and edible quality of hydroponically grown lettuce (Lactuca sativa L.) under short-term continuous light from red and blue light- emitting diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Jankauskienė, J.; Viršilė, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Open Life Sci. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Council Regulation of the European Economic Community 1881/2006 of December 19, 2006 on the Establishment of Maximum Permissible Contents of Certain Pollutants (Contaminants) in Food Products Appendix. Maximum Levels of Contaminants in Food Section 1: Nitrates. Available online: https://base.garant.ru/2568274/baf8d0298b9a3923e3794eecbe3d1996/ (accessed on 7 September 2023).
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Sun, J.; Nishio, J.N.; Vogelmann, T.C. Green Light Drives CO2 Fixation Deep within Leaves. Plant Cell Physiol. 1998, 39, 1020–1026. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.A.; Schwartz, J.M.; Johnson, G.N. From empirical to theoretical models of light response curves—Linking photosynthetic and metabolic acclimation. Photosynth. Res. 2020, 145, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Li, B.; Zhang, H.; Hu, J. Optimization and Control of the Light Environment for Greenhouse Crop Production. Sci. Rep. 2019, 9, 8650. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of Lettuce Growth under an Increasing Daily Light Integral Depends on the Combination of the Photosynthetic Photon Flux Density and Photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]


| Experimental Variant | Photon Flux, µmol Photons m−2 s−1 of Spectral Ranges (nm) | ||||
|---|---|---|---|---|---|
| Total PFD (400–800) | Blue (400–500) | Green (500–600) | Red (600–700) | Far-red (700–800) | |
| (µmol m−2 s−1) | (µmol m−2 s−1) | (µmol m−2 s−1) | (µmol m−2 s−1) | (µmol m−2 s−1) | |
| Control | 120 | 18.0 | 38.4 | 50.4 | 13.2 |
| 180 | 27.0 | 57.6 | 75.6 | 19.8 | |
| V1 | 120 | 14.4 | 24.0 | 75.6 | 6.0 |
| 180 | 21.6 | 36.0 | 113.4 | 9.0 | |
| V2 | 120 | 18.0 | 36.0 | 58.8 | 7.2 |
| 180 | 27.0 | 54.0 | 88.2 | 10.8 | |
| V3 | 120 | 36.0 | 1.2 | 81.6 | 1.2 |
| 180 | 54.0 | 1.8 | 122.4 | 1.8 | |
| Experimental Variant | PFD | Fresh Weight | Dry Weight | Plant Height | Number of Leaves | Leaf Surface Area |
|---|---|---|---|---|---|---|
| (µmol m−2 s−1) | (g) | (%) | (cm) | (pcs.) | (cm2) | |
| 30th day of cultivation | ||||||
| Control | 120 | 5.2 ± 1.0 c | 7.7 ± 1.7 b | 9.6 ± 1.9 b | 6.6 ± 0.5 b | 93.1 ± 17.8 c |
| 180 | 4.2 ± 1.3 c | 10.6 ± 3.5 a | 13.3 ± 2.5 a | 6.1 ± 0.8 b | 96.2 ± 12.7 c | |
| V1 | 120 | 8.0 ± 1.4 b | 6.4 ± 1.0 b | 9.9 ± 1.2 b | 7.9 ± 1.0 a | 140.2 ± 17.7 b |
| 180 | 11.1 ± 1.9 a | 7.3 ± 1.0 b | 15.5 ± 1.3 a | 7.4 ± 0.7 a | 179.3 ± 25.8 a | |
| V2 | 120 | 6.5 ± 0.9 bc | 7.0 ± 0.5 b | 8.6 ± 1.2 b | 6.7 ± 0.7 b | 111.2 ± 13.2 bc |
| 180 | 7.7 ± 1.2 b | 8.0 ± 0.6 ab | 14.0 ± 1.6 a | 7.0 ± 0.8 b | 127.6 ± 19.9 b | |
| V3 | 120 | 5.4 ± 0.5 c | 7.8 ± 0.5 b | 9.3 ± 0.9 b | 7.4 ± 0.5 a | 103.7 ± 7.9 c |
| 180 | 10.0 ± 2.0 ab | 7.9 ± 0.4 b | 12.9 ± 1.7 a | 7.9 ± 0.6 a | 179.6 ± 31.1 a | |
| 45th day of cultivation | ||||||
| Control | 120 | 19.7 ± 6.4 c | 7.5 ± 1.0 ab | 20.0 ± 1.9 | 9.6 ± 0.9 b | 328.9 ± 79.8 |
| 180 | 29.0 ± 6.0 a | 7.8 ± 0.8 a | 21.5 ± 2.6 | 10.6 ± 1.0 ab | 410.1 ± 70.3 | |
| V1 | 120 | 31.71 ± 6.0 a | 6.3 ± 0.3 b | 20.0 ± 3.7 | 11.1 ± 1.7 ab | 524.1 ± 112.2 |
| 180 | 32.9 ± 7.6 a | 7.4 ± 0.6 ab | 19.2 ± 3.7 | 12.3 ± 1.9 a | 460.5 ± 101.4 | |
| V2 | 120 | 22.2 ± 5.3 c | 7.2 ± 0.6 ab | 21.4 ± 3.1 | 10.1 ± 1.4 ab | 422.8 ± 110.9 |
| 180 | 29.0 ± 10.8 b | 7.5 ± 0.9 ab | 19.8 ± 2.7 | 11.9 ± 2.5 a | 421.8 ± 122.0 | |
| V3 | 120 | 22.5 ± 3.3 c | 6.6 ± 0.3 b | 19.0 ± 1.6 | 10.7 ± 1.2 ab | 391.5 ± 61.38 |
| 180 | 33.8 ± 12.9 a | 7.3 ± 1.3 ab | 21.4 ± 1.3 a | 12.9 ± 2.2 a | 492.6 ± 174.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, N.A.; Proshkin, Y.A.; Smirnov, A.A.; Dorokhov, A.S.; Ivanitskikh, A.S.; Burynin, D.A.; Dorokhov, A.A.; Uyutova, N.I.; Chilingaryan, N.O. The Influence of the Spectral Composition and Light Intensity on the Morphological and Biochemical Parameters of Spinach (Spinacia oleracea L.) in Vertical Farming. Horticulturae 2023, 9, 1130. https://doi.org/10.3390/horticulturae9101130
Semenova NA, Proshkin YA, Smirnov AA, Dorokhov AS, Ivanitskikh AS, Burynin DA, Dorokhov AA, Uyutova NI, Chilingaryan NO. The Influence of the Spectral Composition and Light Intensity on the Morphological and Biochemical Parameters of Spinach (Spinacia oleracea L.) in Vertical Farming. Horticulturae. 2023; 9(10):1130. https://doi.org/10.3390/horticulturae9101130
Chicago/Turabian StyleSemenova, Natalya A., Yuri A. Proshkin, Alexandr A. Smirnov, Alexey S. Dorokhov, Alina S. Ivanitskikh, Dmitry A. Burynin, Artem A. Dorokhov, Nadezhda I. Uyutova, and Narek O. Chilingaryan. 2023. "The Influence of the Spectral Composition and Light Intensity on the Morphological and Biochemical Parameters of Spinach (Spinacia oleracea L.) in Vertical Farming" Horticulturae 9, no. 10: 1130. https://doi.org/10.3390/horticulturae9101130






