Exploring the Identity and Properties of Two Bacilli Strains and their Potential to Alleviate Drought and Heavy Metal Stress
Abstract
:1. Introduction
2. Materials and Methods
- Biochemical characteristics
- Plant Growth Promoting Rhizobacterial traits evaluation
2.1. Identification of Bacterial Strains Based on 16s rRNA Sequence
2.2. Screening ACC Deaminase Activity from Isolated Rhizobacteria
2.2.1. ACC Deaminase Activity (Qualitative)
2.2.2. ACC Deaminase Quantified from Selected Bacterial Isolates
2.2.3. Measurement of ACC Deaminase (Enzyme) Activity
2.2.4. Standard Curve of α-Ketobutyrate
2.3. Portrayal of Partially Purified ACC Deaminase Enzyme
2.4. Quantification of Partially Purified Protein by Bradford Method
2.5. Determination of Molecular Weight of ACC Deaminase
2.6. Estimation of Exopolysaccharide (EPS)
2.7. Protein Content in EPS: PGPRs Potentials at Various Levels of Drought
2.8. Heavy Metal Tolerance Test by Plate Method
3. Results
3.1. Soil Properties
3.2. Bacterial Properties
3.3. Identification of PGPRs by 16s rRNA Sequence
3.4. Screening ACC Deaminase Activity from Rhizobacteria Isolates
3.4.1. ACC Deaminase Qualitative Test
3.4.2. Quantification of ACC Deaminase
3.4.3. Measurement of ACC Deaminase Activity
3.4.4. Characterization of Partially Purified ACC Deaminase Enzyme
3.4.5. Quantification of Partially Purified Protein Extraction by Bradford Method
3.4.6. Determination of Molecular Weight of ACC Deaminase
3.5. Quantification of EPS
Protein content in EPS
3.6. Heavy Metal Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnawal, D.; Singh, R.; Singh, R.P. Role of Plant Growth Promoting Rhizobacteria in Drought Tolerance: Regulating Growth Hormones and Osmolytes. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 107–128. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Sopory, S.K. Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 2008, 3, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kour, D.; Khan, S.S.; Kaur, T.; Kour, H.; Singh, G.; Yadav, A.; Yadav, A.N. Drought adaptive microbes as bioinoculants for the horticultural crops. Heliyon 2022, 8, e09493. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Shahid, M.; Syed, A.; Rajput, V.D.; Elgorban, A.M.; Minkina, T.; Bahkali, A.H.; Lee, J. Drought tolerant enterobacter sp./Leclercia adecarboxylata secretes indole-3-acetic acid and other biomolecules and enhances the biological attributes of Vigna radiata (L.) R. Wilczek in water deficit conditions. Biology 2021, 10, 1149. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Rajput, V.D.; Kumari, A.; Espinosa-Saiz, D.; Menendez, E.; Minkina, T.; Dwivedi, P.; Mandzhieva, S. Plant growth-promoting rhizobacteria: A potential bio-asset for restoration of degraded soil and crop productivity with sustainable emerging techniques. Environ. Geochem. Health 2022. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Sheikh, I.; Kumar, V.; Yadav, A.N.; Dhaliwal, H.S.; Saxena, A.K. Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, adrought-adaptive phosphorus-solubilizing bacterium. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 785–795. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Rao, L.V. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann. Microbiol. 2014, 64, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moenne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dye, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Fulekar, M.H.; Sharma, J.; Tendulkar, A. Bioremediation of heavy metals using biostimulation in laboratory bioreactor. Environ. Monit. Assess. 2012, 184, 7299–7307. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Kumar, S.; Patil, B.S.; Bhat, J.S.; Sharma, M.; Kemal, S.; Ontagodi, T.P.; Datta, S.; Patil, P.; Chaturvedi, S.K.; et al. Narrowing yield gaps through genetic improvement for Fusarium wilt resistance in three pulse crops of the semi-arid tropics. SABRAO J. Breed. Genet. 2013, 45, 341–370. [Google Scholar]
- Araujo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; Gonzalez, E.M.; Gruber, V.; Lejeune Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic stress responses in strategies used to cope with environmental challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 7 September 2022).
- Sharma, P.; Khanna, V.; Kumar, P.I. Efficacy of aminocyclopropane-1-carboxylic acid (ACC)-deaminase-producing rhizobacteria in ameliorating water stress in chickpea under axenic conditions. Afr. J. Microbiol. Res. 2013, 7, 5749–5757. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef] [Green Version]
- Andy, A.K.; Masih, S.A.; Gour, V.S. Isolation, screening and characterization of plant growth promoting rhizobacteria from rhizospheric soils of selected pulses. Biocatal. Agric. Biotechnol. 2020, 27, 101685. [Google Scholar] [CrossRef]
- Sharma, K. Manual of Microbiology: Tools & Techniques; Ane Books: Delhi, India; pp. 141–171.
- Bhaskara, R.K.V.; Ashwini, K.; Gaurav, K.; Karthik, L. Optimization, production and partial purification of extracellular á-amylase from Bacillus sp. marini. Arch. Appl. Sci. Res. 2011, 3, 33–42. [Google Scholar]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 363–370. [Google Scholar]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappuccino, J.C.; Sherman, N. Microbiology: A Laboratory Manual, 3rd ed.; Benjamin/Cummings Pub. Co.: New York, NY, USA, 1992; pp. 125–179. [Google Scholar]
- Lorck, H. Production of hydrocyanic acid by bacteria. Physiol. Plant 1948, 1, 142–146. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, D.; Kachhwaha, S.; Jain, R.; Kothari, S.L. PCR based detection of cry genes in indigenous strains of Bacillus thuringiensis isolated from the soils of Rajasthan. Indian J. Biotechnol. 2012, 11, 491–494. [Google Scholar]
- Deharvengt, S.J.; Petersen, L.M.; Jung, H.S.; Tsongalis, G.J. Chapter 13—Nucleic acid analysis in the clinical laboratory. In Contemporary Practice in Clinical Chemistry, 4th ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 215–234. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, M.; Foster, J.W. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592. [Google Scholar] [CrossRef] [Green Version]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant 2003, 118, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Maxton, A.; Singh, P.; Masih, S.A. ACC deaminase-producing bacteria mediated drought and salt tolerance in Capsicum annuum. J. Plant Nutr. 2018, 41, 574–583. [Google Scholar] [CrossRef]
- Jia, Y.J.; Kakuta, Y.; Sugawara, M.; Igarashi, T.; Oki, N.; KisAKi, M.; Shoji, T.; Kanetuna, Y.; Horita, T.; Matsui, H.; et al. Synthesis and degradation of 1-aminocyclopropane-1-carboxylic acid by Penicillium citrinum. Biosci. Biotechnol. Biochem. 1999, 63, 542–549. [Google Scholar] [CrossRef]
- Chug, R.; Mathur, S.; Kothari, S.; Gour, V.S. Maximizing EPS production from Pseudomonas aeruginosa and its application in Cr and Ni sequestration. Biochem. Biophys. Rep. 2021, 26, 100972. [Google Scholar] [CrossRef]
- Dunlap, C.A.; Schisler, D.A.; Perry, E.B.; Connor, N.; Cohan, F.M.; Rooney, A.P. Bacillus swezeyi sp. nov. and Bacillus haynesii sp. nov., isolated from desert soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Alemneh, A.A.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Is phosphate solubilizing ability in plant growth-promoting rhizobacteria isolated from chickpea linked to their ability to produce ACC deaminase? J. Appl. Microbiol. 2021, 131, 2416–2432. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Li, L.; Chen, G.; Su-Zhou, C.; Li, Y.; Merkeryan, H.; Liu, W.; Liu, X. 1-Aminocyclopropane1-carboxylate deaminase-producing plant growth-promoting rhizobacteria improve drought stress tolerance in grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 12, 706990. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11, e1071004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, B.R.; Nascimento, F.X. Pseudomonas 1-Aminocyclopropane-1-carboxylate (ACC) Deaminase and Its Role in Beneficial Plant-Microbe Interactions. Microorganisms 2021, 9, 2467. [Google Scholar] [CrossRef]
- Hontzeas, N.; Zoidakis, J.; Glick, B.R.; Abu-Omar, M.M. Expression and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the rhizobacterium Pseudomonas putida UW4: A key enzyme in bacterial plant growth promotion. Biochim. Biophys. Acta 2004, 1703, 11–19. [Google Scholar] [CrossRef]
- Fedorov, D.N.; Ekimova, G.A.; Doronina, N.V.; Trotsenko, Y.A. 1-Aminocyclopropane-1-carboxylate (ACC) deaminases from Methylobacterium radiotolerans and Methylobacterium nodulans with higher specificity for ACC. FEMS Microbiol. Lett. 2013, 343, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ekimova, G.A.; Fedorov, D.N.; Doronina, N.V.; Trotsenko, Y.A. 1-aminocyclopropane-1-carboxylate deaminase of the aerobic facultative methylotrophic actinomycete Amycolatopsis methanolica 239. Microbiology 2015, 84, 584–586. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkatesvarlu, B. Alleviation of drought stress effects in sunflower seedlings by exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Syed, S.; Chinthala, P. Heavy metal detoxification by different Bacillus species isolated from solar salterns. Scientifica 2015, 2015, 319760. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Sun, H.; Jiang, C.; Mao, H.; Zhang, Y. Immobilization of Cd in soil and changes of soil microbial community by bioaugmentation of UV-mutated Bacillus subtilis 38 assisted by biostimulation. Eur. J. Soil Biol. 2014, 65, 62–69. [Google Scholar] [CrossRef]
- Panyushkina, A.E.; Babenko, V.V.; Nikitina, A.S.; Selezneva, O.V.; Tsaplina, I.A.; Letarova, M.A.; Kostryukova, E.S.; Letarov, A.V. Sulfobacillus thermotolerans: New insights into resistance and metabolic capacities of acidophilic chemolithotrophs. Sci. Rep. 2019, 9, 15069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govarthanan, M.; Mythili, R.; Selvankumar, T. Bioremediation of heavy metals using an endophytic bacterium Paenibacillus sp. RM isolated from the roots of Tridax procumbens. 3 Biotech 2016, 6, 242. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
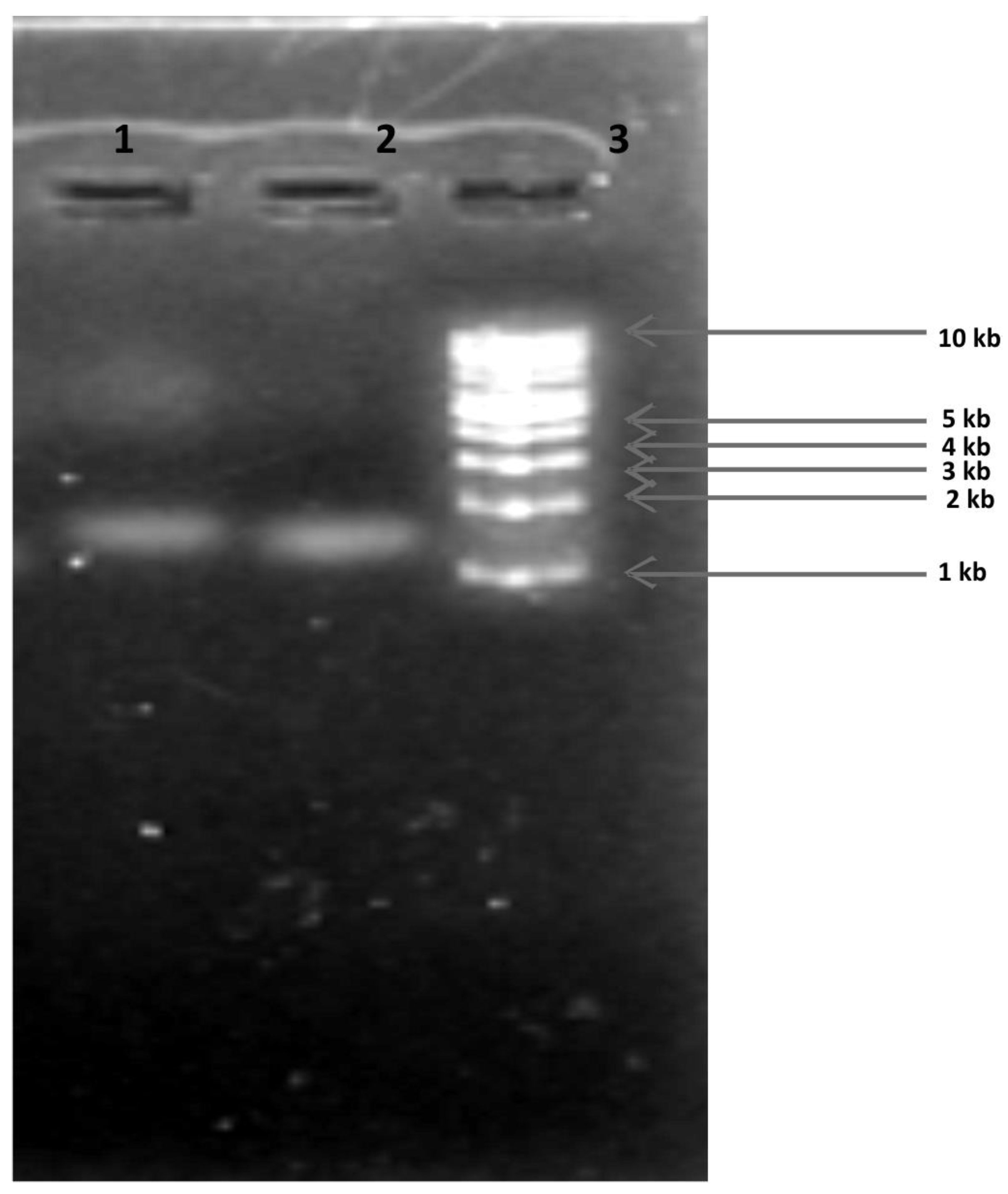
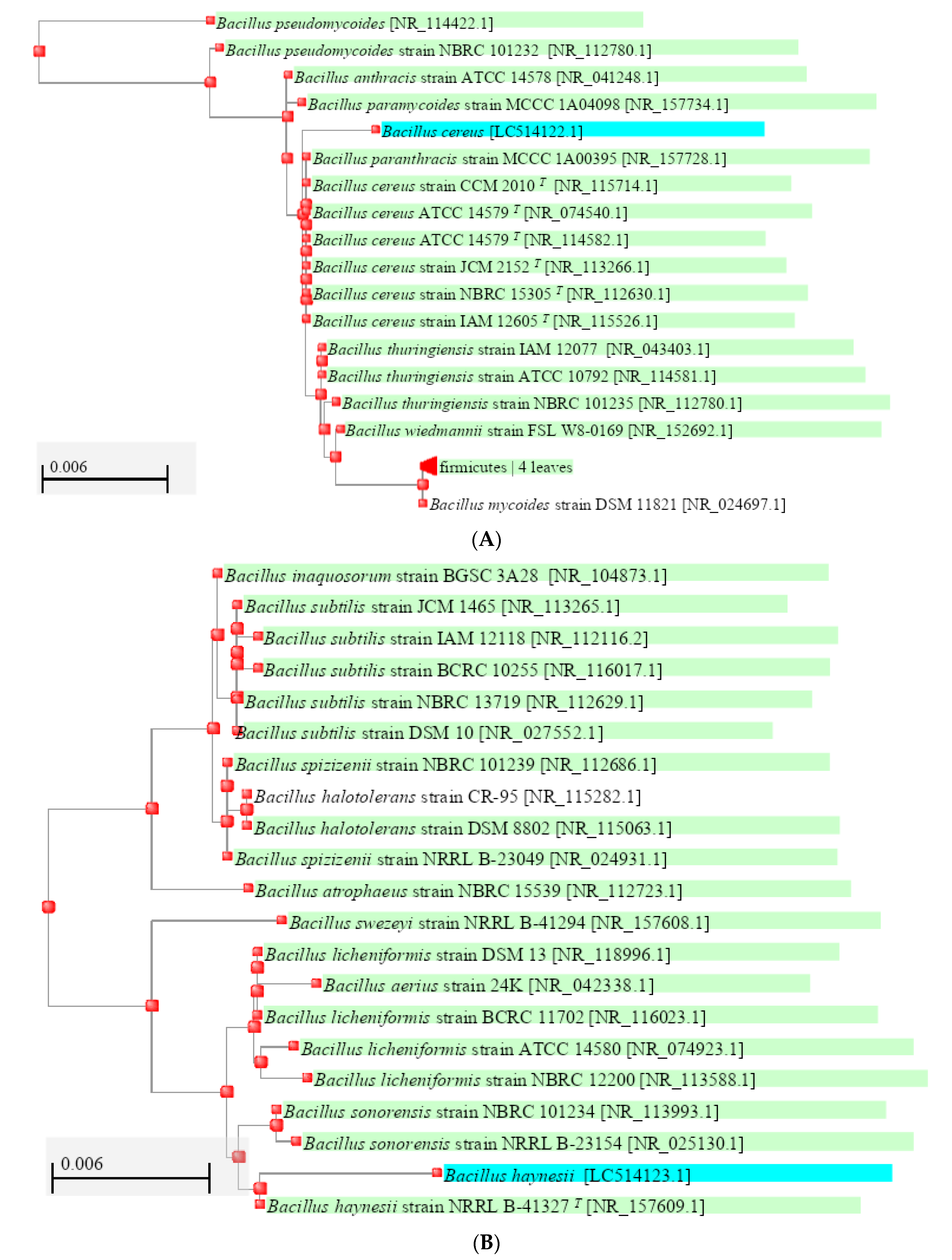
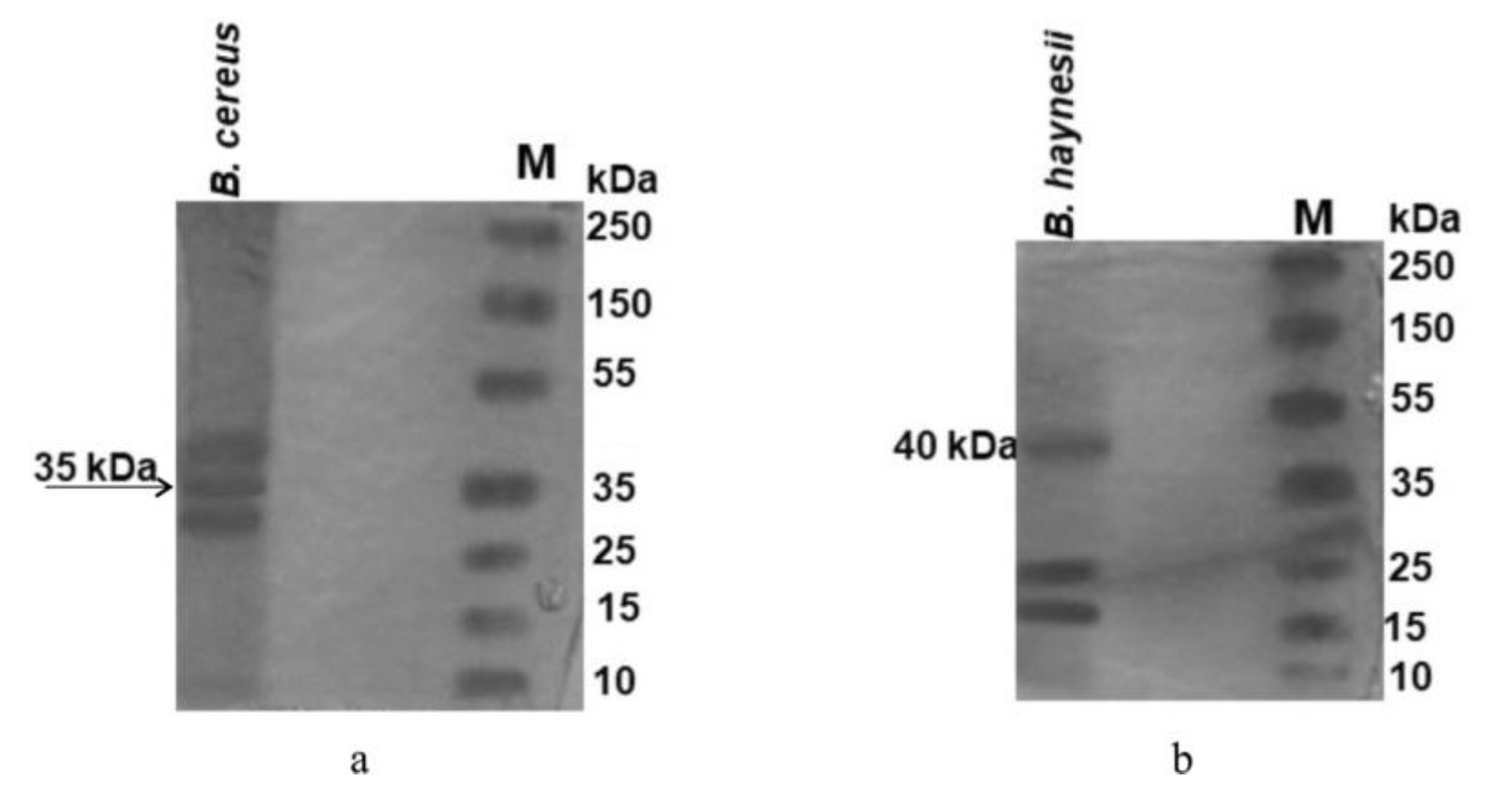
| Characteristics | AV-12 | AV-7 |
|---|---|---|
| Soil sample source | Vigna mungo | Phaseolus vulgaris |
| Morphology | ||
| Cell morphology | Round and Smooth colonies | Round and Smooth colonies# |
| Gram reaction | Gram-Negative | Gram-Negative # |
| Shape of organism | Bacilli (rod) | Cocci # |
| Spore formation | Observed | Observed |
| Arrangement of cells | Cells form clusters | Cells form clusters |
| Culture | ||
| Colony colour | White | White |
| Elevation | Convex | Convex |
| Biochemical tests | ||
| Indole test | Negative | Negative |
| Methyl red test | Positive | Positive # |
| VP test | Positive | Positive # |
| Catalase test | Positive | Positive # |
| Citrate utilization test | Positive | Positive |
| Phosphate solubilization | Positive | Positive # |
| Amylase hydrolysis | Positive | Positive |
| Carbohydrate production test | Positive | Positive |
| Carbohydrate fermentation | ||
| D-Glucose | Negative | Positive |
| Sucrose | Negative | -- |
| Maltose | Negative | Positive |
| PGP Traits | ||
| Phosphate solubilization (mg/mL) | 0.063 | 1.881 |
| Ammonia production (µmol/mL) | 0.518 | 0.413 |
| HCN production (µmol/mL) | 21.30 | 30.58 |
| Siderophore production (%) | 48.71 | 42.30 |
| Exopolysaccharide yield (mg/10mL) | 18 | 15 |
| ACC deaminase production (µmol/mL) | 5.484 | 6.008 |
| Molecular | ||
| BLAST Comparison (16S rDNA) | Bacillus cereus | Bacillus haynesii |
| Accession Number | LC514122 | LC514123 |
| Properties | B. cereus | B. haynesii |
|---|---|---|
| Cell density (Number/mL) | 108 × 106 | 108 × 106 |
| EPS (mg/mg protein) No Stress | 2.26 | 1.29 |
| EPS (mg/mg protein) Stress | 5.88 | 4.84 |
| ACC deaminase activity (µM αKB/mg/min) | 12.6 | 11.0 |
| Total protein in crude extract (mg) | 2.87 | 1.98 |
| After purification | ||
| ACC deaminase activity (µM/mg/min) | 3.33 | 2.85 |
| Mol weight of ACC deaminase (35-42 kDa) | 35 | 40 |
| Heavy metal stress at two concentrations of each heavy metal | ||
| Arsenic (As) [14.74 mg/L] | Resistant | Sensitive |
| Arsenic (As) [29.48 mg/L] | Resistant | Sensitive |
| Barium (Ba) [6.28 mg/L] | Resistant | Sensitive |
| Barium (Ba) [12.56 mg/L] | Resistant | Sensitive |
| Nickel (Ni) [2.963 mg/L] | Resistant | Sensitive |
| Nickel (Ni) [5.926 mg/L] | Resistant | Sensitive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andy, A.K.; Rajput, V.D.; Burachevskaya, M.; Gour, V.S. Exploring the Identity and Properties of Two Bacilli Strains and their Potential to Alleviate Drought and Heavy Metal Stress. Horticulturae 2023, 9, 46. https://doi.org/10.3390/horticulturae9010046
Andy AK, Rajput VD, Burachevskaya M, Gour VS. Exploring the Identity and Properties of Two Bacilli Strains and their Potential to Alleviate Drought and Heavy Metal Stress. Horticulturae. 2023; 9(1):46. https://doi.org/10.3390/horticulturae9010046
Chicago/Turabian StyleAndy, Aruna Kumari, Vishnu D. Rajput, Marina Burachevskaya, and Vinod Singh Gour. 2023. "Exploring the Identity and Properties of Two Bacilli Strains and their Potential to Alleviate Drought and Heavy Metal Stress" Horticulturae 9, no. 1: 46. https://doi.org/10.3390/horticulturae9010046






