Effects of NIR Reflective Film as a High Tunnel-Covering Material on Fruit Cracking and Biomass Production of Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Condition PAR
2.2. Destructive Measurements and Growth Analysis
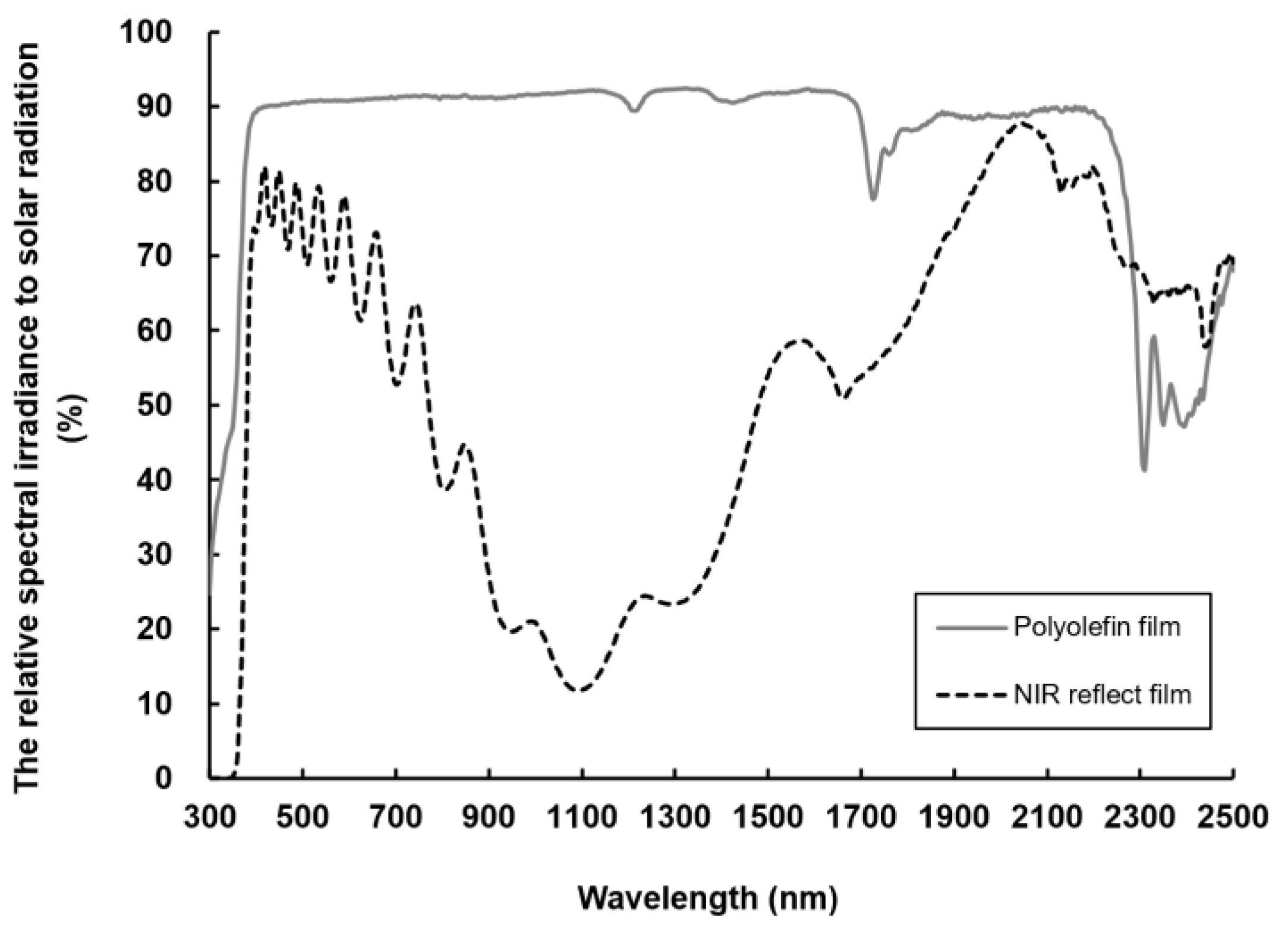
2.3. Measurement of Temperature and Fruit Quality
2.4. Determination of Chl and Total N per Leaf Area
2.5. Statistical analysis
3. Results
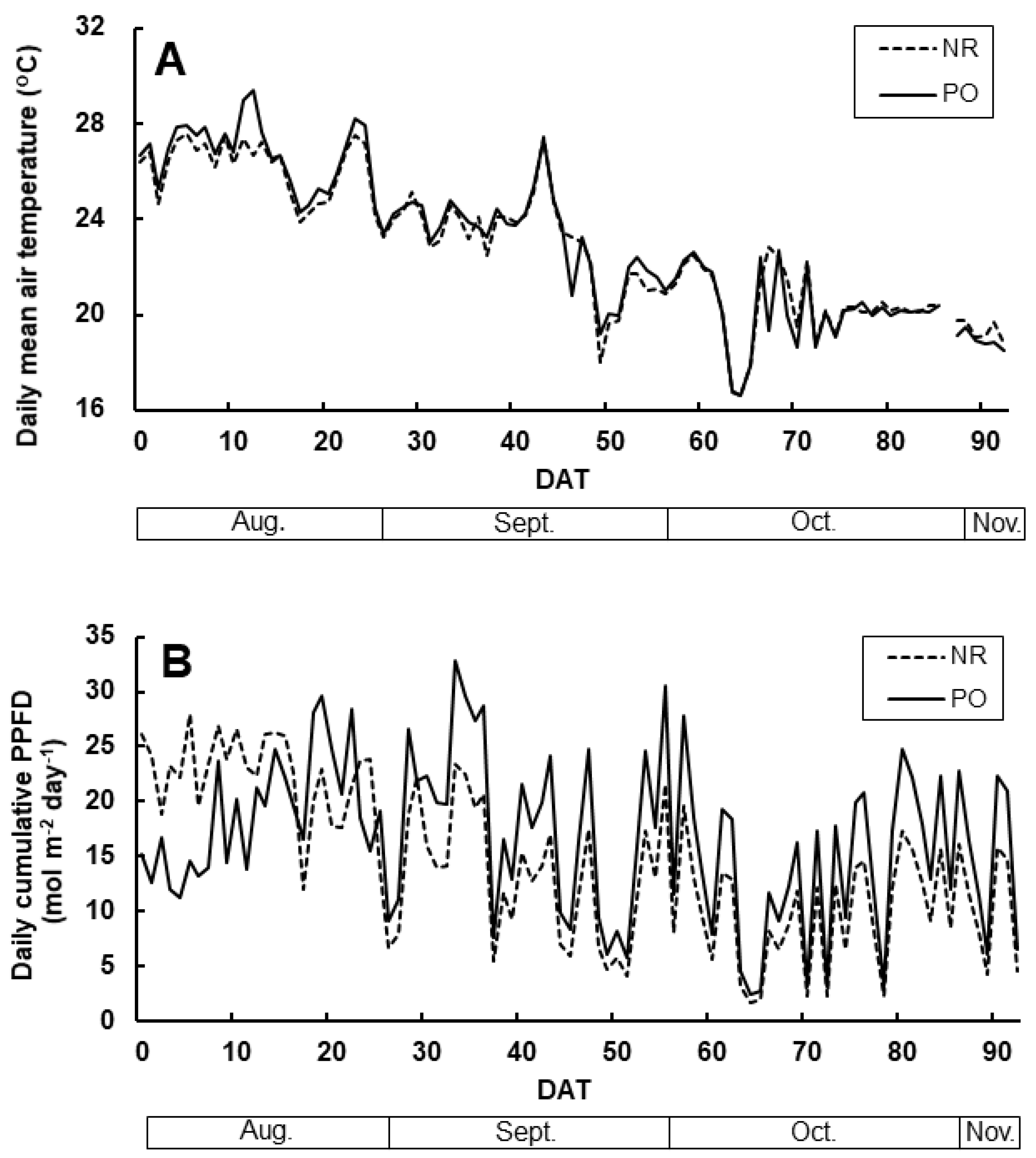
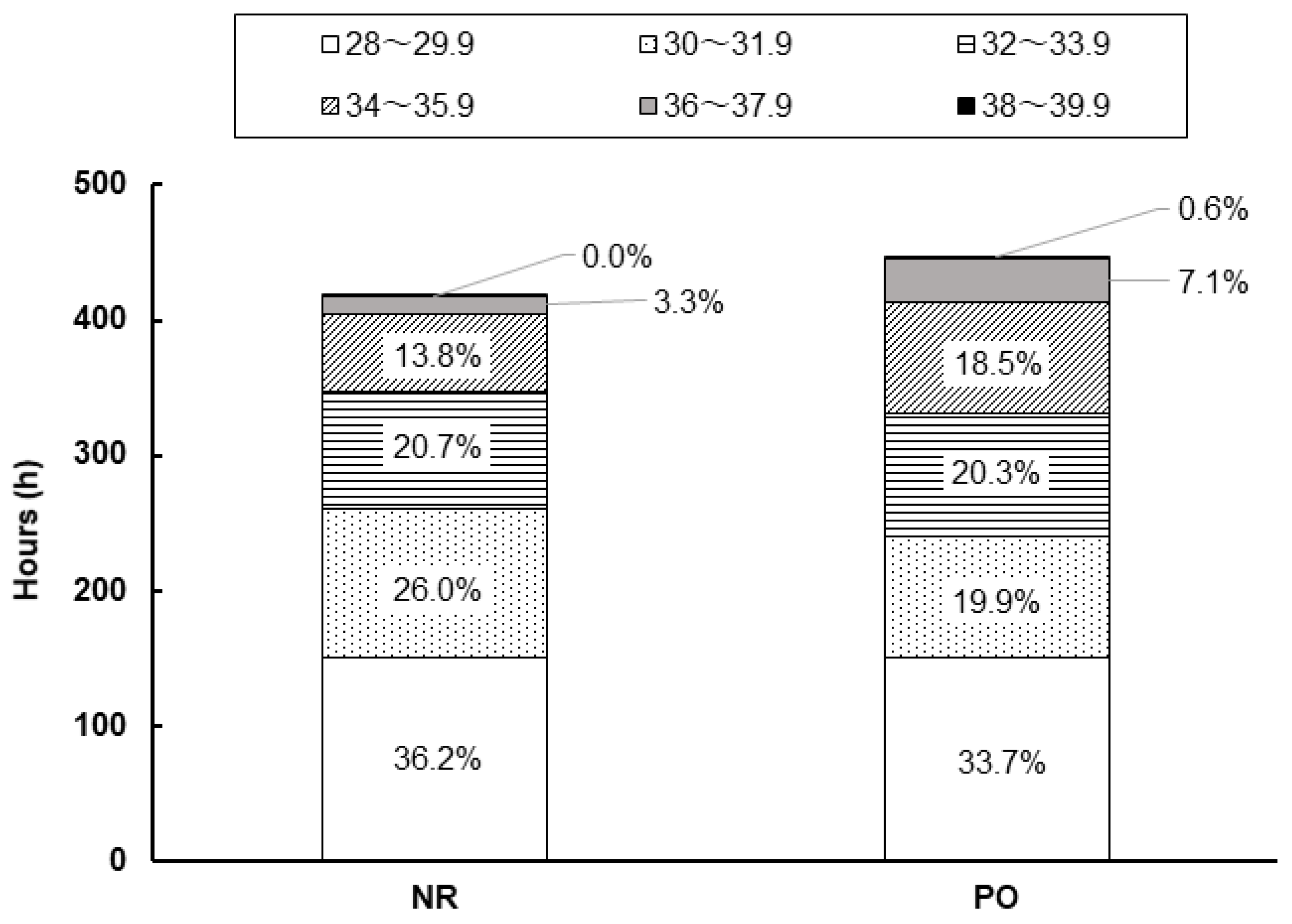
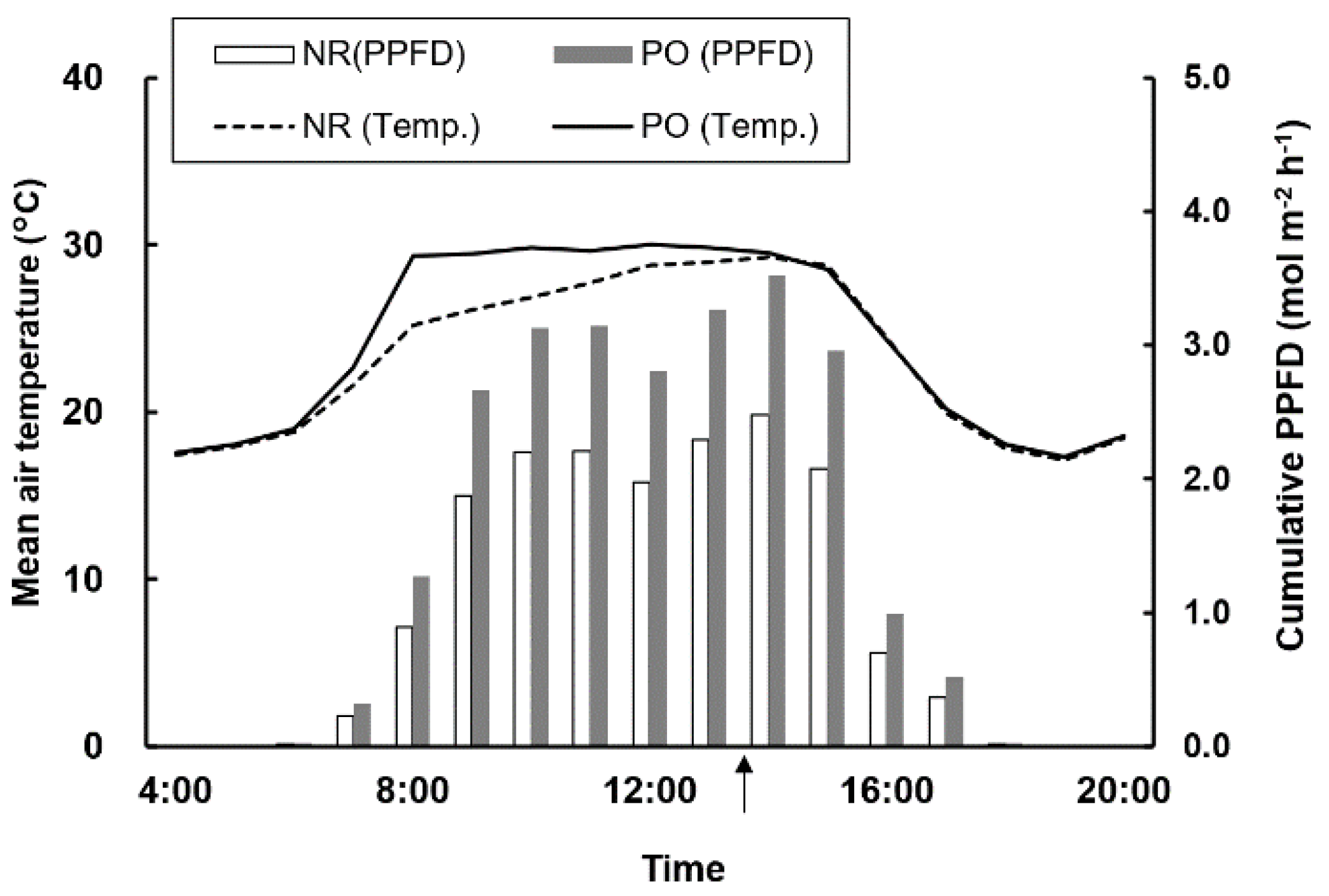
3.1. The Climate in High Tunnel
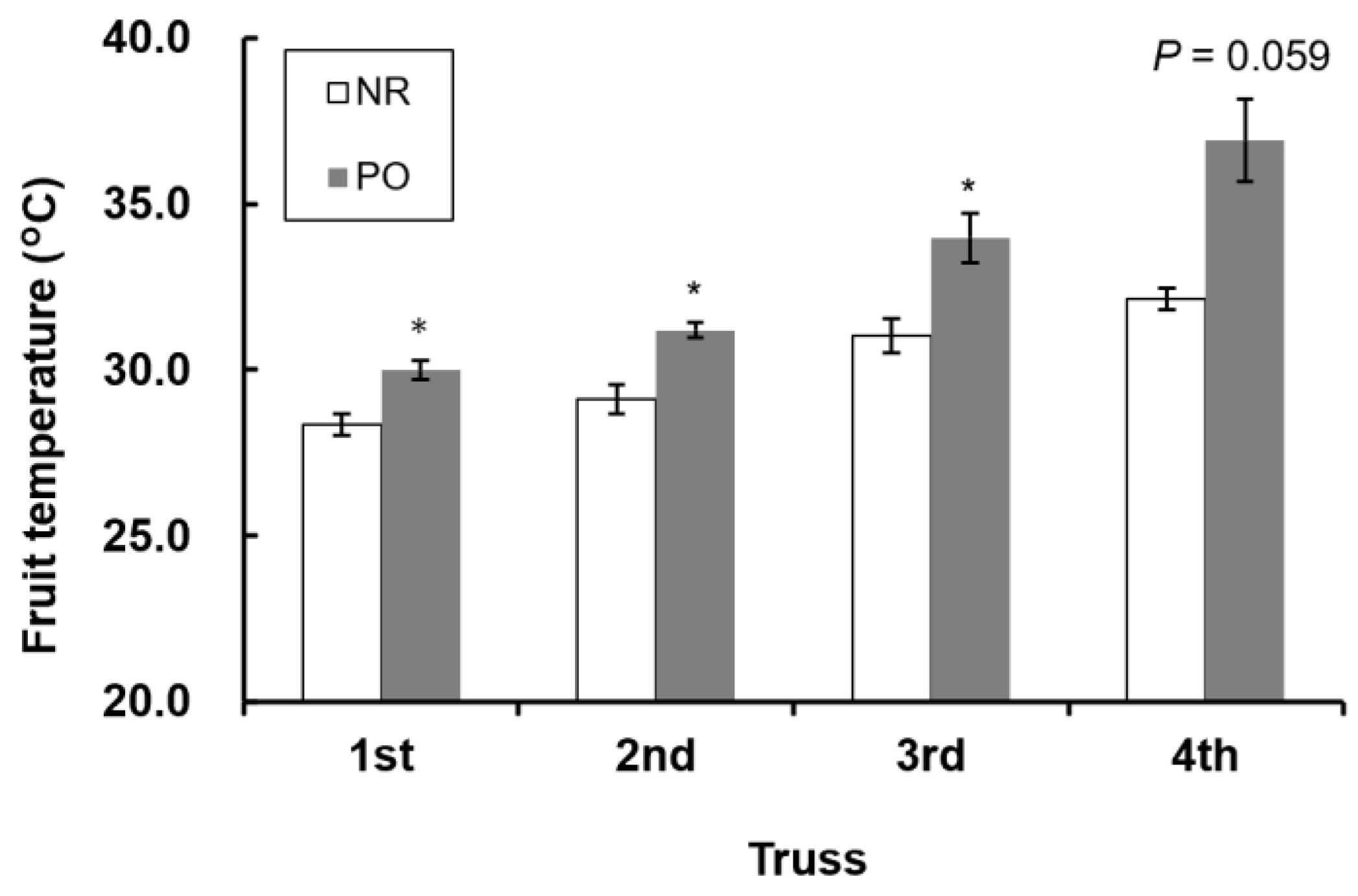
3.2. Fruit Yield and Quality
3.3. Plant Growth and Leaf Properties
4. Discussion
4.1. NIR Reflective Film Contributes to Fruit Quality
4.2. Changes in Plant Growth under NR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faostat. 2021. Available online: https://www.fao.org/faostat/ (accessed on 17 November 2021).
- Kinet, J.M.; Peet, M.M. 1997 Tomato. In The Physiology of Vegetable Crops; Wien, H.C., Ed.; C.A.B. International: Wallingford, UK, 1997; pp. 207–258. ISBN 9780851991467. [Google Scholar]
- Suzuki, K. Physiological disorders and their management in greenhouse tomato cultivation at high temperatures. In Adaptation to Climate Change in Agriculture; Izumi, T., Hirata, R., Matsuda, R., Eds.; Springer: Singapore, 2019; pp. 81–96. ISBN 978-981-13-9234-4. [Google Scholar] [CrossRef]
- Hemming, S.; Kempkes, F.; Van der Braak, N.; Dueck, T.; Marissen, N. Greenhouse cooling by NIR-reflection. Acta Hortic. 2006, 719, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Mutwiwa, U.N.; Von Elsner, B.; Tantau, H.J.; Max, J.F.J. Cooling naturally ventilated greenhouses in the tropics by near-infra red reflection. Acta Hortic. 2008, 801, 259–266. [Google Scholar] [CrossRef]
- López-Marín, J.; González, A.; García-Alonso, Y.; Espí, E.; Salmerón, A.; Fontecha, A.; Real, A.I. Use of cool plastic films for greenhouse covering in Southern Spain. Acta Hortic. 2008, 801, 181–186. [Google Scholar] [CrossRef]
- Stanghellini, C.; Dai, J.; Kempkes, F. Effect of near-infrared-radiation reflective screen materials on ventilation requirement, crop transpiration and water use efficiency of a greenhouse rose crop. Biosyst. Eng. 2011, 110, 261–271. [Google Scholar] [CrossRef]
- Mutwiwa, U.N.; Von Elsner, B.; Tantau, H.J.; Max, J.F.J. Effects of near infrared reflection greenhouse cooling on blossom-end rot and fruit cracking in tomato (Solanum lycopersicum L.). Afr. J. Hortic. Sci. 2008, 1, 33–43. [Google Scholar]
- Ikeda, T.; Ishigami, Y.; Goto, E. The effect of CO2 enrichment in a closed greenhouse equipped with NIR-reflecting film and EHP cooling on the yield and quality of tomato fruits during the summer season. J. Agric. Meteorol. 2020, 76, 104–110. [Google Scholar] [CrossRef]
- Nakayama, M.; Fujita, S.; Watanabe, Y.; Ando, T.; Isozaki, M.; Iwasaki, Y. The effect of greenhouse cultivation under a heat insulation film covering on tomato growth, yield, and fruit quality in a subtropical area. Hort. J. 2021, 90, 304–313. [Google Scholar] [CrossRef]
- Dorais, M.; Papadopoulos, A.; Gosselin, A. Greenhouse tomato fruit quality. Hort. A Rev. 2001, 26, 239–319. [Google Scholar]
- Frazier, W.A.; Bowers, J.L. A report on studies of tomato fruit cracking in Maryland. Proc. Soc. Hortic. Sci. 1947, 49, 241–255. [Google Scholar]
- Lang, A.; Düring, H. Grape berry splitting and some mechanical properties of the skin. Vitis 1990, 29, 61–70. [Google Scholar] [CrossRef]
- Peet, M.M. Fruit cracking in tomato. Horttechnology 1992, 2, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, M.G.; Runkle, E.S. Influence of NIR-reflecting shading paint on greenhouse environment, plant temperature, and growth and flowering of bedding plants. Trans. ASABE 2010, 53, 939–944. [Google Scholar] [CrossRef]
- Lobos, G.A.; Acevedo-Opazo, C.; Guajardo-Moreno, A.; Valdés-Gómez, H.; Taylor, J.; Laurie, V.F. Effects of kaolin-based particle film and fruit zone netting on Cabernet Sauvignon physiology and fruit quality. J. Int. Sci. Vigne Vin 2015, 49, 137–144. [Google Scholar] [CrossRef]
- Spicher, L.; Glauser, G.; Kessler, F. Lipid antioxidant and galactolipid remodeling under temperature stress in tomato plants. Front. Plant Sci. 2016, 7, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.S.; Husna, M.T.; Uddin, M.N.; Hossain, M.S.; Ali, A.K.M.G.; Abdel, O.M.; Latef, A.A.H.A.; Hossain, A. Heat Stress at Early Reproductive Stage Differentially Alters Several Physiological and Biochemical Traits of Three Tomato Cultivars. Horticulturae 2021, 7, 330. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Y.; Wang, L.; Ding, J.; Cao, Y.; Qin, G.; Yan, L.; Xi, L.; Zhang, J.; Zou, Z. Increased CO2 and light intensity regulate growth and leaf gas exchange in tomato. Physiol. Plant 2020, 168, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Heuvelink, E. Influence of day and night temperature on the growth of young tomato plants. Sci. Hortic. 1989, 38, 11–22. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X.L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Gent, M.P.N. Carbohydrate level and growth of tomato plants: II. The effect of irradiance and temperature. Plant Physiol. 1986, 81, 1075–1079. [Google Scholar] [CrossRef] [Green Version]
- Bruggink, G.T.; Heuvelink, E. Influence of light on the growth of young tomato, cucumber and sweet pepper plants in the greenhouse: Effects on relative growth rate, net assimilation rate and leaf area ratio. Sci. Hortic. 1987, 31, 161–174. [Google Scholar] [CrossRef]
- Abdel-Ghany, A.M.; Al-Helal, I.M.; Alzahrani, S.M.; Alsadon, A.A.; Ali, I.M.; Elleithy, R.M. Covering materials incorporating radiation-preventing techniques to meet greenhouse cooling challenges in arid regions: A review. Sci. World J. 2012, 2012, 906360. [Google Scholar] [CrossRef] [PubMed]
- Japan Meteorological Agency. 2020 Past Weather Data Search. Available online: https://www.jma.go.jp/jma/index.html (accessed on 19 February 2021).
- Saeki, T. Growth analysis of plants. Shokubutsugaku Zasshi 1965, 78, 111–119. [Google Scholar] [CrossRef]
- Watson, D.J. The dependence of net assimilation rate on leaf-area index. Ann. Bot. 1958, 22, 37–54. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and Simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Yasuba, K.; Suzuki, K.; Sasaki, H.; Higashide, T.; Takaichi, M. Fruit yield and environmental condition under integrative environment control for high yielding production at long-time culture of tomato. Bull. Naro Veg. Flor. Sci. 2011, 3, 19–28. (In Japanese) [Google Scholar]
- Hemming, S.; Kempkes, F.; van der Braak, N.; Dueck, T.; Marissen, N. Filtering natural light at the greenhouse covering—Better greenhouse climate and higher production by filtering out NIR? Acta Hortic. 2006, 711, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Kempkes, F.L.K.; Stanghellini, C.; Hemming, S. Cover materials excluding near infrared radiation: What is the best strategy in mild climates? Acta Hortic. 2009, 807, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Corey, K.A.; Tan, Z.Y. Induction of changes in internal gas pressure of bulky plant organs by temperature gradients. JASHS 1990, 115, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.J.; Ho, L.C. Carbon translocation in the tomato: Carbon import and fruit growth. Ann. Bot. 1977, 41, 813–823. [Google Scholar] [CrossRef]
- Pearce, B.D.; Grange, R.I.; Hardwick, K. The growth of young tomato fruit. I. Effects of temperature and irradiance on fruit grown in controlled environments. J. Hortic. Sci. 1993, 68, 1–11. [Google Scholar] [CrossRef]
- Pearce, B.D.; Grange, R.I.; Hardwick, K. The growth of young tomato fruit. II. Environmental influences on glasshouse crops grown in rock wool or nutrient film. J. Hortic. Sci. 1993, 68, 13–23. [Google Scholar] [CrossRef]
- Bakker, J.C.; Janse, J. Lage etmaaltemperatuur geeft meer kans op zwelscheuren bij tomaat. Weekbl. Groenten Fruit 1988, 26, 30–31. [Google Scholar]
- Schilstra-van Veelen, I.M.; Bakker, J.C. Cracking of Tomato Fruits. In Annual Report 1985 Glasshouse Crops Research Station Naaldwijk; Glasshouse Crops Research Station: Naaldwijk, The Netherlands, 1985; p. 39. [Google Scholar]
- Higashide, T.; Yasuba, K.; Suzuki, K.; Nakano, A.; Ohmori, H. Yield of Japanese tomato cultivars has been hampered by a breeding focus on flavor. HortScience 2012, 47, 1408–1411. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.; Goto, C.; Iwasaki, Y.; Sugeno, W.; Ahn, D.; Higashide, T. Production of high soluble Solids fruits without reducing dry matter production in tomato plants grown in salinized nutrient solution controlled by electrical conductivity. Hort. J. 2020, 89, 403–409. [Google Scholar] [CrossRef]
- Kimura, M.; Fujitani, S.; Itimanda, K. Mitigation techniques on fruit cracking in tomato cultivation under rain shelter in summer and autumn. Bull. Oita Pref. Agric. Forest. Fish. Res. Cent. 2012, 2, 23–42. (In Japanese) [Google Scholar]
- Makino, A.; Sato, T.; Nakano, H.; Mae, T.T. Leaf photosynthesis, plant growth and nitrogen allocation in rice under different irradiances. Planta 1997, 203, 390–398. Available online: https://link.springer.com/content/pdf/10.1007/s004250050205.pdf (accessed on 17 January 2021). [CrossRef]
- Kaneko, S.; Higashide, T.; Yasuba, K.; Ohmori, H.; Nakano, A. Effects of planting stage and density of tomato seedlings on growth and yield component in low-truss cultivation. Hort. Res. 2015, 14, 163–170. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Higashide, T. Review of dry matter production and light interception by plants for yield improvement of greenhouse tomatoes in Japan. Hort. Res. (Jpn.) 2018, 17, 133–146. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Makino, A.; Mae, T. The effects of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiol. 1997, 115, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Film | Total Yield (kg m−2) | No. Fruits (Fruit m−2) | Fresh Fruit Weight (g Fruit−1) | DM Content (%) | Marketable Fruit Yield (kg m−2) | Fruit Cracking Rate (%) |
|---|---|---|---|---|---|---|
| NR | 6.0 ± 0.3 | 74.0 ± 2.4 | 80.9 ± 3.0 | 4.76 ± 0.03 | 3.9 ± 0.1 | 13.4 ± 2.2 |
| PO | 6.6 ± 0.4 | 74.0 ± 3.5 | 89.6 ± 2.7 | 5.13 ± 0.02 | 3.8 ± 0.5 | 29.6 ± 3.6 |
| p-value | 0.18 | 0.68 | <0.05 * | <0.01 ** | 0.87 | <0.01 ** |
| Film | 42DAT | 92DAT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TDM (g m−2) | DM Allocation to Each Organ (%) | TDM (g m−2) | DM Allocation to Each Organ (%) | |||||||
| Stem | Leaves | Fruits | Others | Stem | Leaves | Fruits | Others | |||
| NR | 409.2 ± 7.2 | 30.6 ± 0.4 | 42.7 ± 0.7 | 4.7 ± 0.2 | 3.4 ± 0.2 | 720.9 ± 26.7 | 20.3 ± 0.6 | 31.9 ± 0.6 | 40.6 ± 1.2 | 7.1 ± 0.3 |
| PO | 447.1 ± 4.8 | 31.7 ± 0.4 | 42.0 ± 1.1 | 4.7 ± 0.3 | 2.9 ± 0.2 | 891.2 ± 21.0 | 20.2 ± 0.4 | 32.0 ± 0.5 | 38.3 ± 1.0 | 9.5 ± 0.7 |
| p-value | <0.01 ** | 0.08 | 0.63 | 1.00 | 0.09 | <0.01 ** | 0.81 | 0.88 | 0.14 | <0.01 ** |
| Film | 0-42DAT | 42-92DAT | ||||
|---|---|---|---|---|---|---|
| CGR (g m−2 Day−1) | NAR (g m−2 Day−1) | LAI (m2 m−2) | CGR (g m−2 Day−1) | NAR (g m−2 Day−1) | LAI (m2 m−2) | |
| NR | 9.57 ± 0.17 | 6.80 ± 0.17 | 3.17 ± 0.10 | 6.11 ± 0.52 | 1.74 ± 0.14 | 3.86 ± 0.18 |
| PO | 10.50 ± 0.12 | 7.36 ± 0.23 | 3.35 ± 0.16 | 8.71 ± 0.41 | 2.26 ± 0.08 | 4.37 ± 0.13 |
| p-value | <0.01 ** | 0.08 | 0.38 | <0.01 ** | <0.01 ** | <0.05 * |
| Film | Total N (mmol m−2) | chl (mmol m−2) | chl a/b |
|---|---|---|---|
| NR | 87.6 ± 1.2 | 0.187 ± 0.006 | 3.19 ± 0.05 |
| PO | 107.4 ± 2.0 | 0.229 ± 0.006 | 3.11 ± 0.10 |
| p-value | <0.01 ** | <0.01 ** | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaura, H.; Furuyama, S.; Takano, N.; Nakano, Y.; Kanno, K.; Ando, T.; Amasaki, I.; Watanabe, Y.; Iwasaki, Y.; Isozaki, M. Effects of NIR Reflective Film as a High Tunnel-Covering Material on Fruit Cracking and Biomass Production of Tomatoes. Horticulturae 2022, 8, 51. https://doi.org/10.3390/horticulturae8010051
Yamaura H, Furuyama S, Takano N, Nakano Y, Kanno K, Ando T, Amasaki I, Watanabe Y, Iwasaki Y, Isozaki M. Effects of NIR Reflective Film as a High Tunnel-Covering Material on Fruit Cracking and Biomass Production of Tomatoes. Horticulturae. 2022; 8(1):51. https://doi.org/10.3390/horticulturae8010051
Chicago/Turabian StyleYamaura, Hiroko, Shinichi Furuyama, Nobuo Takano, Yuka Nakano, Keiichi Kanno, Takashi Ando, Ichiro Amasaki, Yukie Watanabe, Yasunaga Iwasaki, and Masahide Isozaki. 2022. "Effects of NIR Reflective Film as a High Tunnel-Covering Material on Fruit Cracking and Biomass Production of Tomatoes" Horticulturae 8, no. 1: 51. https://doi.org/10.3390/horticulturae8010051






