Influence of Flue Gas Desulfurization Gypsum on Phosphorus Loss from a Horticultural Growth Medium
Abstract
:1. Introduction
2. Materials and Methods
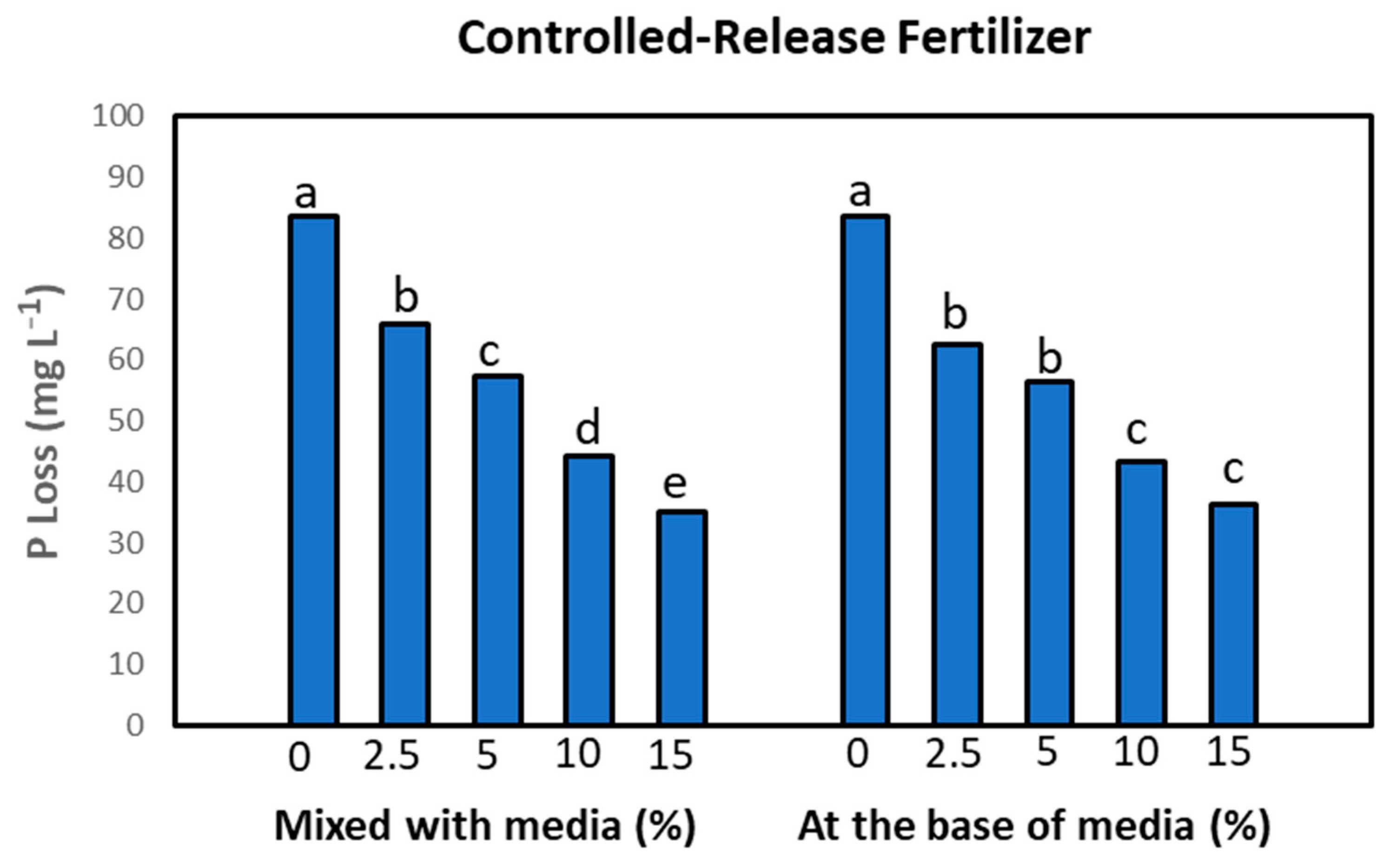
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- US EPA. Safe and Sustainable Water Resources—Strategic Research Action Plan 2019–2022; EPA 601K20005; US Environmental Protection Agency: Washington, DC, USA, 2020. Available online: https://www.epa.gov/research/safe-and-sustainable-water-resources-strategic-research-action-plan-2019-2022 (accessed on 21 June 2021).
- Sharpley, A.; Daniel, T.; Sims, T.; Lemunyon, J.L.; Stevens, R.; Parry, R. Agricultural Phosphorus and Eutrophication; United States Department of Agriculture: Washington, DC, USA, 2003.
- Michaud, A.R.; Lauzier, R.; Laverdiere, M.R. Temporal and spatial variability in non-point source phosphorus in relation to agricultural production and terrestrial indicators: The Beaver Brook case study, Pike River basin, Quebec. In Lake Champlain: Partnership and Research in the New Millennium; Mihuc, T., Ed.; Kluwer Academic/Plenum Pub: Dordrecht, The Netherlands, 2004; pp. 97–121. [Google Scholar]
- Joosse, P.J.; Baker, D.B. Context for re-evaluating agricultural source phosphorus loadings to the great Lakes. Can. J. Soil Sci. 2011, 91, 317–327. [Google Scholar] [CrossRef]
- Bunting, L.; Leavitt, P.R.; Simpson, G.L.; Wissel, B.; Laird, K.R.; Cumming, B.F.; St Amand, A.; Engstrom, D.R. Increased variability and sudden ecosystem state change in Lake Winnipeg, Canada, caused by 20th century agriculture. Limnol. Oceanogr. 2016, 61, 2090–2107. [Google Scholar] [CrossRef] [Green Version]
- US EPA. Nutrient Pollution: Sources and Solutions; US Environmental Protection Agency: Washington, DC, USA, 2017. Available online: https://www.epa.gov/nutrientpollution/sources-and-solutions (accessed on 21 June 2021).
- Boesch, D.F. Barriers and bridges in abating coastal Eutrophication. Front. Mar. Sci. 2019, 6, 123. [Google Scholar] [CrossRef] [Green Version]
- USDA-NASS. 2019 Census of Horticultural Specialties. Volume 3, Special Studies, Part 3 AC-17-SS-3. 2020. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/Census_of_Horticulture_Specialties/HORTIC.pdf (accessed on 18 June 2021).
- Scheiber, S.; Wang, Q.; Pearson, B.; Beeson, R.; Chen, J. Evaluation of irrigation frequency and quantity on leaf gas exchange, growth, and nitrate leaching of coleus in a simulated landscape. HortScience 2008, 43, 881–884. [Google Scholar] [CrossRef]
- Bayer, A.; Whitaker, K.; Chappell, M.; Ruter, J.; van Iersel, M. Effect of irrigation duration and fertilizer rate on plant growth, substrate EC, and leaching volume. Acta Hortic. 2014, 1034, 477–484. [Google Scholar] [CrossRef]
- Sanders, K.R.; Beasley JSBush, E.W.; Conger, S.L. Fertilizer source and irrigation depth affect nutrient leaching during coleus container production. J. Environ. Hortic. 2019, 37, 113–119. [Google Scholar] [CrossRef]
- Weaver, D.; Summers, R. Soil factors influencing eutrophication. In Soilguide. A Handbook for Understanding and Managing Agricultural Soil; Moore, G., Ed.; Bulletin 4343; Department of Agriculture and Food: Perth, WA, Australia, 2001; pp. 243–250. Available online: https://www.agric.wa.gov.au/sites/gateway/files/Soil%20Guide%20-%20a%20handbook.pdf (accessed on 21 June 2021).
- Oh, Y.-M.; Nelson, P.V.; Hesterberg, D.L.; Niedziela, C.E. Efficacy of a phosphate-charged soil material in supplying phosphate for plant growth in soilless root media. Int. J. Agron. 2016, 10, doi. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.A.; Nelson, P.V. Low, controlled nutrient availability provided by organic waste materials for chrysanthemum. J. Am. Soc. Hortic. Sci. 1992, 117, 422–429. [Google Scholar] [CrossRef]
- Ku, C.S.M.; Hershey, D.R. Growth response, nutrient leaching, and mass balance for potted poinsettia. II. Phosphorus. J. Am. Soc. Hortic. Sci. 1997, 122, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Norton, L.D. Gypsum soil amendment as a management practice in con-servation tillage to improve water quality. J. Soil Water Conserv. 2008, 63, 46A–48A. [Google Scholar] [CrossRef]
- Watts, D.B.; Torbert, H.A. Impact of gypsum applied to buffer strips on reducing soluble P in surface water runoff. J. Environ. Qual. 2009, 38, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Endale, D.M.; Schomberg, H.H.; Fisher, D.S.; Franklin, D.H.; Jenkins, M.B. Flue gas desulfurization gypsum: Implication for runoff and nutrient losses associated with broiler litter use on pastures on Ultisols. J. Environ. Qual. 2014, 43, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Torbert, H.A.; Watts, D.B. Impact of flue gas desulfurization gyp-sum application on water quality in a Coastal Plain soil. J. Environ. Qual. 2014, 43, 273–280. [Google Scholar] [CrossRef]
- Watts, D.B.; Torbert, H.A. Influence of flue gas desulfurization gypsum on reducing soluble phosphorus in successive runoff events from a coastal plain bermudagrass pasture. J. Environ. Qual. 2016, 45, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.B.; Torbert, H.A. Three annual flue gas desulfurization gypsum applications on macronutrient and micronutrient losses in runoff from bermudagrass fertilized with poultry litter. Soil Sci. 2017, 182, 18–27. [Google Scholar] [CrossRef]
- Watts, D.B.; Dick, W.A. Sustainable Uses of FGD gypsum in agricultural systems: Introduction. J. Environ. Qual. 2014, 43, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Dick, W.A. Gypsum as an Agricultural Amendment: GENERAL Use Guidelines; Extension Bulletin 945; The Ohio State University: Columbus, OH, USA, 2011; Available online: https://fabe.osu.edu/sites/fabe/files/imce/files/Soybean/Gypsum%20Bulletin.pdf (accessed on 21 June 2021).
- Watts, D.B.; Torbert, H.A.; Mitchell, C.C. Gypsum Use to Reduce P Loss from Agricultural Fields; Alabama Experiment Station: Auburn, AL, USA, 2013; Available online: https://aurora.auburn.edu/handle/11200/44267 (accessed on 21 June 2021).
- USDA-NRCS. Phosphorus Index for Alabama—A Planning Tool to Assess & Manage P Movement. Agronomic Technical Note AL-72. 2014. Available online: https://efotg.sc.egov.usda.gov/references/public/AL/AGRON-AL-72_Phosphorus_Index.pdf (accessed on 21 June 2021).
- USDA-NRCS. Amending Soil Properties with Gypsum Products. Conservation Practice Standard, Code 333. 2015. Available online: http://www.nrcs.usda.gov/wps/portal/nrcs/detailfull/national/techni-cal/cp/ncps/?cid=nrcs143_026849 (accessed on 21 June 2021).
- Kovar, J.L.; Pierzynski, G.M. Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters; Southern Cooperative Series Bull. 408; Virginia Tech University: Blacksburg, VA, USA, 2009; Available online: https://sera17dotorg.files.wordpress.com/2015/02/sera-17-methods-for-p-2000.pdf (accessed on 21 June 2021).
- SAS Institute. SAS/STAT for Windows. Release 9.4; SAS Institute: Cary, NC, USA, 2014. [Google Scholar]
- Broschat, T.K. Rates of ammonium-nitrogen, nitrate-nitrogen, phosphorus, and potassium from two controlled-release fertilizers under different substrate environments. HortTechnology 2005, 15, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Zhou, J.; Shaviv, A. Release characteristics of nutrients from polymer-coated compound controlled release fertilizers. J. Polym. Environ. 2006, 14, 223–230. [Google Scholar] [CrossRef]
- Newman, J.P.; Albano, J.P.; Merhaut, D.J.; Blythe, E.K. Nutrient release from controlled-release fertilizers in a neutral-pH substrate in an outdoor environment: I. Leachate electrical conductivity, pH, and nitrogen, phosphorus, and potassium concentrations. HortScience 2006, 41, 1674–1682. [Google Scholar] [CrossRef]
- Broschat, T.K.; Moore, K.K. Release rates of ammonium-nitrogen, nitrate-nitrogen, phosphorus, potassium, magnesium, iron, and manganese from seven controlled-release fertilizers. Commun. Soil Sci. Plant Anal. 2007, 38, 843–850. [Google Scholar] [CrossRef]
- Liu, G.; Zotarelli, L.; Li, Y.; Dinkins, D.; Wang, Q.; Ozores-Hampton, M. Controlled-Release and Slow-Release Fertilizers as Nutrient Management Tools; HS1255. UF/IFAS Extension; University of Florida: Gainesville, FL, USA, 2014; Available online: http://edis.ifas.ufl.edu/hs1255 (accessed on 21 June 2021).
- Mikkelsen, R.L.; Williams, H.M.; Behel, A.D.J. Nitrogen leaching and plant uptake from controlled-released fertilizers. Fert. Res. 1994, 37, 43–50. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Benlloch, M.; Herrera, E.; García-Novelo, J.M. Effect of traditional and slow-release N fertilizers on growth of olive nursery plants and N losses by leaching. Sci. Hortic. 2004, 101, 39–49. [Google Scholar] [CrossRef]
- Morgan, K.T.; Cushman, K.E.; Sato, S. Release mechanisms for slow- and controlled-release fertilizers and strategies for their use in vegetable production. HortTechnology 2009, 19, 10–12. [Google Scholar] [CrossRef]
- Brauer, D.; Aiken, G.E.; Pote, D.H.; Livingston, S.J.; Norton, L.D.; Way, T.R.; Edwards, J.H. Amendments effects on soil test phosphorus. J. Environ. Qual. 2005, 34, 1682–1686. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley & Sons: New York, NY, USA, 1979. [Google Scholar]
- Shreckhise, J.H.; Owen Jr, J.S.; Eick, M.J.; Niemiera, A.X.; Atland, J.E.; White, S.A. Dolomite and micronutrient fertilizer affect phosphorus fate in pine bark substrate used for containerized nursery crop production. Soil Sci. Soc. Am. J. 2019, 83, 1410–1420. [Google Scholar] [CrossRef] [Green Version]
- King, K.W.; Williams, M.R.; Dick, W.A.; LaBarge, G.A. Decreasing phosphorus loss in tile-drained landscapes using flue gas desulfurization gypsum. J. Environ. Qual. 2016, 45, 1722–1730. [Google Scholar] [CrossRef]
- Stout, W.L.; Sharpley, A.N.; Landa, J. Effectiveness of coal combustion by-products in controlling phosphorus export from soils. J. Environ. Qual. 2000, 29, 1239–1244. [Google Scholar] [CrossRef] [Green Version]




| Application Method | Gypsum Rate | Sampling Day of Year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 171 | 172 | 176 | 179 | 183 | 189 | 196 | 234 | ||
| Inorganic P (mg L−1) | |||||||||
| mixed | 0 | 167.82 a | 90.38 a | 13.37 a | 27.01 a | 62.09 a | 72.12 a | 95.95 a | 62.86 a |
| 2.5 | 115.10 ab | 80.62 a | 4.88 b | 8.50 b | 17.19 b | 27.42 b | 29.76 b | 26.33 b | |
| 5 | 108.18 ab | 72.65 ab | 3.82 b | 4.66 bc | 13.42 b | 21.38 bc | 29.21 b | 22.09 b | |
| 10 | 95.79 b | 67.01 ab | 3.21 b | 2.33 c | 9.84 b | 21.94 bc | 25.83 b | 17.66 b | |
| 15 | 62.98 b | 52.53 b | 2.91 b | 3.64 bc | 9.30 b | 17.03 c | 26.22 b | 5.17 c | |
| p > F | 0.048 | 0.067 | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| separated | 0 | 167.82 a | 90.38 a | 13.37 a | 27.01 a | 62.09 a | 72.12 a | 95.95 a | 62.86 a |
| 2.5 | 145.80 a | 82.86 a | 8.18 ab | 13.68 b | 50.38 b | 70.87 a | 94.23 a | 60.10 a | |
| 5 | 121.10 a | 69.79 ab | 4.18 bc | 10.14 bc | 33.33 c | 69.72 a | 93.78 a | 44.27 b | |
| 10 | 103.99 a | 55.42 b | 2.67 c | 7.24 bc | 12.60 d | 56.43 a | 79.64 a | 27.81 c | |
| 15 | 95.81 a | 50.59 b | 1.17 c | 5.63 c | 12.01 d | 52.27 a | 76.15 a | 25.89 c | |
| p > F | 0.269 | 0.016 | 0.001 | 0.001 | <0.001 | 0.171 | 0.365 | <0.001 | |
| Application Method | Gypsum Rate | Sampling Day of Year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 171 | 172 | 176 | 179 | 183 | 189 | 196 | 234 | ||
| Inorganic P (mg L−1) | |||||||||
| mixed | 0 | 105.13 a | 96.96 a | 99.25 a | 76.94 a | 91.73 a | 73.57 a | 66.43 a | 58.54 a |
| 2.5 | 98.81 a | 86.94 a | 67.30 b | 49.03 b | 76.70 a | 60.63 b | 46.58 b | 40.21 b | |
| 5 | 87.40 ab | 80.64 a | 62.57 b | 49.65 b | 48.84 b | 54.19 b | 42.03 b | 33.70 c | |
| 10 | 69.05 bc | 67.22 a | 46.61 c | 36.77 bc | 35.38 b | 34.87 c | 34.59 c | 29.77 c | |
| 15 | 62.17 c | 63.85 a | 38.14 c | 22.65 c | 13.42 c | 23.78 d | 26.00 d | 30.86 c | |
| p > F | <0.001 | 0.452 | <0.001 | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | |
| separated | 0 | 105.13 a | 96.96 a | 99.25 a | 76.94 a | 91.73 a | 73.57 a | 66.43 a | 58.54 a |
| 2.5 | 82.66 ab | 84.68 ab | 55.24 b | 66.25 ab | 47.02 b | 61.01 ab | 62.07 a | 41.82 b | |
| 5 | 69.05 bc | 72.65 ab | 53.17 b | 55.15 b | 49.63 b | 58.46 bc | 55.36 a | 37.21 bc | |
| 10 | 58.35 bc | 55.14 bc | 19.13 c | 40.33 c | 41.39 b | 50.58 bc | 56.00 a | 27.45 c | |
| 15 | 55.53 c | 43.04 c | 7.66 c | 17.63 d | 36.06 b | 45.79 c | 54.77 a | 29.60 c | |
| p > F | 0.007 | 0.022 | 0.001 | <0.001 | <0.001 | 0.013 | 0.568 | 0.001 | |
| Application Method | Gypsum Rate | Sampling Day of Year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 171 | 172 | 176 | 179 | 183 | 189 | 196 | 234 | ||
| Inorganic P Load (mg) | |||||||||
| mixed | 0 | 62.26 a | 23.55 a | 3.35 a | 7.23 a | 16.41 a | 33.56 a | 42.17 a | 19.31 a |
| 2.5 | 38.57 b | 24.29 a | 1.57 b | 2.84 b | 5.91 b | 12.91 b | 13.83 b | 8.94 b | |
| 5 | 36.27 b | 22.15 a | 1.15 b | 1.49 bc | 3.01 bc | 10.33 bc | 13.17 b | 7.16 b | |
| 10 | 40.56 ab | 21.11 a | 1.11 b | 0.83 c | 3.59 bc | 10.67 b | 12.09 b | 6.04 b | |
| 15 | 27.15 b | 18.55 a | 1.00 b | 1.28 bc | 5.97 c | 8.07 c | 11.84 b | 1.56 c | |
| p > F | 0.056 | 0.669 | 0.022 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| separated | 0 | 62.26 a | 23.55 a | 3.35 a | 7.23 a | 16.41 a | 33.56 a | 42.17 a | 19.31 a |
| 2.5 | 49.24 a | 23.29 a | 2.69 a | 4.48 b | 16.70 a | 33.19 a | 41.42 ab | 19.89 a | |
| 5 | 39.73 a | 19.52 ab | 1.28 b | 3.11 b | 10.07 b | 32.06 ab | 39.32 ab | 13.17 b | |
| 10 | 41.24 a | 17.20 ab | 0.84 b | 2.25 b | 3.97 c | 25.23 ab | 31.04 bc | 7.48 c | |
| 15 | 37.28 a | 15.85 b | 0.39 b | 1.84 b | 3.67 c | 23.06 b | 28.31 c | 6.62 c | |
| p > F | 0.302 | 0.111 | 0.002 | 0.004 | <0.001 | 0.090 | 0.049 | <0.001 | |
| Application Method | Gypsum Rate | Sampling Day of Year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 171 | 172 | 176 | 179 | 183 | 189 | 196 | 234 | ||
| Inorganic P Load (mg) | |||||||||
| mixed | 0 | 36.90 a | 26.12 a | 28.03 a | 21.54 a | 25.94 a | 33.24 a | 27.93 a | 17.93 a |
| 2.5 | 28.82 ab | 25.12 a | 22.87 ab | 16.43 ab | 26.72 a | 28.31 b | 21.15 b | 14.03 b | |
| 5 | 27.11 ab | 23.42 a | 20.58 bc | 16.38 ab | 16.57 b | 25.53 b | 19.04 b | 10.91 c | |
| 10 | 25.02 b | 21.26 a | 15.65 cd | 13.14 ab | 13.07 b | 17.00 c | 16.06 c | 9.69 cd | |
| 15 | 23.41 b | 21.50 a | 13.24 d | 8.07 b | 4.69 c | 11.54 d | 11.85 d | 8.87 d | |
| p > F | 0.086 | 0.884 | 0.001 | 0.069 | <0.001 | <0.001 | <0.001 | <0.001 | |
| separated | 0 | 36.90 a | 26.12 a | 28.03 a | 21.54 ab | 25.94 a | 33.24 a | 27.93 a | 17.93 a |
| 2.5 | 25.90 b | 23.67 a | 20.06 b | 22.76 a | 16.09 b | 29.29 ab | 28.24 a | 15.11 ab | |
| 5 | 20.55 b | 21.63 ab | 17.88 b | 16.97 b | 16.26 b | 26.89 abc | 23.48 a | 12.67 b | |
| 10 | 21.59 b | 17.64 ab | 6.13 c | 12.11 c | 12.19 bc | 22.75 bc | 22.32 a | 7.92 c | |
| 15 | 22.38 b | 13.74 b | 2.41 c | 4.99 d | 9.83 c | 20.79 c | 21.37 a | 8.38 c | |
| p > F | 0.011 | 0.103 | <0.001 | <0.001 | <0.001 | 0.013 | 0.258 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watts, D.B.; Runion, G.B.; Torbert, H.A. Influence of Flue Gas Desulfurization Gypsum on Phosphorus Loss from a Horticultural Growth Medium. Horticulturae 2021, 7, 199. https://doi.org/10.3390/horticulturae7070199
Watts DB, Runion GB, Torbert HA. Influence of Flue Gas Desulfurization Gypsum on Phosphorus Loss from a Horticultural Growth Medium. Horticulturae. 2021; 7(7):199. https://doi.org/10.3390/horticulturae7070199
Chicago/Turabian StyleWatts, Dexter Brown, George Brett Runion, and Henry Allen Torbert. 2021. "Influence of Flue Gas Desulfurization Gypsum on Phosphorus Loss from a Horticultural Growth Medium" Horticulturae 7, no. 7: 199. https://doi.org/10.3390/horticulturae7070199





