Combined Effect of Salinity and LED Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Lighting Treatments
2.3. Agronomical and Biochemical Parameters
2.4. Statistical Analysis
3. Results and Discussion
3.1. Yield
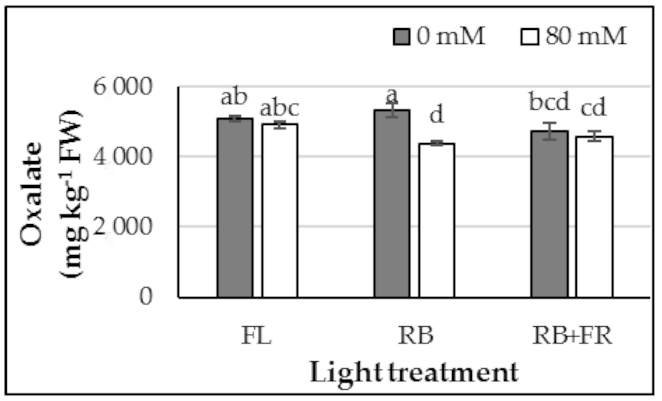
3.2. Anions and Cations
3.3. Total Phenol Content, Total Flavonoids Content and Total Antioxidant Capacity
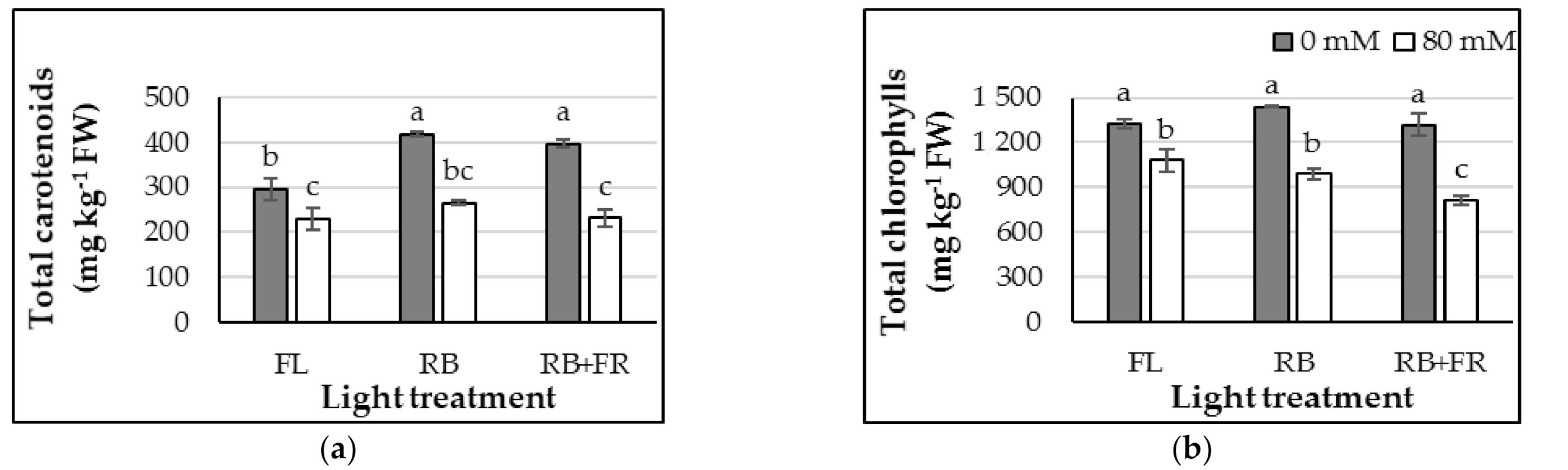
3.4. Chlorophylls and Carotenoids
3.5. Fatty Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cros, V.; Martínez-Sánchez, J.J.; Franco, J.A. Good Yields of Common Purslane with a High Fatty Acid Content Can Be Obtained in a Peat-based Floating System. HortTechnology 2007, 17, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Egea-Gilabert, C.; Ruiz-Hernández, M.V.; Parra, M.Á.; Fernández, J.A. Characterization of purslane (Portulaca oleracea L.) accessions: Suitability as ready-to-eat product. Sci. Hortic. 2014, 172, 73–81. [Google Scholar] [CrossRef]
- Gonnella, M.; Charfeddine, M.; Conversa, G.; Santamaria, P. Purslane: Review of its Potential for Health and Agricultural Aspect. The European. J. Sci. Biotech. 2010, 4, 131–136. [Google Scholar]
- Lara, L.J.; Egea-Gilabert, C.; Niñirola, D.; Conesa, E.; Fernández, J.A. Effect of aeration of the nutrient solution on the growth and quality of purslane (Portulaca oleracea). J. Hortic. Sci. Biotechnol. 2011, 86, 603–610. [Google Scholar] [CrossRef]
- He, J.; You, X.; Qin, L. High Salinity Reduces Plant Growth and Photosynthetic Performance but Enhances Certain Nutritional Quality of C4 Halophyte Portulaca oleracea L. Grown Hydroponically Under LED Lighting. Front. Plant Sci. 2021, 12, 651341. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Carvalho, I.S. Effects of salt stress on purslane (Portulaca oleracea) nutrition. Ann. Appl. Biol. 2008, 154, 77–86. [Google Scholar] [CrossRef]
- Karakaş, S.; Çullu, M.A.; Dikilitaş, M. Comparison of two halophyte species (Salsola soda and Portulaca oleracea)for salt removal potential under different soil salinity conditions. Turk. J. Agric. For. 2017, 41, 183–190. [Google Scholar] [CrossRef]
- Alam, A.; Juraimi, A.; Rafii, M.; Hamid, A.; Aslani, F.; Alam, M. Effects of salinity and salinity-induced augmented bioactive compounds in purslane (Portulaca oleracea L.) for possible economical use. Food Chem. 2015, 169, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.A.; Cros, V.; Vicente, M.J.; Martínez-Sánchez, J.J. Effects of salinity on the germination, growth, and nitrate contents of purslane (Portulaca oleracea L.) cultivated under different climatic conditions. J. Hortic. Sci. Biotechnol. 2011, 86, 1–6. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Teixeira, M.; Brodelius, M. Effect of Salt Stress on Purslane and Potential Health Benefits: Oxalic Acid and Fatty Acids Profile. 2009. Available online: https://escholarship.org/uc/item/4cc78714 (accessed on 20 April 2021).
- Anastácio, A.; Carvalho, I.S. Accumulation of fatty acids in purslane grown in hydroponic salt stress conditions. Int. J. Food Sci. Nutr. 2012, 64, 235–242. [Google Scholar] [CrossRef]
- Alam, A.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Hakim, M.A. Salinity-induced changes in the morphology and major mineral nutrient composition of purslane (Portulaca oleracea L.) accessions. Biol. Res. 2016, 49, 24. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernández, J.A.; Orsini, F.; Artés-Hernández, F. Postharvest LED lighting: Effect of red, blue and far red on quality of minimally processed broccoli sprouts. J. Sci. Food Agric. 2020, 101, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Orsini, F.; Castillejo, N.; Gómez, P.A.; Crepaldi, A.; Fernández, J.A.; Egea-Gilabert, C.; Artés-Hernández, F.; Gianquinto, G. Spectral composition from led lighting during storage affects nutraceuticals and safety attributes of fresh-cut red chard (Beta vulgaris) and rocket (Diplotaxis tenuifolia) leaves. Postharvest Biol. Technol. 2021, 175, 111500. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Duchovskis, P. Nutrient Levels in Brassicaceae Microgreens Increase Under Tailored Light-Emitting Diode Spectra. Front. Plant Sci. 2019, 10, 1475. [Google Scholar] [CrossRef] [Green Version]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current Review of the Modulatory Effects of LED Lights on Photosynthesis of Secondary Metabolites and Future Perspectives of Microgreen Vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-Specific Modulatory Effects of Select Spectral Bandwidths on the Nutritive and Phytochemical Composition of Microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef]
- Egea-Gilabert, C.; Fernández, J.A.; Migliaro, D.; Martínez-Sánchez, J.J.; Vicente, M.J. Genetic variability in wild vs. cultivated Eruca vesicaria populations as assessed by morphological, agronomical and molecular analyses. Sci. Hortic. 2009, 121, 260–266. [Google Scholar] [CrossRef]
- Pennisi, G.; Sanyé-Mengual, E.; Orsini, F.; Crepaldi, A.; Nicola, S.; Ochoa, J.; Fernandez, J.; Gianquinto, G. Modelling Environmental Burdens of Indoor-Grown Vegetables and Herbs as Affected by Red and Blue LED Lighting. Sustainability 2019, 11, 4063. [Google Scholar] [CrossRef] [Green Version]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin−Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labiad, M.; Giménez, A.; Varol, H.; Tüzel, Y.; Egea-Gilabert, C.; Fernández, J.; Martínez-Ballesta, M. Effect of Exogenously Applied Methyl Jasmonate on Yield and Quality of Salt-Stressed Hydroponically Grown Sea Fennel (Crithmum maritimum L.). Agronomy 2021, 11, 1083. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting Broccoli Accumulate Higher Concentrations of Nutritionally Important Metabolites under Narrow-band Light-emitting Diode Lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.H. Growth and Anthocyanins of Lettuce Grown under Red or Blue Light-emitting Diodes with Distinct Peak Wavelength. Korean J. Hortic. Sci. Technol. 2014, 32, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding Far-Red to Red-Blue Light-Emitting Diode Light Promotes Yield of Lettuce at Different Planting Densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Yazici, I.; Türkan, I.; Sekmen, A.H.; Demiral, T. Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ. Exp. Bot. 2007, 61, 49–57. [Google Scholar] [CrossRef]
- Camalle, M.; Standing, D.; Jitan, M.; Muhaisen, R.; Bader, N.; Bsoul, M.; Ventura, Y.; Soltabayeva, A.; Sagi, M. Effect of Salinity and Nitrogen Sources on the Leaf Quality, Biomass, and Metabolic Responses of Two Ecotypes of Portulaca oleracea. Agronomy 2020, 10, 656. [Google Scholar] [CrossRef]
- Bantis, F.; Fotelli, M.; Ilić, Z.; Koukounaras, A. Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution. Agriculture 2020, 10, 574. [Google Scholar] [CrossRef]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.-C. Red Light Is Effective in Reducing Nitrate Concentration in Rocket by Increasing Nitrate Reductase Activity and Contributes to Increased Total Glucosinolates Content. Front. Plant Sci. 2020, 11, 604. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Yanagisawa, S. Light signalling-induced regulation of nutrient acquisition and utilisation in plants. Semin. Cell Dev. Biol. 2018, 83, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.-H.; Lei, B.; Cheng, R.-F.; Wang, Y.; Li, T.; Yang, Q.-C. Selenium distribution and nitrate metabolism in hydroponic lettuce (Lactuca sativa L.): Effects of selenium forms and light spectra. J. Integr. Agric. 2020, 19, 133–144. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Sakalauskienė, S.; Jankauskienė, J.; Miliauskienė, J.; Vaštakaitė, V. LED lighting for reduced nitrate contents in green vegetables. Acta Hortic. 2018, 1227, 669–676. [Google Scholar] [CrossRef]
- Chandok, M.R.; Sopory, S.K. Phosphorylation/dephosphorylation steps are key events in the phytochrome-mediated enhancement of nitrate reductase mRNA levels and enzyme activity in maize. Mol. Gen. Genet. 1996, 251, 599–608. [Google Scholar] [CrossRef]
- Aslam, M.; Huffaker, R.C.; Rains, W.D. Early effects of salinity on nitrate assimilation in barley seedlings. Plant Physiol. 1984, 76, 321–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinigg, M.; Posthumus, F.; Ferschke, M.; Elzenga, J.M.; Stulen, I. Effects of NaCl salinity on 15N-nitrate fluxes and specific root length in the halophyte Plantago maritima L. Plant Soil 2003, 250, 201–213. [Google Scholar] [CrossRef]
- Varma, S.; Rogers, D.M.; Pratt, L.; Rempe, S.B. Design principles for K+ selectivity in membrane transport. J. Gen. Physiol. 2011, 137, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Corrado, G.; Lucini, L.; Miras-Moreno, B.; Chiaiese, P.; Colla, G.; De Pascale, S.; Rouphael, Y. Metabolic Insights into the Anion-Anion Antagonism in Sweet Basil: Effects of Different Nitrate/Chloride Ratios in the Nutrient Solution. Int. J. Mol. Sci. 2020, 21, 2482. [Google Scholar] [CrossRef] [Green Version]
- Mapson, L.W. Metabolism of Ascorbic Acid in Plants: Part I. Function. Annu. Rev. Plant Physiol. 1958, 9, 119–150. [Google Scholar] [CrossRef]
- Hayashi, R.; Morohashi, Y. Phytochrome Control of the Development of Ascorbate Oxidase Activity in Mustard (Sinapis alba L.) Cotyledons. Plant Physiol. 1993, 102, 1237–1241. [Google Scholar] [CrossRef] [Green Version]
- Bienger, I.; Schopfer, P. Photomodulation by phytochrome of the rate of accumulation of ascorbic acid in mustard seedlings (Sinapis alba L.). Planta 1970, 93, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ge, C.; Xu, C.; Wang, X.; Wang, S.; Wang, Q. Expression Analysis of Oxalate Metabolic Pathway Genes Reveals Oxalate Regulation Patterns in Spinach. Molecules 2018, 23, 1286. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Shiqi, L.; Li, X.; Wenyan, Y.; Liang Qingling, L.; Shuqin, H. Effects of light qualities on accumulation of oxalate, tannin and nitrate in spinach. Trans. CSAE 2007, 23, 201–205. [Google Scholar] [CrossRef]
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Singh, K.; Chatrath, R. Salinity tolerance. In Application of Physiology in Wheat Breeding; Reynolds, M.P., Ortiz-Monasterio, J.I., McNab, A., Eds.; CIMMYT Crop Improvement Division: México DF, Mexico, 2001; Chapter 8; pp. 101–110. [Google Scholar]
- Yang, C.; Chong, J.; Li, C.; Kim, C.; Shi, D.; Wang, D. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Kafi, M.; Rahimi, Z. Effect of salinity and silicon on root characteristics, growth, water status, proline content and ion accumulation of purslane (Portulaca oleracea L.). Soil Sci. Plant Nutr. 2011, 57, 341–347. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Jean, Y.-K.; Kabir, I.; Tolay, I.; Torun, A.A.; Kronzucker, H.J. K+ Efflux and Retention in Response to NaCl Stress Do Not Predict Salt Tolerance in Contrasting Genotypes of Rice (Oryza sativa L.). PLoS ONE 2013, 8, e57767. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.P. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014, 202, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamora, L.; Castillejo, N.; Gómez, P.; Artés-Hernández, F. Amelioration Effect of LED Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting. Agronomy 2021, 11, 304. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, S.; Gu, M.; Chen, X.; Chen, X.; Yang, J.; Zhao, F.; Ye, N. Exploration of the Effects of Different Blue LED Light Intensities on Flavonoid and Lipid Metabolism in Tea Plants via Transcriptomics and Metabolomics. Int. J. Mol. Sci. 2020, 21, 4606. [Google Scholar] [CrossRef] [PubMed]
- Bandaranayake, W. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef]
- Certain, C.; Della Patrona, L.; Gunkel-Grillon, P.; Léopold, A.; Soudant, P.; Le Grand, F. Effect of Salinity and Nitrogen Form in Irrigation Water on Growth, Antioxidants and Fatty Acids Profiles in Halophytes Salsola australis, Suaeda maritima, and Enchylaena tomentosa for a Perspective of Biosaline Agriculture. Agronomy 2021, 11, 449. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.-J.; Lee, Y.-T. Effect of salinity stress on bioactive compounds and antioxidant activity of wheat microgreen extract under organic cultivation conditions. Int. J. Biol. Macromol. 2019, 140, 631–636. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. South Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreyra, M.L.F.; Rius, S.; Emiliani, J.; Pourcel, L.; Feller, A.; Morohashi, K.; Casati, P.; Grotewold, E. Cloning and characterization of a UV-B-inducible maize flavonol synthase. Plant J. 2010, 62, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Sakalauskaitė, J.; Sakalauskienė, S.; Duchovskis, P. The impact of red and blue light-emitting diode illumination on radish physiological indices. Open Life Sci. 2011, 6, 821–828. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Hu, S.; Alam, A.; Du, H.; Che, S. The accumulation of fatty acids in different organs of purslane under salt stress. Sci. Hortic. 2019, 250, 236–242. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Thomas-Hall, S.R.; Chua, E.; Alsenani, F.; Eltanahy, E.; Netzel, M.E.; Netzel, G.; Lu, Y.; Schenk, P.M. Gene expression profiling of astaxanthin and fatty acid pathways in Haematococcus pluvialis in response to different LED lighting conditions. Bioresour. Technol. 2018, 250, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Fleurent, G.; Awwad, F.; Cheng, M.; Meddeb-Mouelhi, F.; Budge, S.M.; Germain, H.; Desgagné-Penix, I. Red Light Variation an Effective Alternative to Regulate Biomass and Lipid Profiles in Phaeodactylum tricornutum. Appl. Sci. 2020, 10, 2531. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Jaffel-Hamza, K.; Sai-Kachout, S.; Harrathi, J.; Lachaâl, M.; Marzouk, B. Growth and Fatty Acid Composition of Borage (Borago officinalis L.) Leaves and Seeds Cultivated in Saline Medium. J. Plant Growth Regul. 2013, 32, 200–207. [Google Scholar] [CrossRef]
- Palaniswamy, U.R.; McAvoy, R.J.; Bible, B.B. Stage of harvest and polyunsaturated essential fatty acid concentrations in purslane (Portulaca oleraceae) leaves. J. Agric. Food Chem. 2001, 49, 3490–3493. [Google Scholar] [CrossRef]
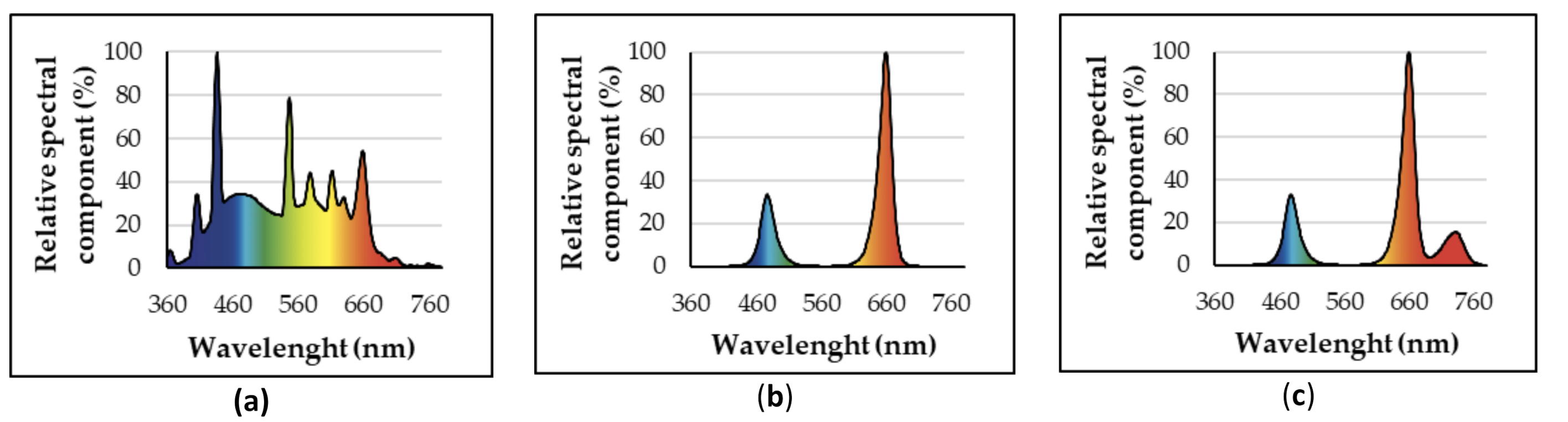


| Yield (kg m−2) | Nitrate (mg kg−1 FW) | |
|---|---|---|
| Light treatment (A) | ||
| FL | 1.47 ± 0.12 a | 6392 ± 120 c |
| RB | 1.88 ± 0.16 b | 1214 ± 64 b |
| RB+FR | 1.87 ± 0.14 b | 556 ± 42 a |
| Salinity (B) | ||
| 0 mM NaCl | 1.51 ± 0.09 a | 2857 ± 501 b |
| 80 mM NaCl | 1.97 ± 0.15 b | 2585 ± 529 a |
| Significant Differences | ||
| A | * | *** |
| B | ** | ** |
| A × B | Ns | ns |
| Total Phenolics (mg GA kg−1 FW) | Total Flavonoids (mg Rutin kg−1 FW) | Antioxidant Capacity (mg DPPHreduced kg−1 FW) | |
|---|---|---|---|
| Treatments (A) | |||
| FL | 1176 ± 21 b | 2829 ± 72 b | 170 ± 3 |
| RB | 1277 ± 27 c | 2819 ± 91 b | 174 ± 5 |
| RB+FR | 931 ± 25 a | 2417 ± 91 a | 177 ± 6 |
| Salinity (B) | |||
| 0 mM NaCl | 1112 ± 64 | 2834 ± 99 b | 175 ± 6 |
| 80 mM NaCl | 1143 ± 69 | 2544 ± 98 a | 172 ± 3 |
| Significant Differences | |||
| A | *** | *** | ns |
| B | ns | ** | ns |
| A × B | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez, A.; Martínez-Ballesta, M.d.C.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined Effect of Salinity and LED Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens. Horticulturae 2021, 7, 180. https://doi.org/10.3390/horticulturae7070180
Giménez A, Martínez-Ballesta MdC, Egea-Gilabert C, Gómez PA, Artés-Hernández F, Pennisi G, Orsini F, Crepaldi A, Fernández JA. Combined Effect of Salinity and LED Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens. Horticulturae. 2021; 7(7):180. https://doi.org/10.3390/horticulturae7070180
Chicago/Turabian StyleGiménez, Almudena, María del Carmen Martínez-Ballesta, Catalina Egea-Gilabert, Perla A. Gómez, Francisco Artés-Hernández, Giuseppina Pennisi, Francesco Orsini, Andrea Crepaldi, and Juan A. Fernández. 2021. "Combined Effect of Salinity and LED Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens" Horticulturae 7, no. 7: 180. https://doi.org/10.3390/horticulturae7070180









