Estimation of Ascorbic Acid in Intact Acerola (Malpighia emarginata DC) Fruit by NIRS and Chemometric Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Fruit Material
2.2. Reference Method for Ascorbic Acid and Morphological Properties
2.3. Near-Infrared Diffuse Reflectance Measurements
2.4. Chemometric Analysis
3. Results
3.1. Experimental Data
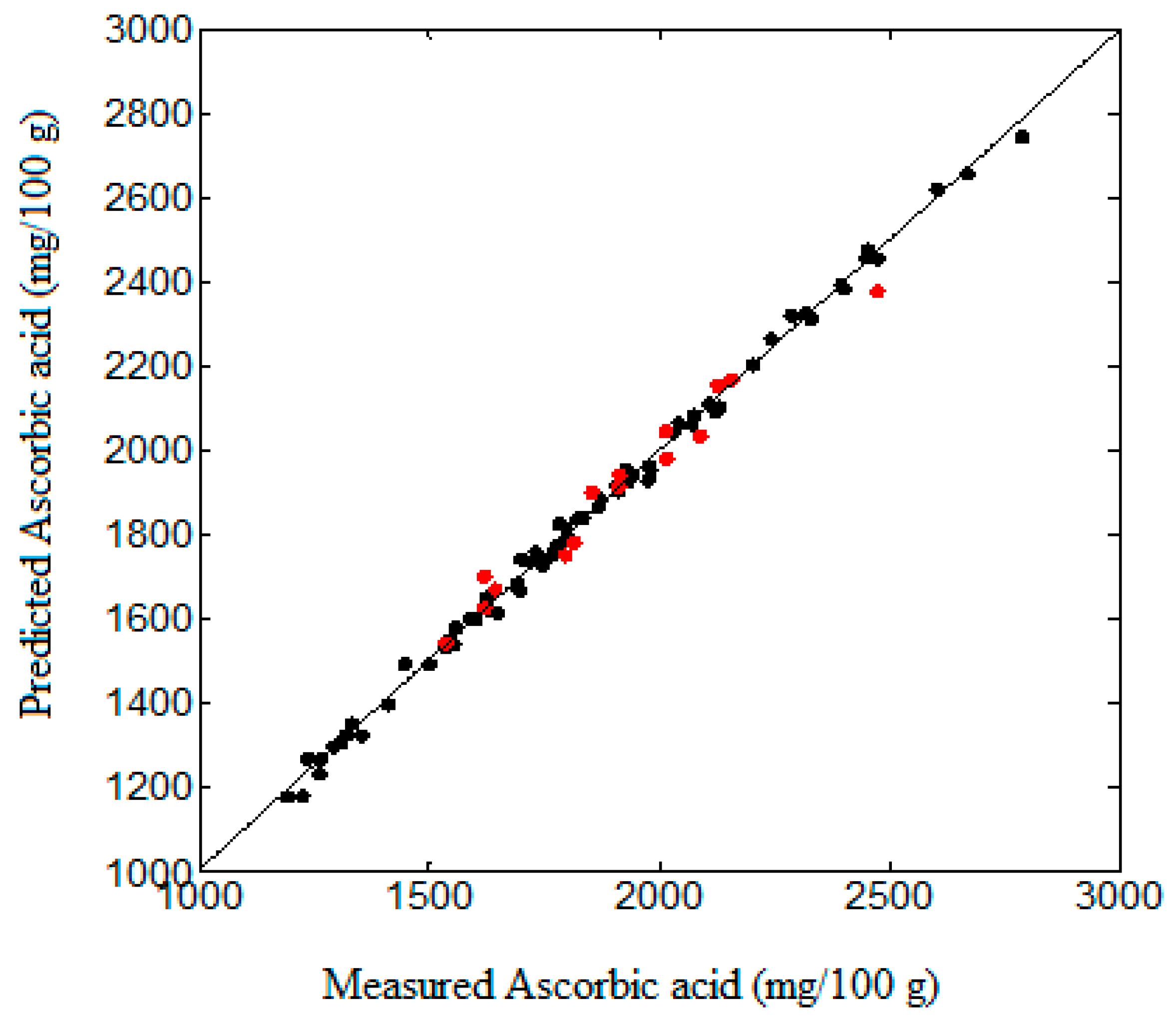
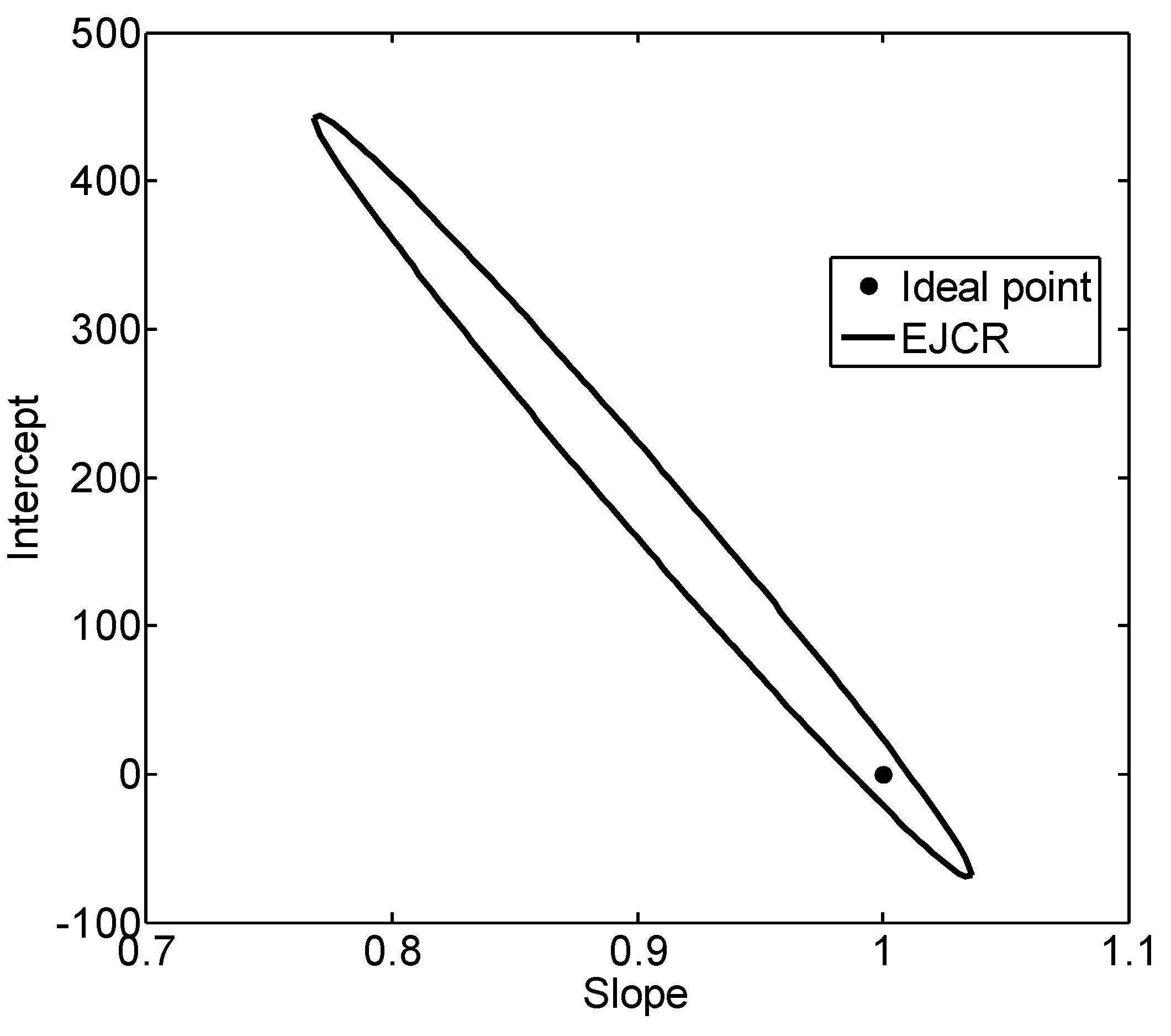
3.2. Ascorbic Acid Model Fitting
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Belwal, T.; Devkota, H.P.; Hassan, H.A.; Ahluwalia, S.; Ramadan, M.F.; Mocan, A.; Atanasov, A.G. Phytopharmacology of Acerola (Malpighia spp.) and its potential as functional food. Trends Food Sci. Technol. 2018, 74, 99–106. [Google Scholar] [CrossRef]
- Future Market Insights. Acerola Extract Market: Rising Global Demand for Natural and Fresh Fruit Derivatives Likely to Boost Market Growth: Global Industry Analysis and Opportunity Assessment, 2016–2026. 2018. Available online: https://www.futuremarketinsights.com/reports/acerola-extract-market (accessed on 1 October 2018).
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.L.; de Oliveira, A.V.; de Brito, E.S.; Ribeiro, P.R.V.; de sá Souza Filho, M.M.; Azeredo, H.M.C. Stabilizing effect of montmorillonite on acerola juice anthocyanins. Food Chem. 2018, 245, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.M.M.; Rodrigues, F.F.G.; Costa, W.D.; Teixeira, R.N.P.; Boligon, A.A.; Sousa, E.O.; Rodrigues, F.F.G.; Coutinho, H.D.M.; da Costa, J.G.M. HPLC and in vitro evaluation of antioxidant properties of fruit from Malpighia glabra (Malpighiaceae) at different stages of maturation. Food Chem. Toxicol. 2018, 119, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Ignat, T.; Schmilovitch, Z.; Fefoldi, J.; Steiner, B.; Alkalai-Tuvia, S. Non-destructive measurement of ascorbic acid content in bell peppers by VIS-NIR and SWIR spectrometry. Postharvest Biol. Technol. 2012, 74, 91–99. [Google Scholar] [CrossRef]
- Blanco-Díaz, M.T.; Del Río-Celestino, M.; Martínez-Valdivieso, D.; Font, R. Use of visible and near-infrared spectroscopy for predicting antioxidant compounds in summer squash (Cucurbita pepo ssp pepo). Food Chem. 2014, 164, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Y.; Xu, J.; Feng, X.; Wu, F.; Zhou, R.; Jin, J.; Xu, K.; Yu, X.; He, Y. SSC and pH for sweet assessment and maturity classification of harvested cherry fruit based on NIR hyperspectral imaging technology. Postharvest Biol. Technol. 2018, 143, 112–118. [Google Scholar] [CrossRef]
- Viegas, T.R.; Mata, A.L.M.L.; Duarte, M.M.L.; Lima, K.M.G. Determination of quality attributes in wax jambu fruit using NIRS and PLS. Food Chem. 2016, 190, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Nieuwoudt, H.; Opara, U.L. Evaluation of biochemical markers associated with the development of husk scald and the use of diffuse reflectance NIR spectroscopy to predict husk scald in pomegranate fruit. Sci. Hortic. 2018, 232, 240–249. [Google Scholar] [CrossRef]
- Moreda, G.P.; Ortiz-Cañavate, J.; García-Ramos, F.J.; Ruiz-Altisent, M. Non-destructive technologies for fruit and vegetable size determination—A review. J. Food Eng. 2009, 92, 119–136. [Google Scholar] [CrossRef]
- Luan, X.; Huang, B.; Sedghi, S.; Liu, F. Probabilistic PCR based near-infrared modeling with temperature compensation. ISA Trans. 2018, 81, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Ichimura, M.; Oshita, S.; Kawagoe, Y.; Yamanaka, H. Estimation of oxygen uptake rate of tomato (Lycopersicon esculentum Mill.) fruits by artificial neural networks modelled using near-infrared spectral absorbance and fruit mass. Food Chem. 2010, 121, 533–539. [Google Scholar] [CrossRef]
- Mariani, N.C.T.; Da Costa, R.C.; De Lima, K.M.G.; Nardini, V.; Cunha Júnior, L.C.; Teixeira, G.H.D.A. Predicting soluble solid content in intact jaboticaba [Myrciaria jaboticaba (Vell.) O. Berg] fruit using near-infrared spectroscopy and chemometrics. Food Chem. 2014, 159, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, W.; Tian, X.; Wang, C.; Fan, S.; Zhao, C. Fast detection and visualization of early decay in citrus using Vis-NIR hyperspectral imaging. Comput. Electron. Agric. 2016, 127, 582–592. [Google Scholar] [CrossRef]
- Moghimi, A.; Aghkhani, M.H.; Sazgarnia, A.; Abbaspour-Fard, M.H. Improvement of NIR transmission mode for internal quality assessment of fruit using different orientations. J. Food Process Eng. 2011, 34, 1759–1774. [Google Scholar] [CrossRef]
- Inácio, M.R.C.; De Lima, K.M.G.; Lopes, V.G.; Pessoa, J.D.C.; De Almeida Teixeira, G.H. Total anthocyanin content determination in intact açaí (Euterpe oleracea Mart.) and palmitero-juçara (Euterpe edulis Mart.) fruit using near infrared spectroscopy (NIR) and multivariate calibration. Food Chem. 2013, 136, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.G.; Godoy, H.T.; Prado, M.A. Otimização de metodologia colorimétrica para a determinação de ácido ascórbico em geleias de frutas. Ciênc. Tecnol. Aliment. 2010, 30, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Pedro, A.M.K.; Ferreira, M.M.C. Simultaneously calibrating solids, sugars and acidity of tomato products using PLS2 and NIR spectroscopy. Anal. Chim. Acta 2007, 595, 221–227. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Altieri, G.; Genovese, F.; Tauriello, A.; Di Renzo, G.C. Models to improve the non-destructive analysis of persimmon fruit properties by VIS/NIR spectrometry. J. Sci. Food Agric. 2017, 97, 5302–5310. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Marín, D.; Torres, I.; Entrenas, J.A.; Vega, M.; Sánchez, M.T. Pre-harvest screening on-vine of spinach quality and safety using NIRS technology. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2019, 207, 242–250. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, F.P.; Gonçalves, A.C.; Miguel, T.B.V.; Borges, K.C.; Correia, R.T.P. Freeze Dried Acerola (Malpighia emarginata) Pulp and Pomace: Physicochemical Attributes, Phytochemical Content and Stability during Storage. J. Food Ind. 2017, 1. [Google Scholar] [CrossRef]
- Oliveira-Folador, G.; de Bicudo, M.O.; de Andrade, E.F.; Renard, C.M.G.C.; Bureau, S.; de Castilhos, F. Quality traits prediction of the passion fruit pulp using NIR and MIR spectroscopy. LWT-Food Sci. Technol. 2018, 95, 172–178. [Google Scholar] [CrossRef]
- Kramchote, S.; Nakano, K.; Kanlayanarat, S.; Ohashi, S.; Takizawa, K.; Bai, G. Rapid determination of cabbage quality using visible and near-infrared spectroscopy. LWT-Food Sci. Technol. 2014, 59, 695–700. [Google Scholar] [CrossRef]
- Alamar, P.D.; Caramês, E.T.S.; Poppi, R.J.; Pallone, J.A.L. Quality evaluation of frozen guava and yellow passion fruit pulps by NIR spectroscopy and chemometrics. Food Res. Int. 2016, 85, 209–214. [Google Scholar] [CrossRef] [PubMed]

| Parameter | N | Min | Max | Mean | S.D. |
|---|---|---|---|---|---|
| Ascorbic acid (mg/100 g FW) | 102 | 1190.65 | 2187.06 | 1833.57 | 374.56 |
| Diameter (mm) | 102 | 10.1 | 25.5 | 19.1 | 3.0 |
| Weight (g) | 102 | 1.5 | 8.2 | 4.2 | 1.4 |
| Model | Number of Selected Wavelengths | Spectral Pre-Treatments | Calibration | Prediction | ||||
|---|---|---|---|---|---|---|---|---|
| RMSECV | RMSEP | RPD | LV | |||||
| PLS | 1553 | 2nd derivative 5 points | 0.88 | 395.7 | 0.17 | 351.5 | 1 | 9 |
| PLS | 1555 | MSC 1st derivative 3points | 0.91 | 402.4 | 0.2 | 355.2 | 1 | 8 |
| PLS | 1555 | MSC 2nd derivative 3points | 0.93 | 510.1 | 0.19 | 358.8 | 1 | 8 |
| iPLS-PLS | 148 | MSC 1st derivative 9 points | 0.73 | 518.9 | 0.16 | 338 | 1.1 | 8 |
| iPLS-PLS | 297 | MSC 2nd derivative 3points | 0.98 | 477.7 | 0.12 | 365.3 | 1 | 8 |
| GA-PLS | 436 | 2nd derivative 5points | 0.98 | 22.9 | 0.54 | 46.3 | 1.6 | 12 |
| GA-PLS | 429 | MSC 2nd derivative 3points | 0.97 | 248.22 | 0.53 | 268.5 | 1.4 | 14 |
| Calibration | Prediction | |||||||
|---|---|---|---|---|---|---|---|---|
| Model | RMSECV | Samples excluded | RMSEP | RPD | Samples excluded | |||
| GA-PLS 9 LV | 0.99 | 20.7 | 4 | 106.2 | 0.85 | 3.5 | 9 | |
| GA-PLS 10 LV | 0.99 | 20.7 | 4 | 101.3 | 0.86 | 3.6 | 10 | |
| GA-PLS 12 LV | 0.99 | 20.7 | 4 | 83.3 | 0.93 | 4.4 | 15 | |
| GA-PLS 14 LV | 0.98 | 22.9 | 3 | 46.3 | 0.96 | 8.0 | 15 | |
| FOM | Ascorbic Acid (mg/100 g FW) |
|---|---|
| RMSECV | 22.9 |
| RMSEP | 46.3 |
| 0.98 | |
| 0.96 | |
| RPD | 8.0 |
| Precision | 11.6 |
| SEN | 1.5598 × 10−6 |
| SEL | 0.047 |
| LD | 1.1397 × 103 |
| LQ | 3.7989 × 103 |
| S/N | 4.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, F.P.d.; Costa, R.C.; Morais, C.d.L.M.d.; Medeiros, F.G.M.d.; Fernandes, T.R.N.; Hoskin, R.T.; Lima, K.M.G.d. Estimation of Ascorbic Acid in Intact Acerola (Malpighia emarginata DC) Fruit by NIRS and Chemometric Analysis. Horticulturae 2019, 5, 12. https://doi.org/10.3390/horticulturae5010012
Moraes FPd, Costa RC, Morais CdLMd, Medeiros FGMd, Fernandes TRN, Hoskin RT, Lima KMGd. Estimation of Ascorbic Acid in Intact Acerola (Malpighia emarginata DC) Fruit by NIRS and Chemometric Analysis. Horticulturae. 2019; 5(1):12. https://doi.org/10.3390/horticulturae5010012
Chicago/Turabian StyleMoraes, Francisca Pereira de, Rosangela Câmara Costa, Camilo de Lelis Medeiros de Morais, Fábio Gonçalves Macêdo de Medeiros, Tássia Rayane Nascimento Fernandes, Roberta Targino Hoskin, and Kássio Michell Gomes de Lima. 2019. "Estimation of Ascorbic Acid in Intact Acerola (Malpighia emarginata DC) Fruit by NIRS and Chemometric Analysis" Horticulturae 5, no. 1: 12. https://doi.org/10.3390/horticulturae5010012
APA StyleMoraes, F. P. d., Costa, R. C., Morais, C. d. L. M. d., Medeiros, F. G. M. d., Fernandes, T. R. N., Hoskin, R. T., & Lima, K. M. G. d. (2019). Estimation of Ascorbic Acid in Intact Acerola (Malpighia emarginata DC) Fruit by NIRS and Chemometric Analysis. Horticulturae, 5(1), 12. https://doi.org/10.3390/horticulturae5010012






